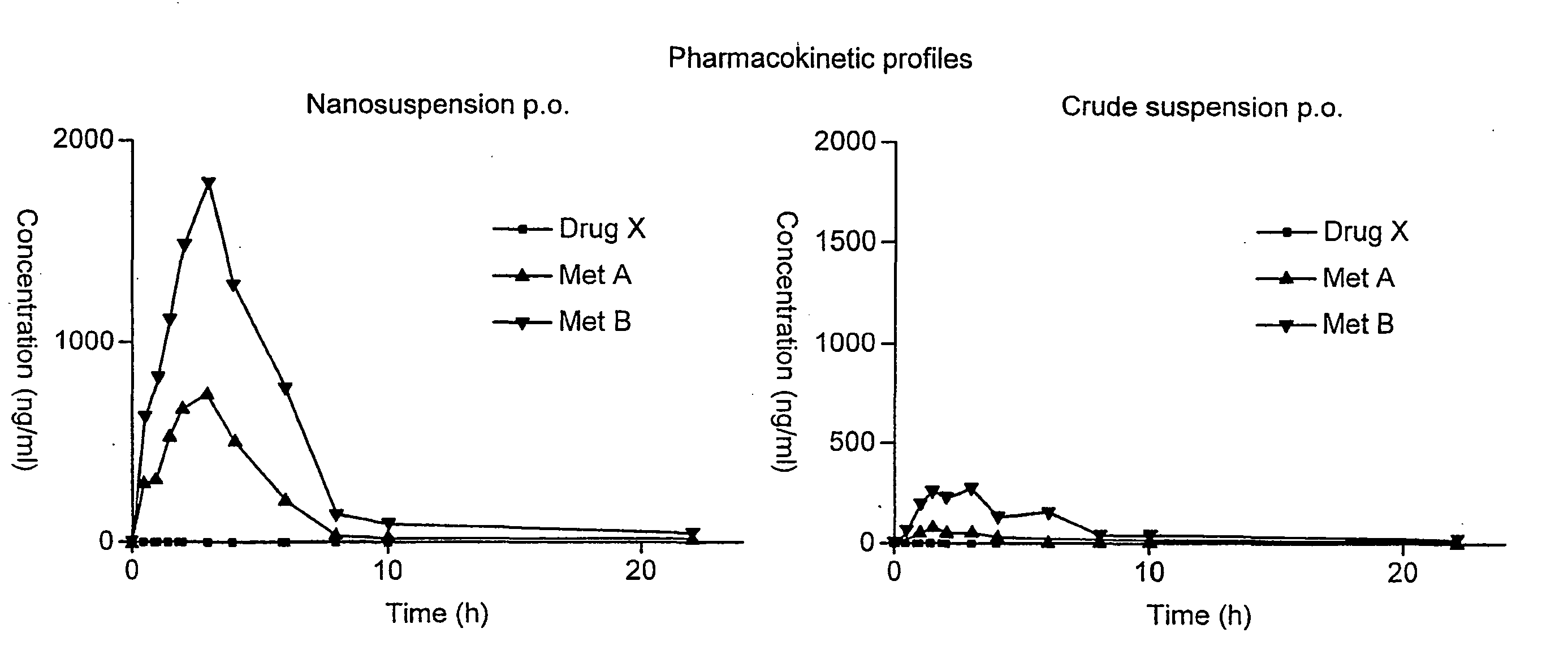
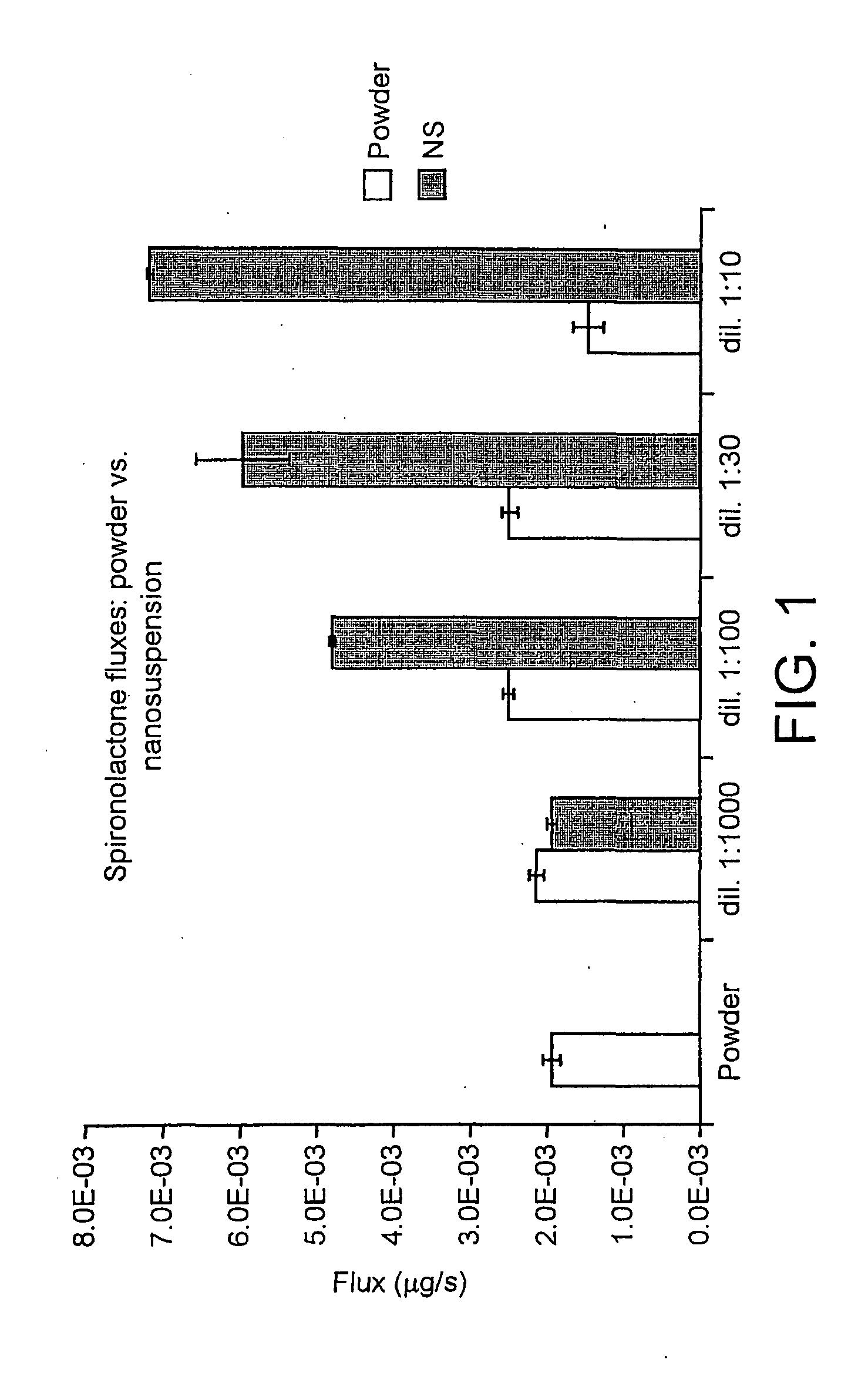
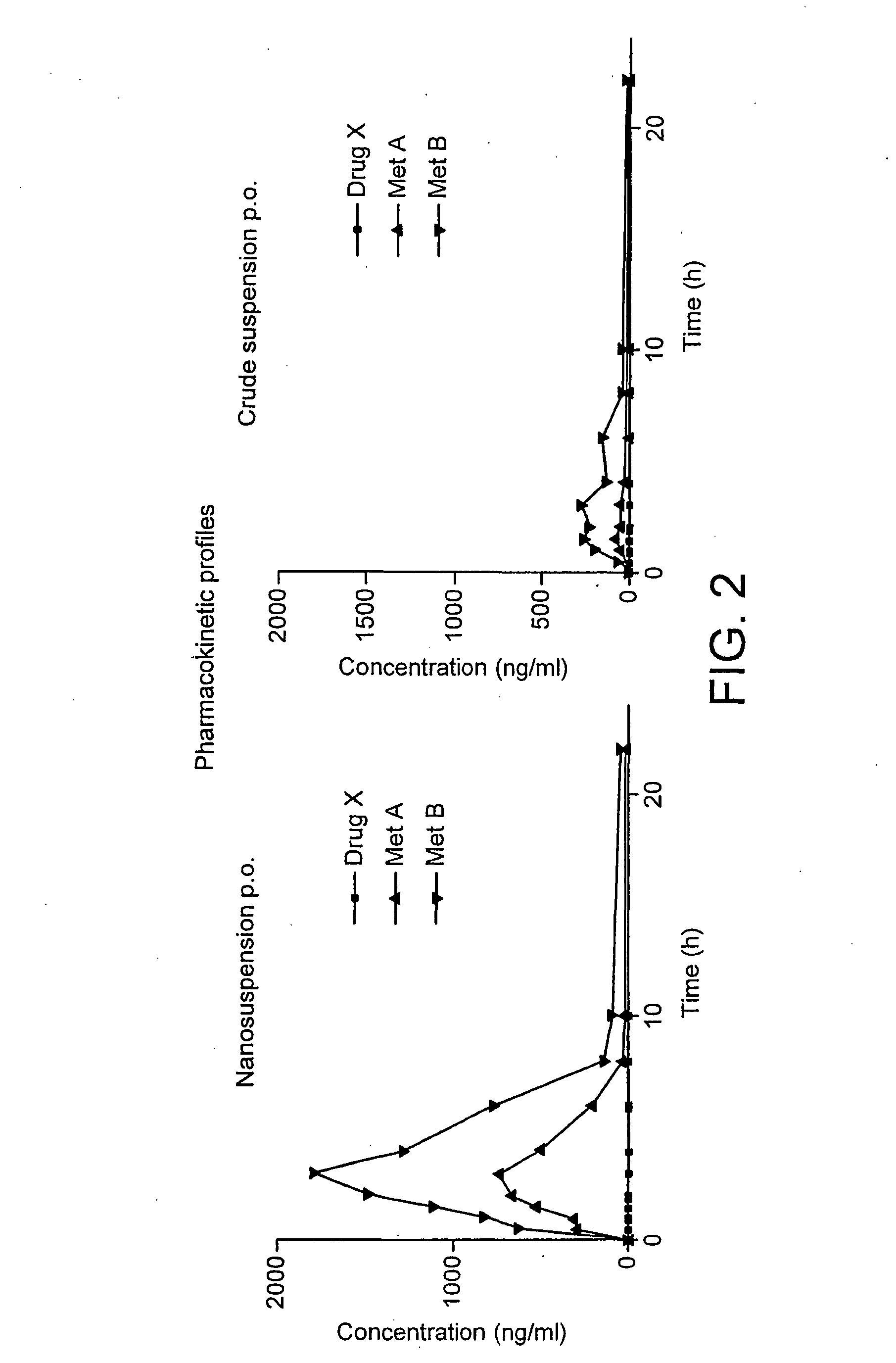
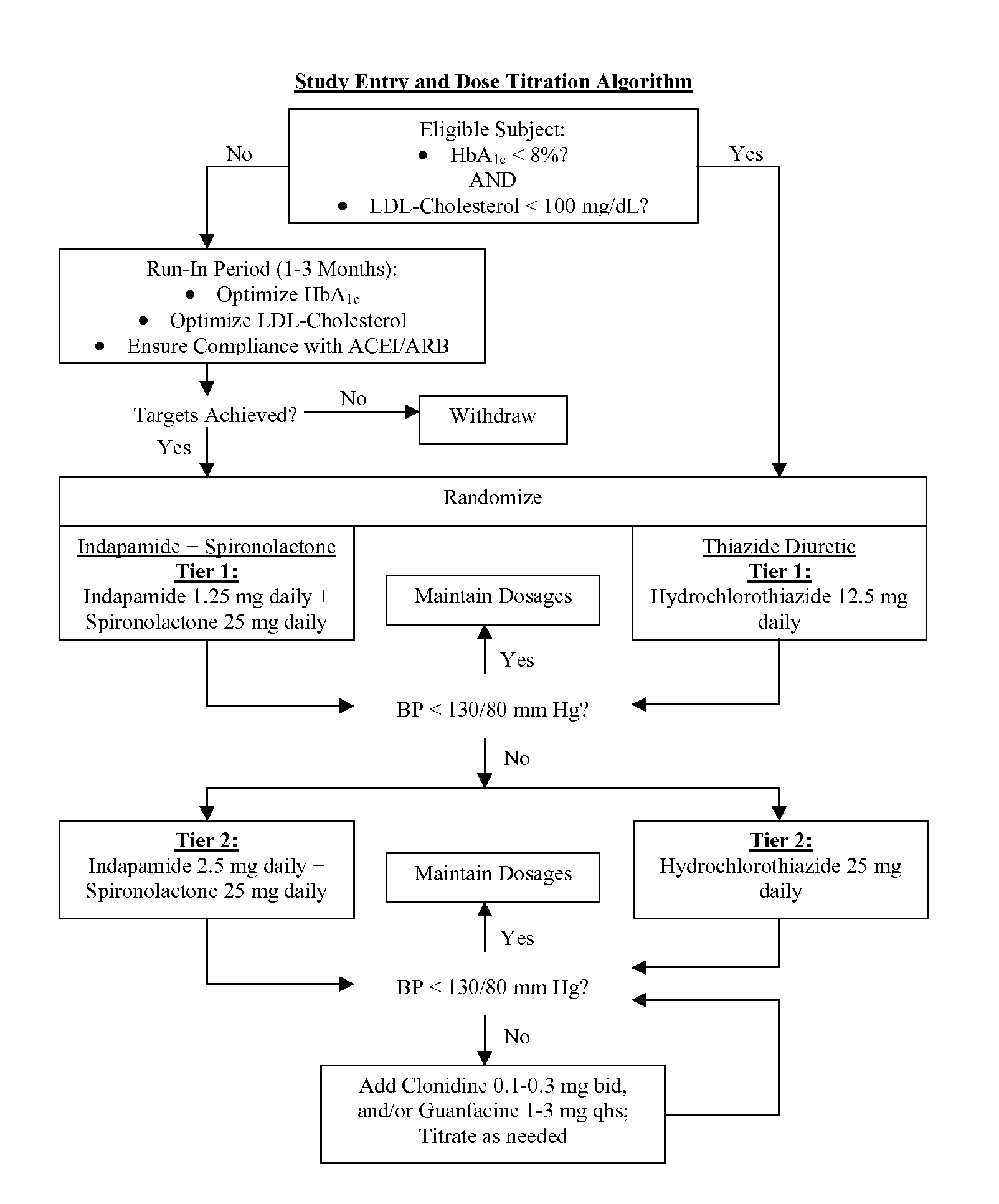
Patents
Literature
85 results about "Spironolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
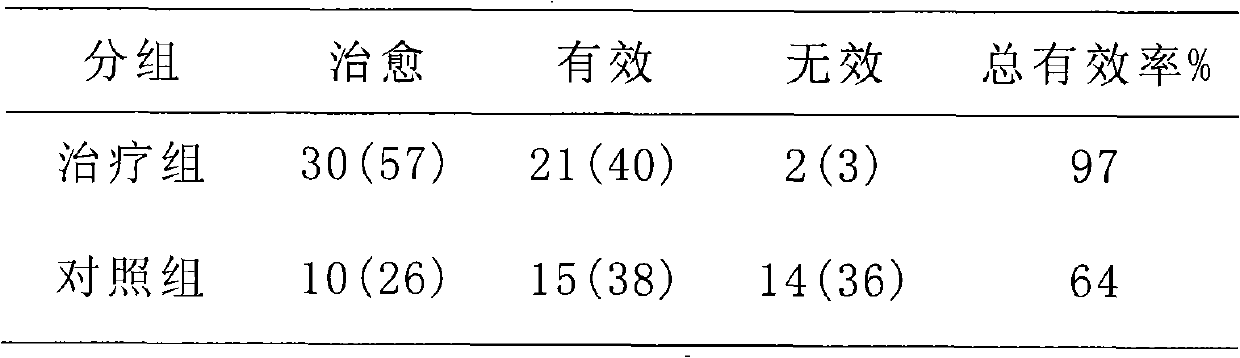
Spironolactone is used to treat high blood pressure and heart failure.
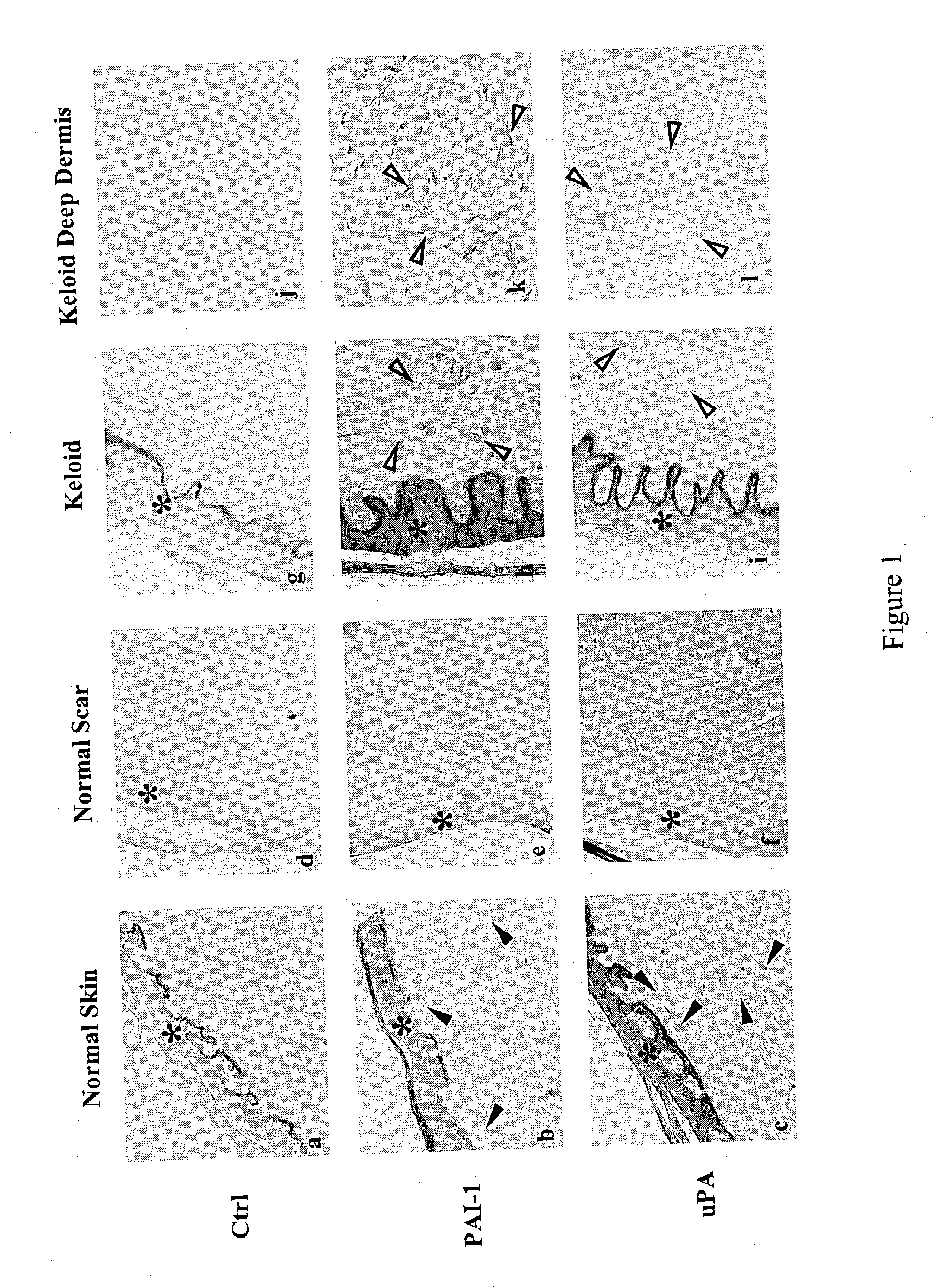
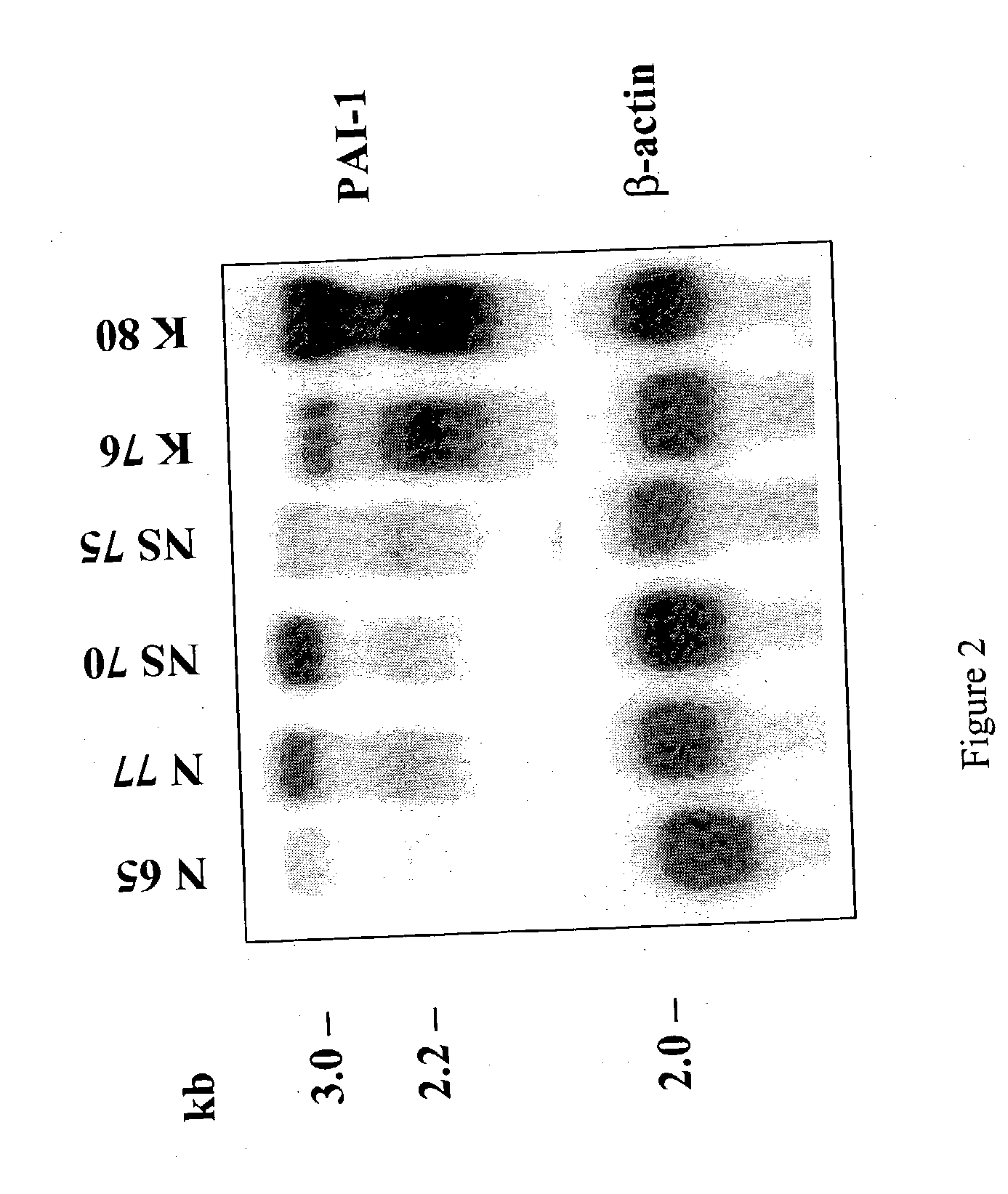
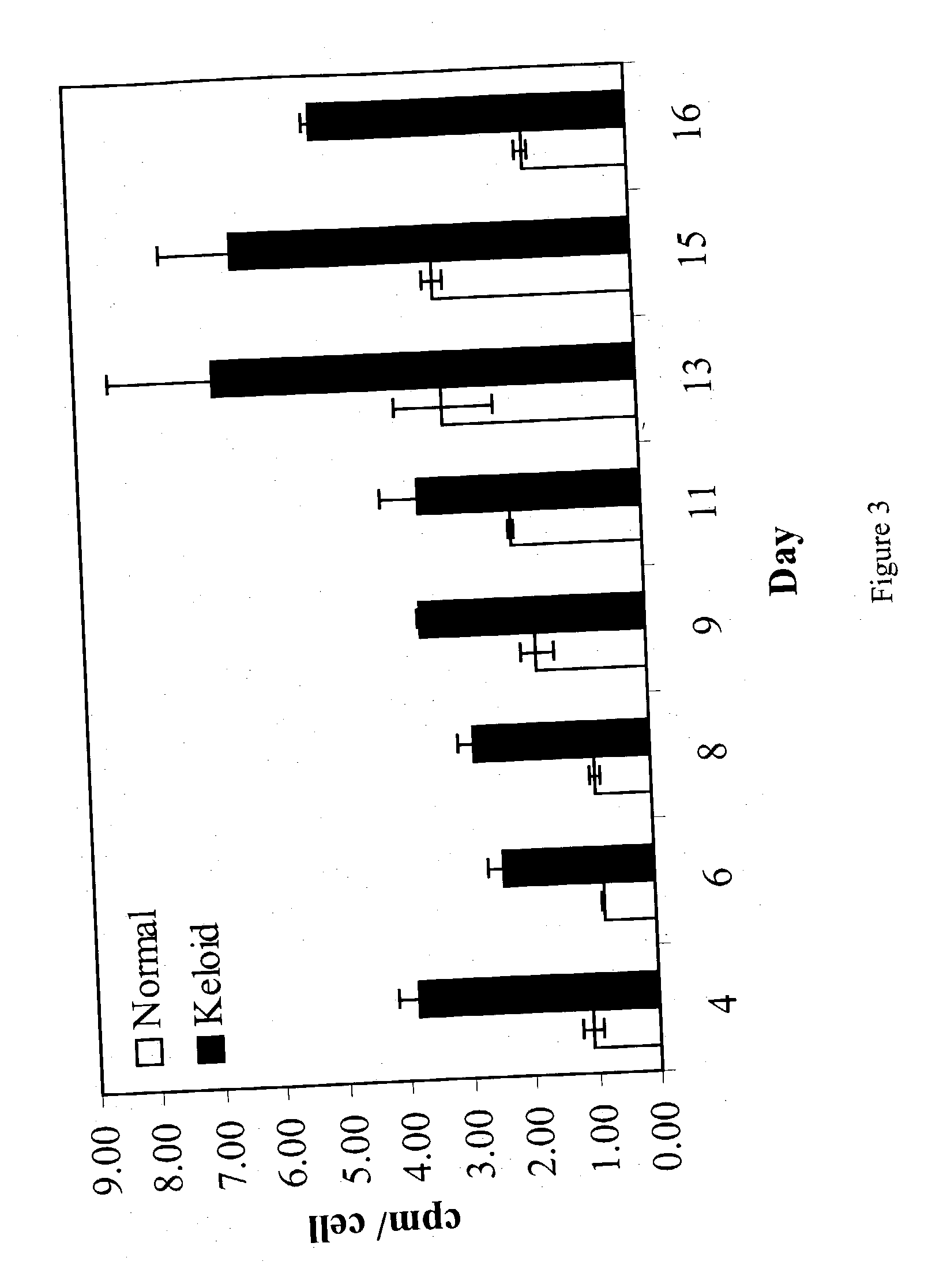
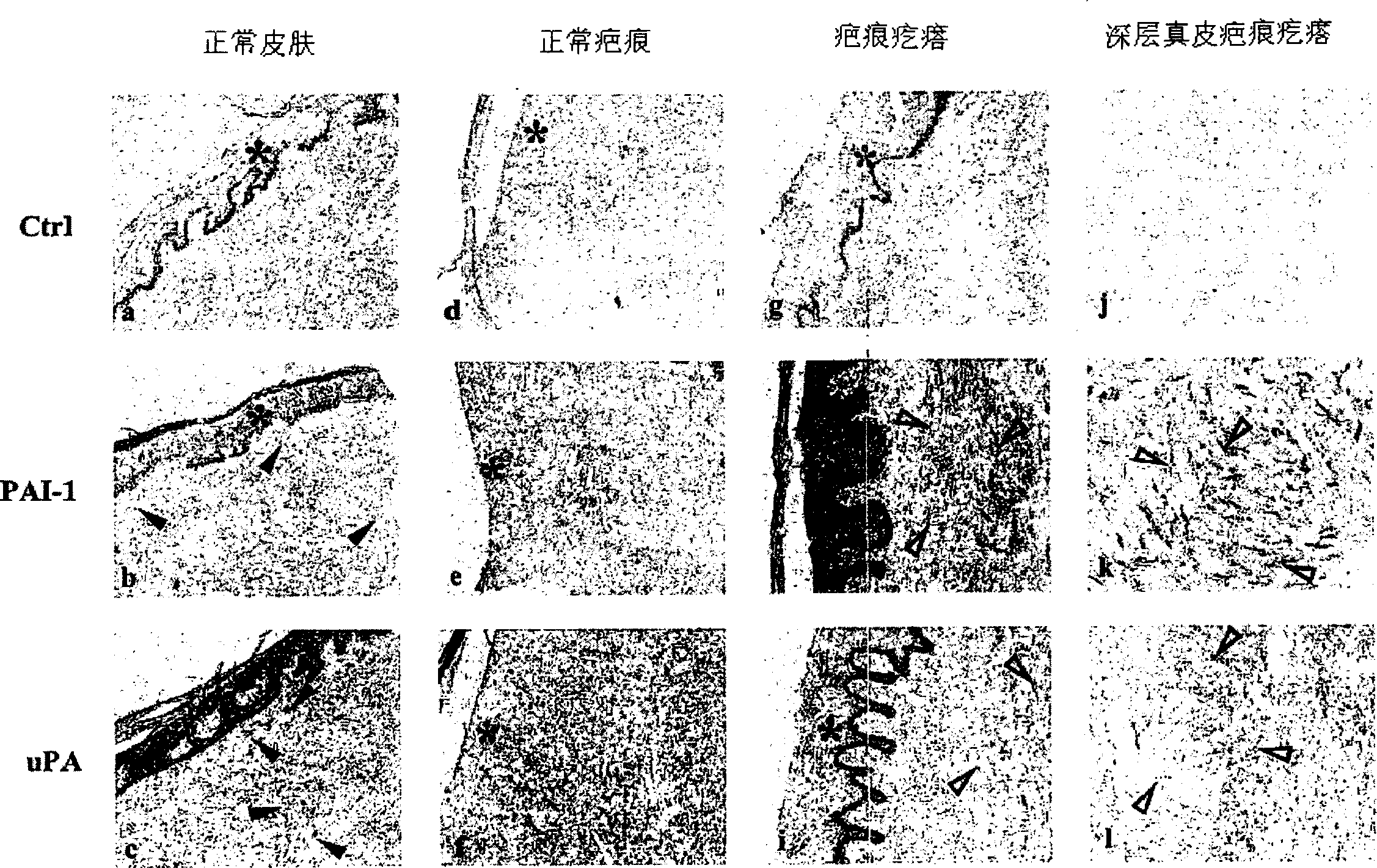
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
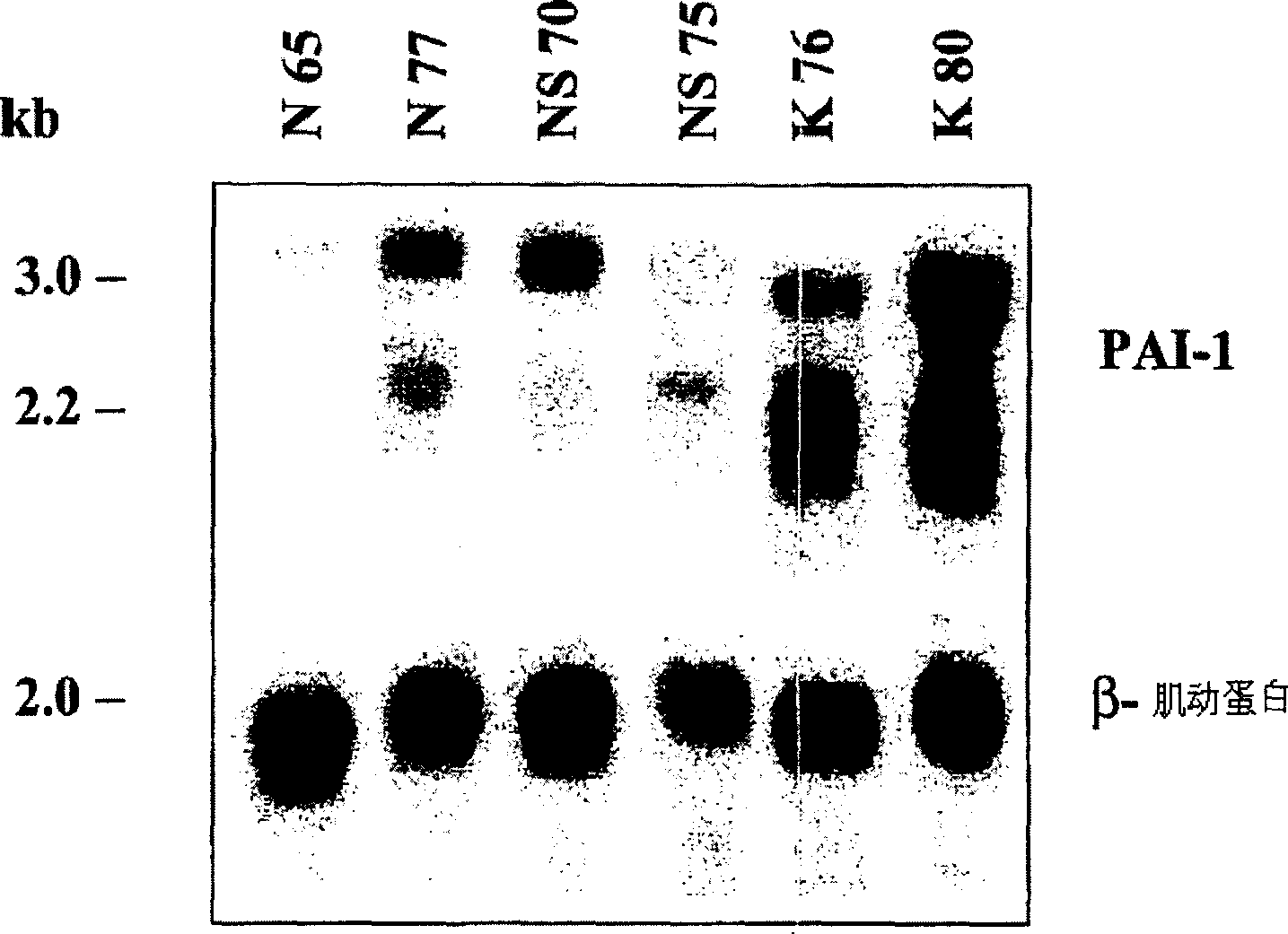
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
InactiveCN1668312AReduced uPA activityImmunoglobulins against animals/humansMuscular disorderVitamin CFibrosis
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Acaricide composition containing spirodiclofen and etoxazole and application thereof
ActiveCN102265836AImprove the effect of prevention and controlSolve the problem of resistanceBiocideAnimal repellantsSpirodiclofenActive component
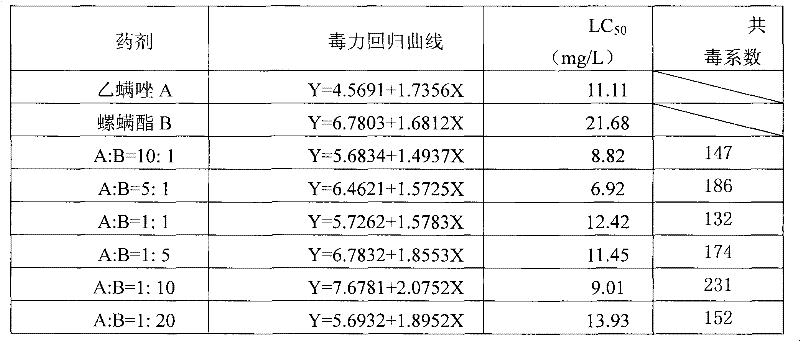
The invention discloses an acaricide composition containing spirodiclofen and etoxazole and application thereof. Spirodiclofen and etoxazole are used as active components of the acaricide composition, and the mass ratio of spirodiclofen to etoxazole is 30:1 to 1:30. According to the invention, a mutual synergy effect of the two active components of the acaricide composition is obtained, and application of the acaricide composition not only enables control efficiency to be improved, but also enables problems of resistance, drug effects, cost and the like caused by utilization of a single component to be overcome, providing a novel acaricide composition for guiding control of pest mites.
Owner:NANJING HUAZHOU PHARMA
Spironolactone nanoparticles, compositions and methods related thereto
InactiveUS20080069886A1Improve throughputImproved profileOrganic active ingredientsPowder deliveryMean diameterNanoparticle
This invention relates to nanoparticles comprising spironolactone. The nanoparticles have a mean diameter, measured by photon spectroscopy, in the range of from about 300 nm to about 900 nm.
Owner:JAGOTEC AG
Compositions and methods for treatment of renal disease
InactiveUS20100249081A1Prevent kidney damageReduce riskBiocideOrganic active ingredientsDiseaseGlomerular diseases
The invention relates to methods, compositions, and kits for the treatment of proteinuria and / or hypertension (e.g., proteinuria and / or hypertension arising from primary renal disease (e.g., focal segmental glomerulosclerosis, glomerular disease) or secondary to other conditions (e.g., diabetes, diabetic nephropathy, liver disease). Specifically, the invention relates to methods involving combination therapy wherein an indoline (e.g., indapamide) is administered in combination with an anti-aldosterone agent (e.g., spironolactone and / or epleronone).
Owner:GUPTA AJAY
Spironolactone aqueous formulations
Disclosed herein is a pharmaceutical composition, comprising: (a) spironolactone; (b) a xanthan gum; (c) an anti-foaming agent; (d) a preservative; (f) a dispersing agent; (g) a sweetening agent; (h) a flavoring agent; (i) optionally a buffer to maintain the pH of the pharmaceutical composition within a range described herein; and (j) a sufficient amount of a water vehicle.
Owner:CMP DEV LLC +1
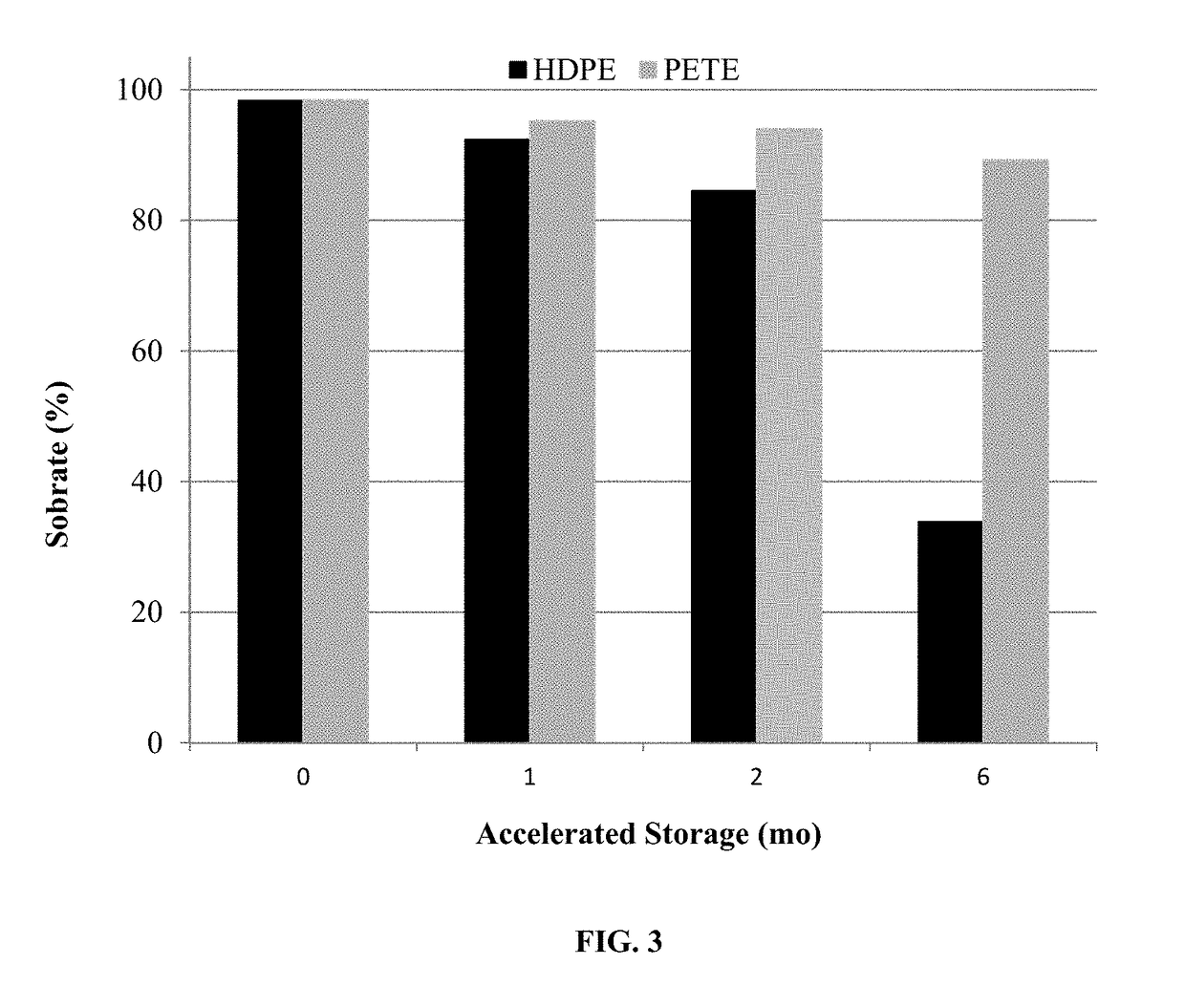
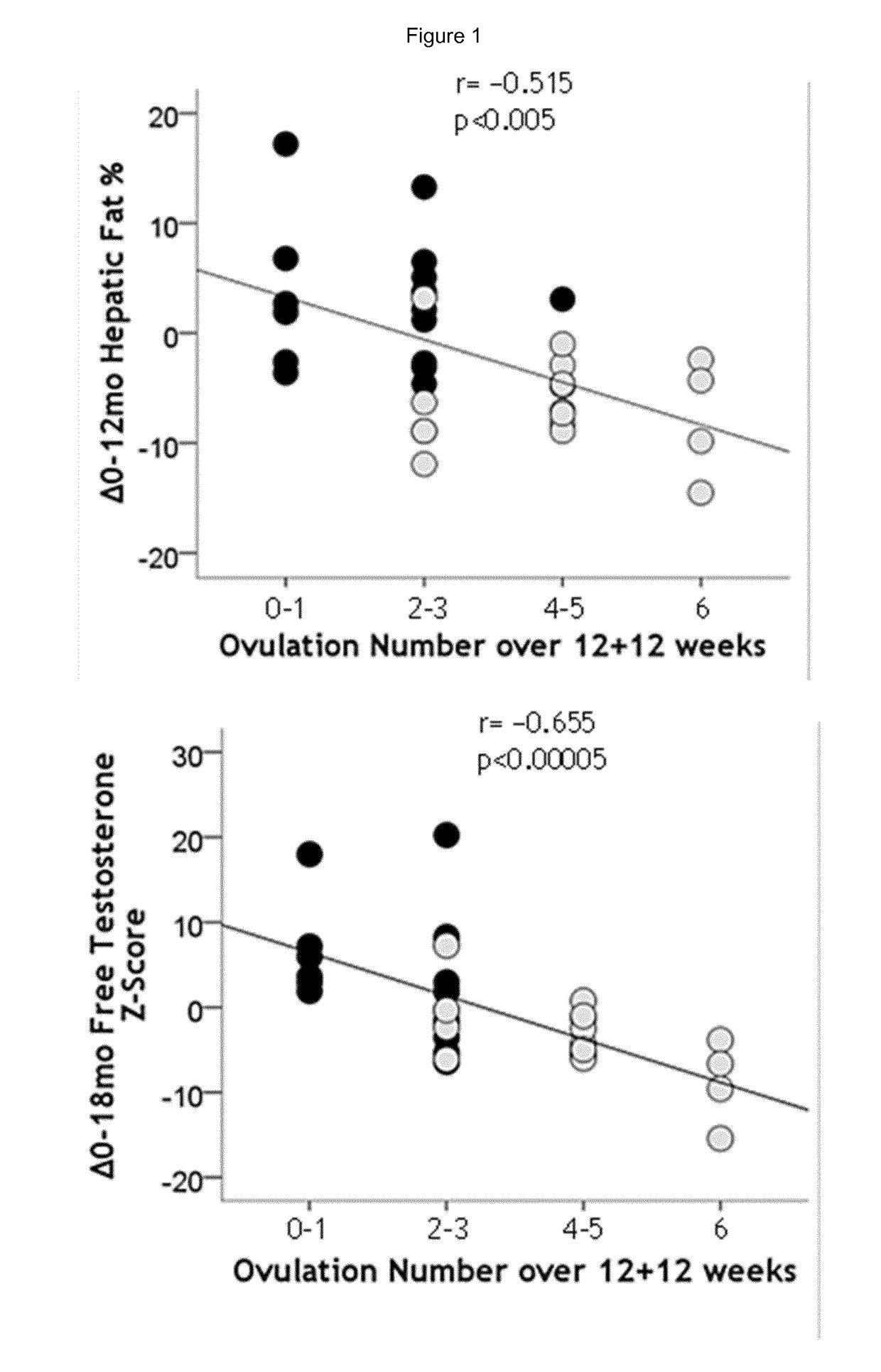
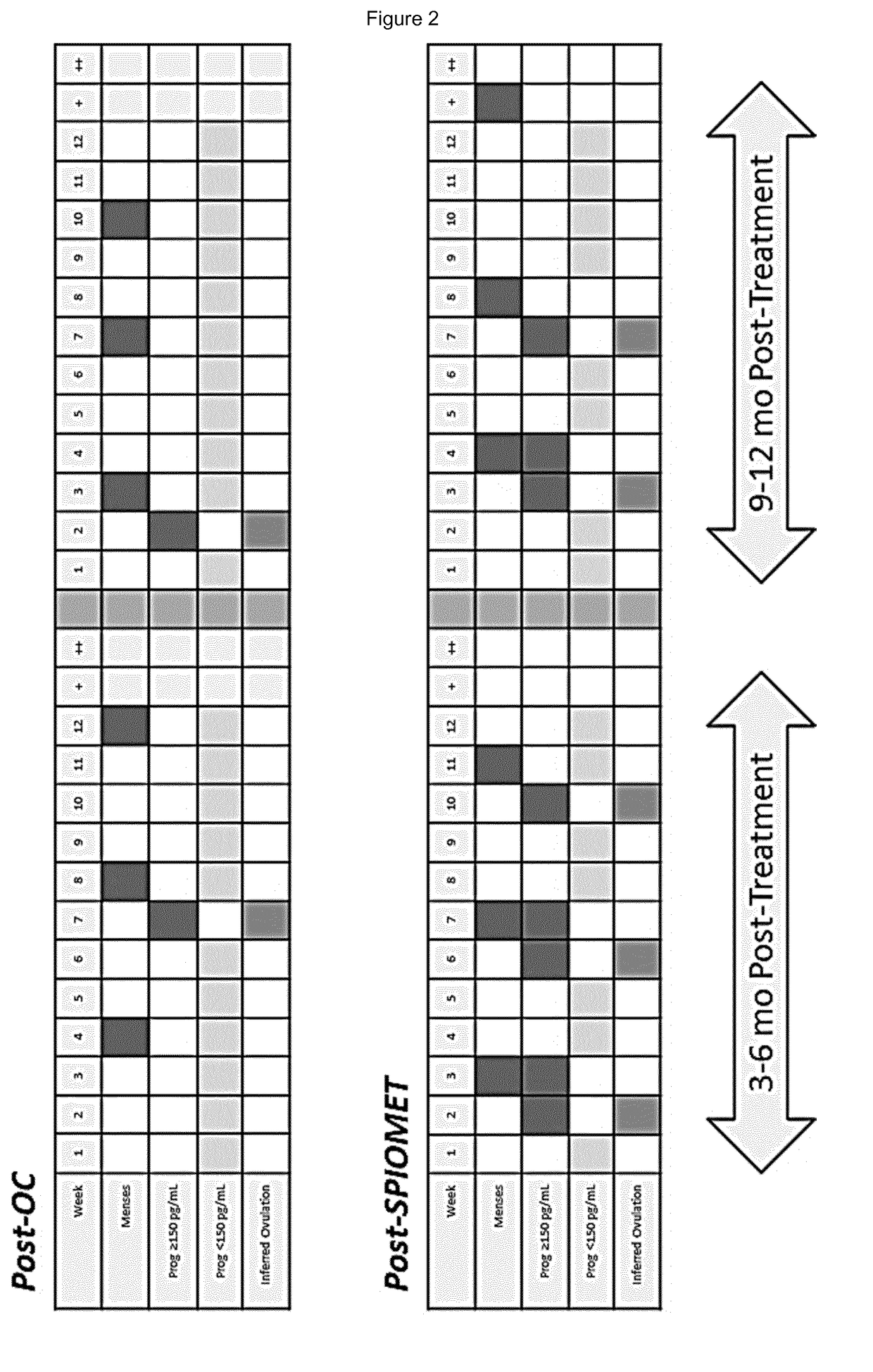
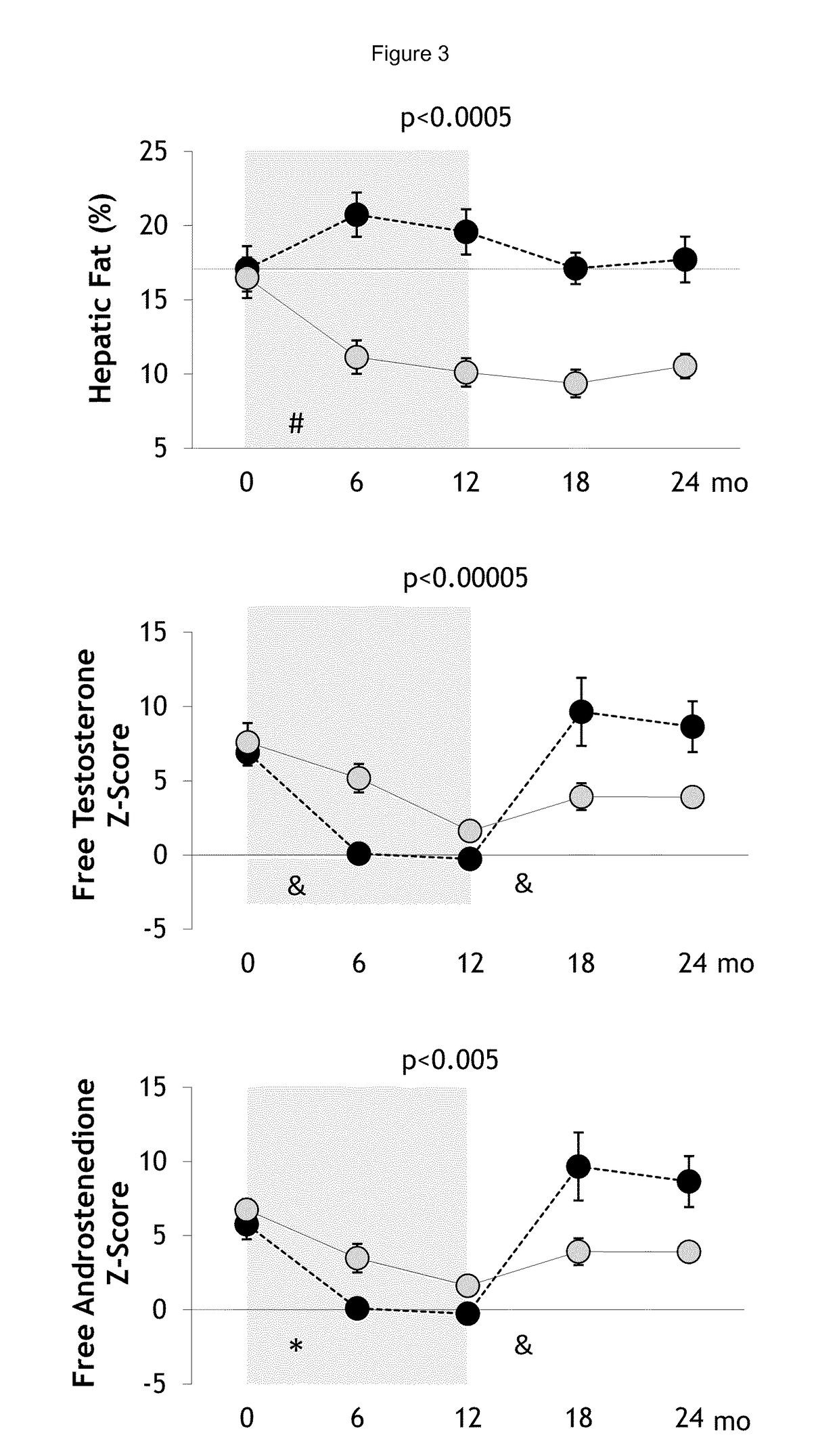
Treatment of hepatic steatosis related oligo-ovulation
PendingUS20190060328A1Decreased visceralReduce liver fat contentOrganic active ingredientsDigestive systemOvulation timesSteatosis
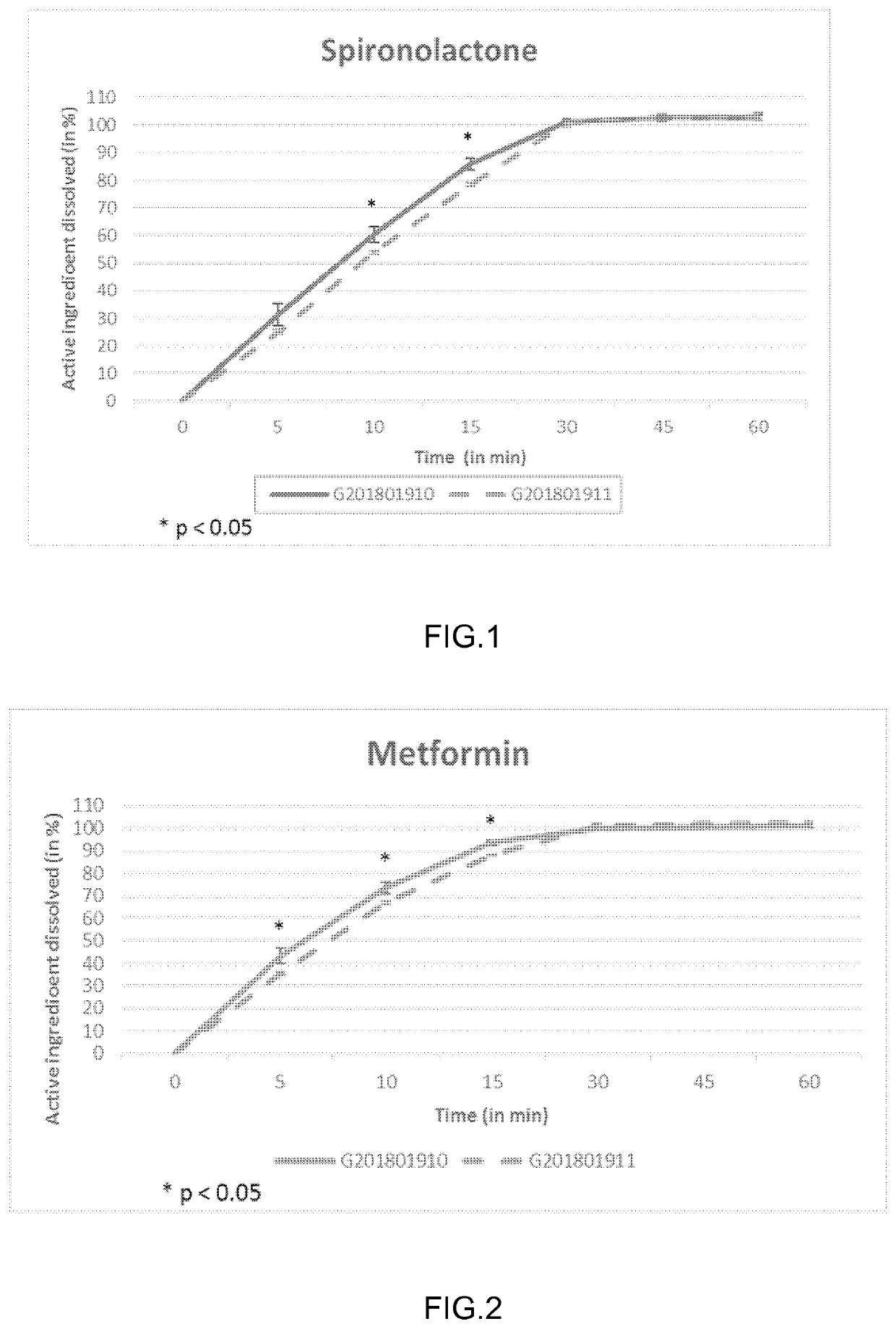
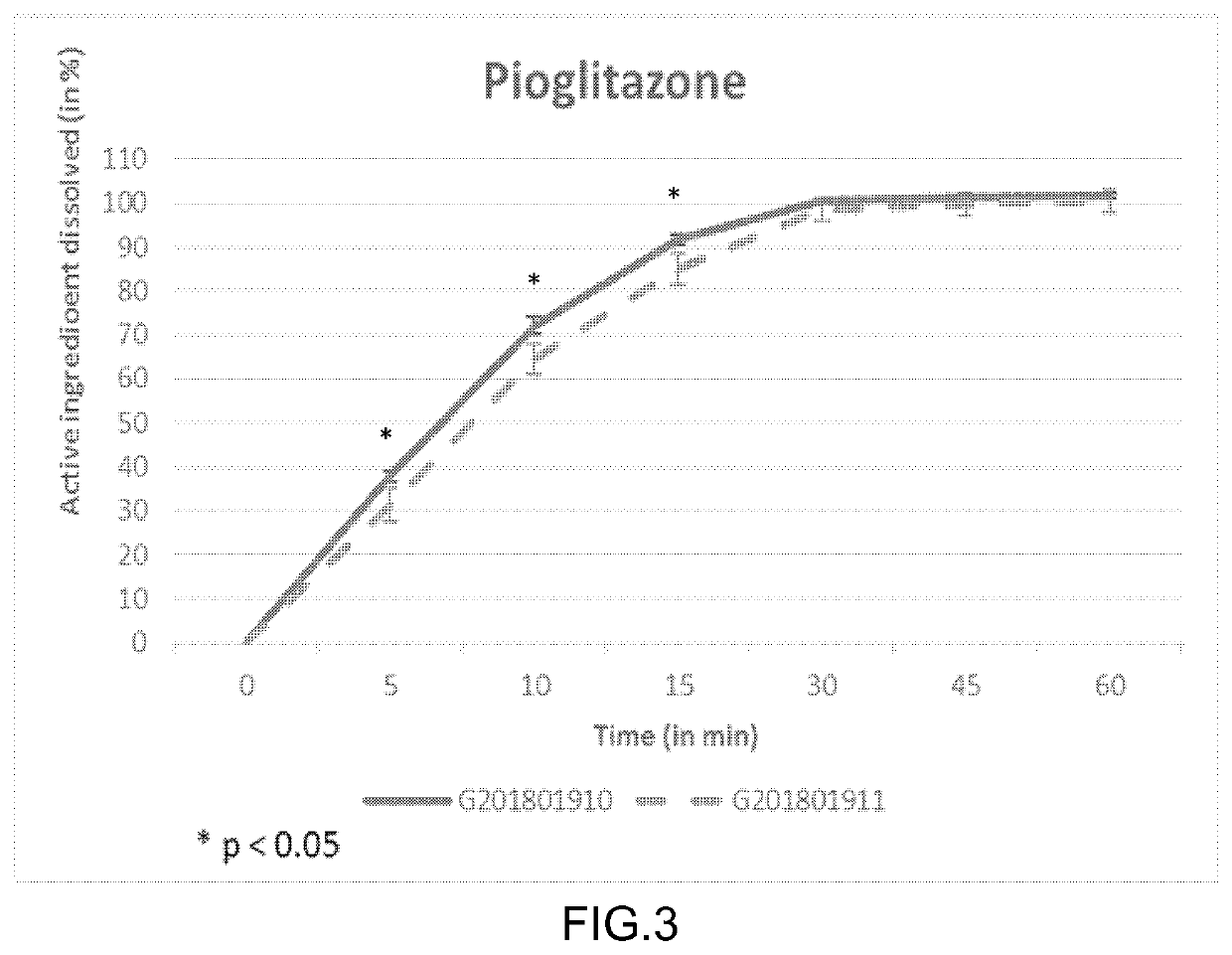
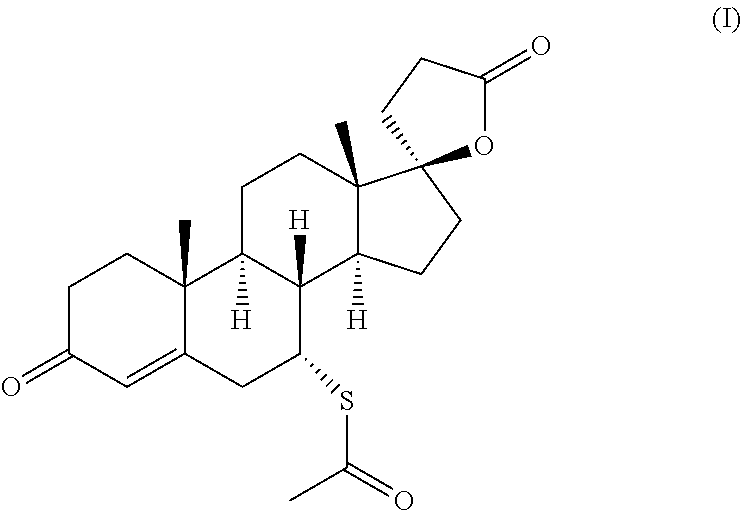
The present invention relates to a method and composition for use in treating a condition that benefits from the reduction of hepatic and / or visceral fat, such as polycystic ovary syndrome in adolescent girls or women of childbearing age, involving the use of spironolactone, pioglitazone and metformin.
Owner:HOSPITAL SANT JOAN DE DEU +1
Spironolactone aqueous compositions
Disclosed herein is a stable, ready-to-use liquid formulation comprising spironolactone and its method of use.
Owner:CMP DEV LLC +1
Pharmaceutical composition for improving in-vitro dissolution and liquidity of spironolactone
ActiveCN105832680AImprove in vitro dissolutionImprove liquidityOrganic active ingredientsPill deliveryOrganic solventMass ratio
The invention belongs to the field of pharmaceutical preparations, and relates to a pharmaceutical composition for improving in-vitro dissolution and liquidity of spironolactone and a preparation method of the pharmaceutical composition. The pharmaceutical composition is mainly characterized by being prepared from an indissolvable drug spironolactone and a carrier material, wherein the mass ratio of the drug to the carrier is 1:3-1:10, the pharmaceutical composition prepared through a hot-melt extrusion technique is solid dispersion, the spironolactone is dispersed into the carrier in a molecular or amorphous mode, and therefore the in-vitro dissolution of the spironolactone is significantly improved. After the spironolactone is smashed, the liquidity of the spironolactone is significantly improved, and the smashed spironolactone can be directly filled into capsules or directly subpackaged by serving as powder and granules or used by being mixed with other pharmaceutical auxiliary materials to prepare tablets. Compared with traditional methods such as the solvent method and the solvent-melt method, the adopted hot-melt extrusion technique has the advantages of being capable of not using an organic solvent, safe, free of pollution, stable in technology, capable of achieving continuous operation, easily achieving industrialized enlarged production and improving the liquidity without needing to add a flow aid and the like.
Owner:SHENYANG PHARMA UNIVERSITY
Medicine for treating Acne and its preparing method
InactiveCN1857376APromote absorptionGood curative effectSalicyclic acid active ingredientsDermatological disorderHigh absorptionAdditive ingredient
The present invention relates to medicine, and especially a kind of acne treating medicine and its preparation process. The acne treating medicine includes dephenhydramine, cimetidine, dexamethasone, metronidazole, spironolactone, salicylic acid and astragalus root in certain weight proportion as main ingredients, and has also the supplementary material including 50% concentration alcohol solution, aloe juice, glycerine and essence in certain proportion. Clinical application shows that the acne treating medicine has the advantages of high absorption, no blocking of pores, fast acting, high treating effect, etc.
Owner:孟祥伟
Compositions and methods for treating ocular diseases
Described methods and compositions for treating Meibomian Gland Disease (MGD) for normalizing gland secretions and improving symptoms of ocular surface diseases associated with MGD. The methods concern treatment of a patient with aldosterone antagonists, such as spironolactone or analogues of spironolactone. Spironolactone is desirably added in a novel treatment composition, preferably in the form of an aqueous solution, emulsion or suspension in small but effective concentrations and in a novel vehicle that adds to the increased solubility of what previously was known to be an insoluble active agent. Moreover, it is believed that the specific lower but effective concentrations of spironolactone, and / or the pluronic vehicle of the treatment composition, permits optimal expression of essential lipids and upregulation of genes that control lipid production necessary to a more normalized lipid component of the tear film for the treatment and / or the prevention of signs and / or symptoms of MGD.
Owner:OCULAR RESOURCES LLC
Compound astragalus polysaccharide liposome long-acting injection and preparation method thereof
InactiveCN102973508AImprove stabilityGood tissue compatibilityOrganic active ingredientsAntiviralsCholesterolPhosphoric acid
The invention discloses a compound astragalus polysaccharide liposome long-acting injection and a preparation method thereof, belonging to the technical field of medicinal preparations. Each 100 ml of the injection comprises the following effective components: 1 to 2 g of astragalus polysaccharide, 5 to 7 g of phosphatide, 1 to 3 g of cholesterol, 0.1 to 1 g of dihexadecyl phosphate and 0.01 to 0.02 g of spironolactone. According to the compound astragalus polysaccharide liposome long-acting injection provided by the invention, inherent properties of phosphatide and cholesterol are utilized and certain external means are used to wrap astragalus polysaccharide by using liposome so as to obtain a bimolecular structure similar to a biomembrane, and the injection has good histocompatibility and cell affinity, improves stability of astragalus polysaccharide and presents a good slow releasing effect; in clinical application, compared with original astragalus polysaccharide injection under the condition of same usage amount, one application of the injection provided by the invention can realize a good effect which is obtained when conventional injection is applied twice.
Owner:HENAN SOAR VETERINARY PHARMA
Topical nanodrug formulation
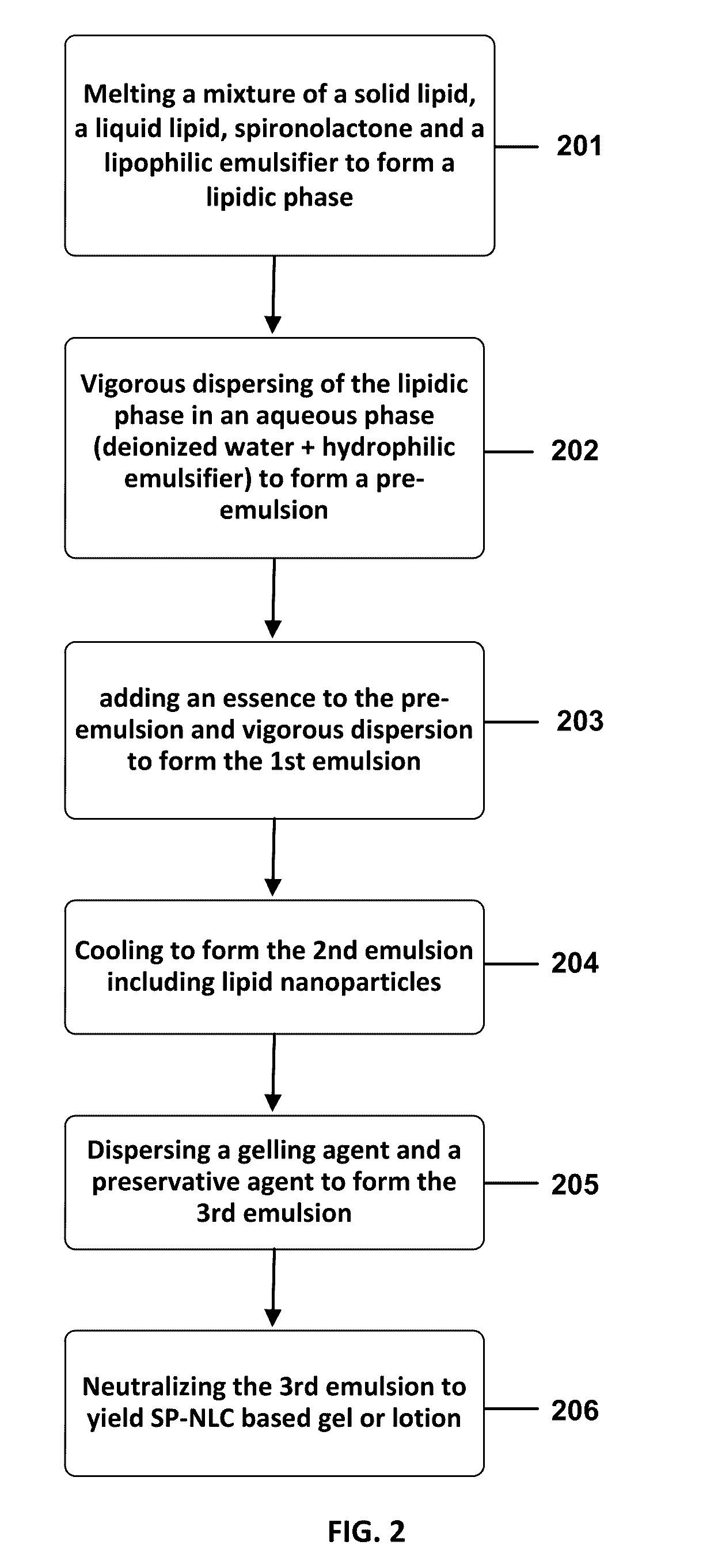
A formulation includes a nanostructured lipid carrier (NLC) matrix and spironolactone as an active ingredient loaded within the NLC matrix, forming a spironolactone-loaded NLC (SP-NLC) gel or lotion.
Owner:KELIDARI HAMIDREZA +1
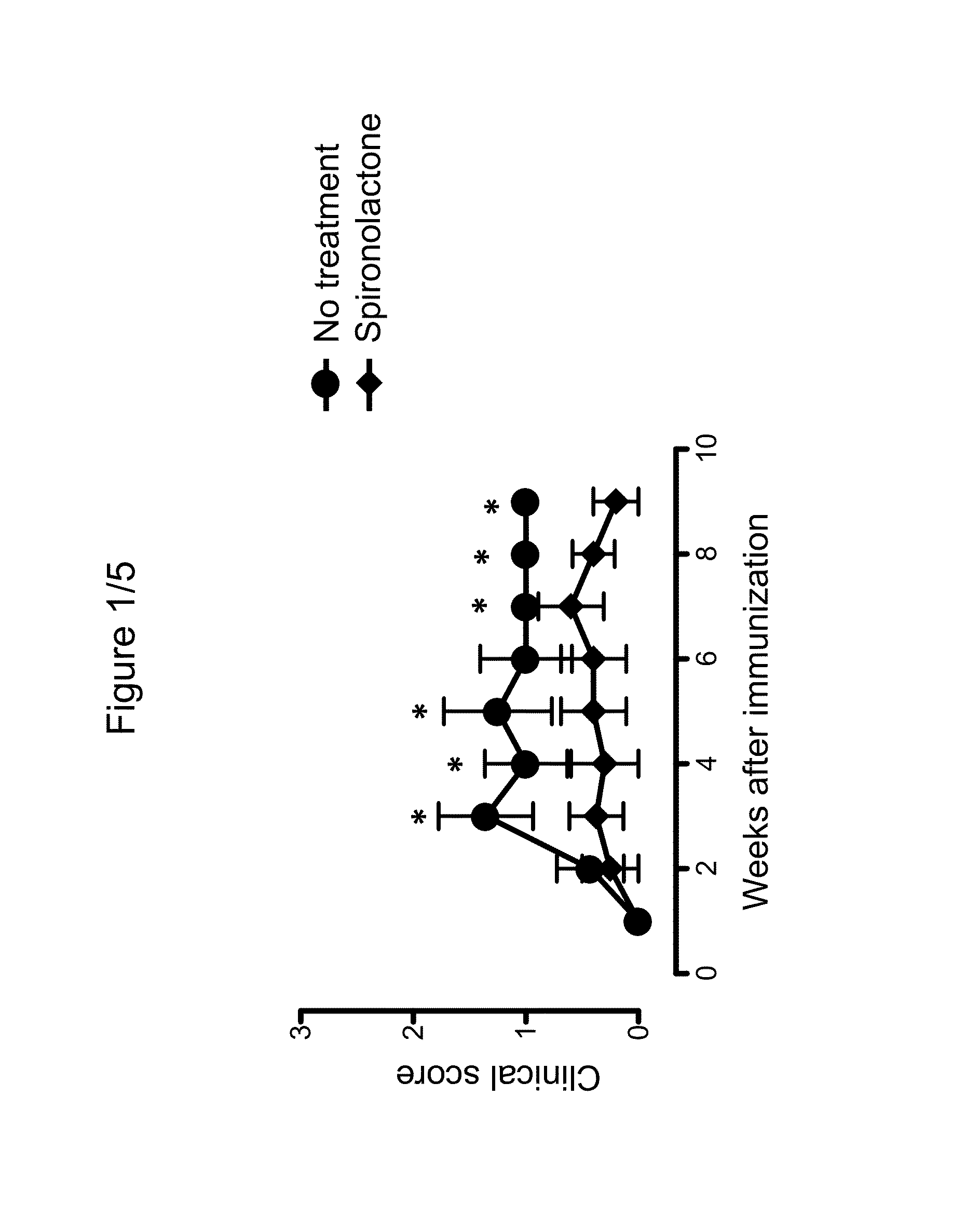
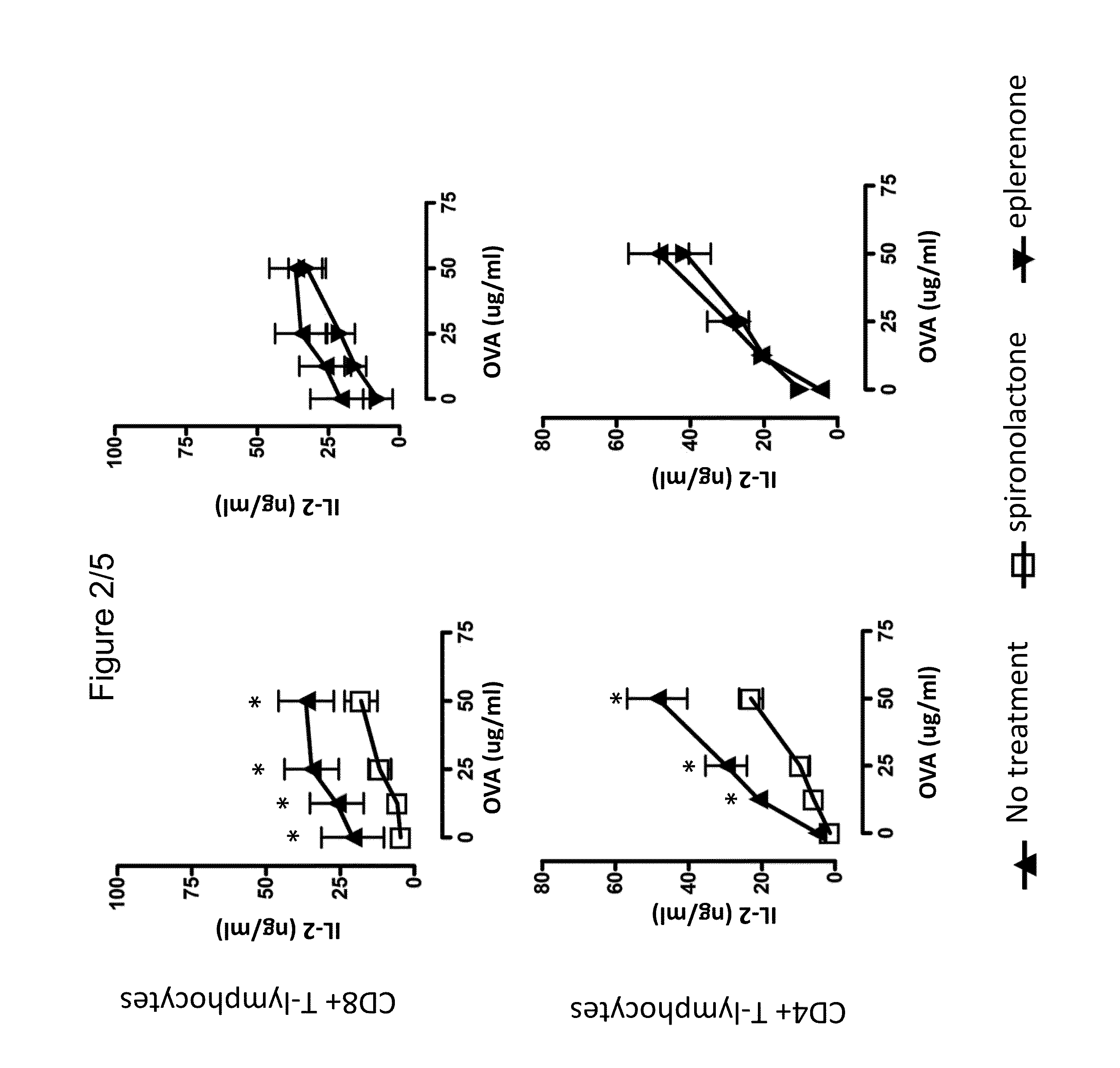
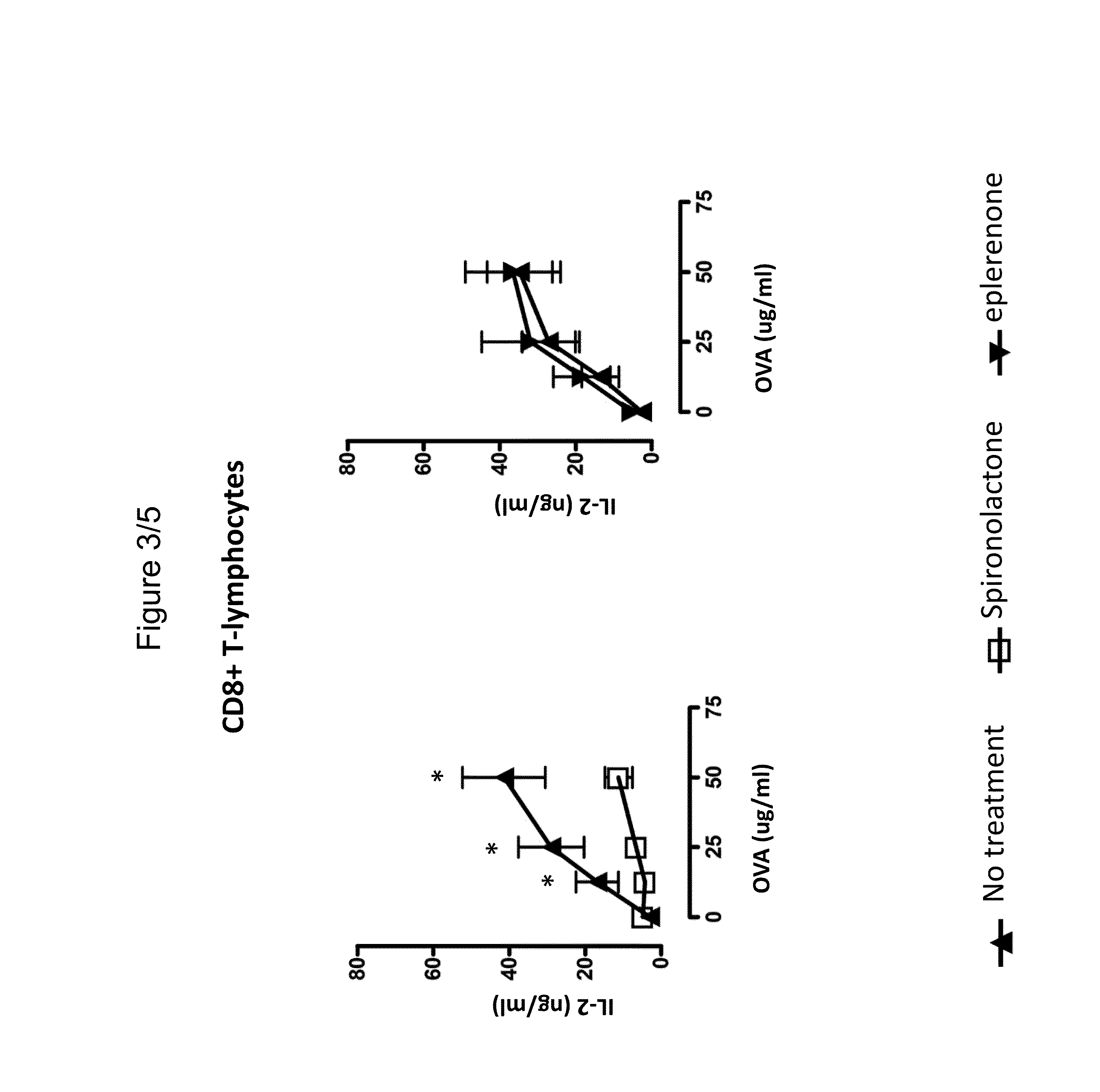
Use of spironolactone-based composition that exhibits an inhibitory action on t-lymphocyte activation which is usefule for preventing and/or treating multiple sclerosis
InactiveUS20130203719A1Less expensiveOrganic active ingredientsNervous disorderDendritic cellClinical study
The present invention relates to the use of spironolactone for the preparation of a pharmaceutical composition intended for preventing and / or treating multiple sclerosis. Alternatively, the invention relates to the use of spironolactone directly in T-lymphocytes or dendritic cells obtained from a blood sample taken from a patient and then injected back into the circulation. Therefore, the present invention relates to the use of a composition comprising spironolactone that can be used in the treatment of multiple sclerosis, which covers the administration of spironolactone directly or lymphocytes pre-treated with spironolactone, or dendritic cells to individuals requiring such treatment. Spironolactone is an orally administered drug that is less expensive than many of the treatments available for MS and, furthermore, has the advantage of being a known compound already used in humans for extended periods and therefore the adverse effects thereof have been described in clinical studies.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
Medicine for curing pain syndrome of late cancer and its preparation
InactiveCN1391952ACurbing rapid growthRelieve painHydroxy compound active ingredientsUnknown materialsCancer cellPain syndrome
The medicine for treating pain syndrome of later stage cancer is one Chinese and Western combined medicine, which is prepared with astragalus root, privet fruit, sealwort and other Chinese medicinal materials as well as Indomethacin, Dexamethasone, Spironolactone, Famotidine and Diazepam. It has the functions of resisting cancer, diminishing inflammation, relieving pain, promoting diuresis and raising immunity; and in can inhibit the growth of cancer cell, relieve pain of the later stage cancer patient, raise survical quality and prolong life.
Owner:潘远志
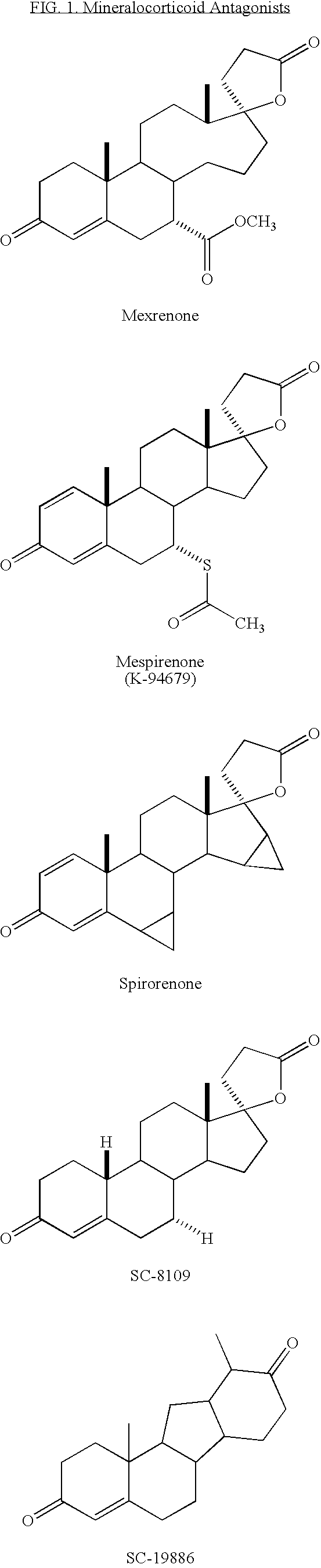
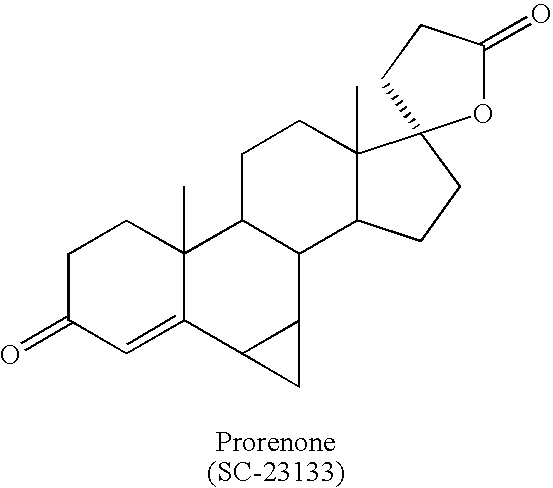
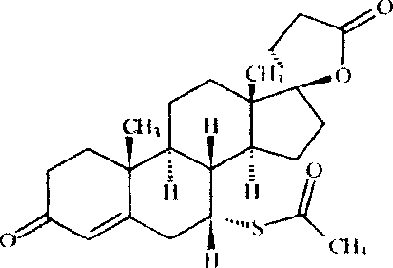
Synthesis and separation of optically active isomers and cyclopropyl derivatives of spironolactone and their biological action
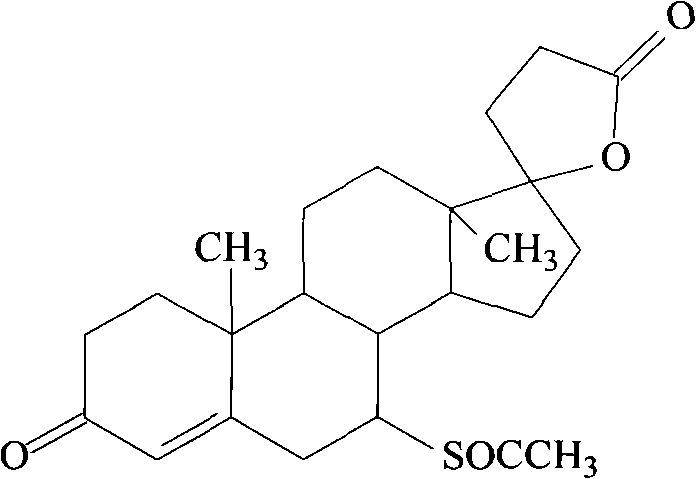
InactiveUS20090325918A1Effective therapyOrganic active ingredientsSteroidsSide effectPR - Progesterone receptor
Methods for separation and synthesis of the optically active 7-thioester isomers and mono or bis-cyclopropyl derivatives of spironolactone are provided. Preferred stereoisomerically purified 7-thioester isomers and mono or bis-cyclopropyl derivatives of spironolactone have fewer effects mediated by gonadal steroid receptors relative to effect mediated by minteralocorticoid progesterone receptors, compared to the stereoisomerically unpurified form of the compound. These optically active compounds can be useful for obtaining reduction in moderate essential hypertension and it the treatment of congestive heart failure in humans with minimized undesirable side effects such as gynecomastia, tender breast enlargement and menstrual irregularities in women, and loss of libido in men.
Owner:SOMBERG JOHN C +1
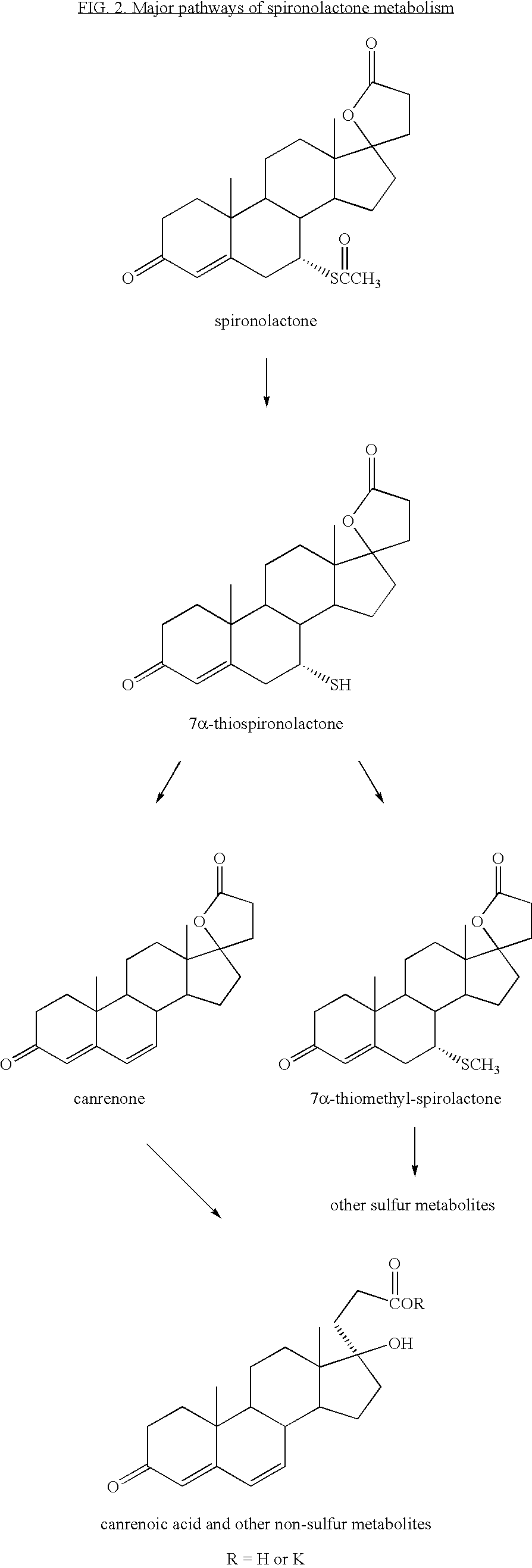
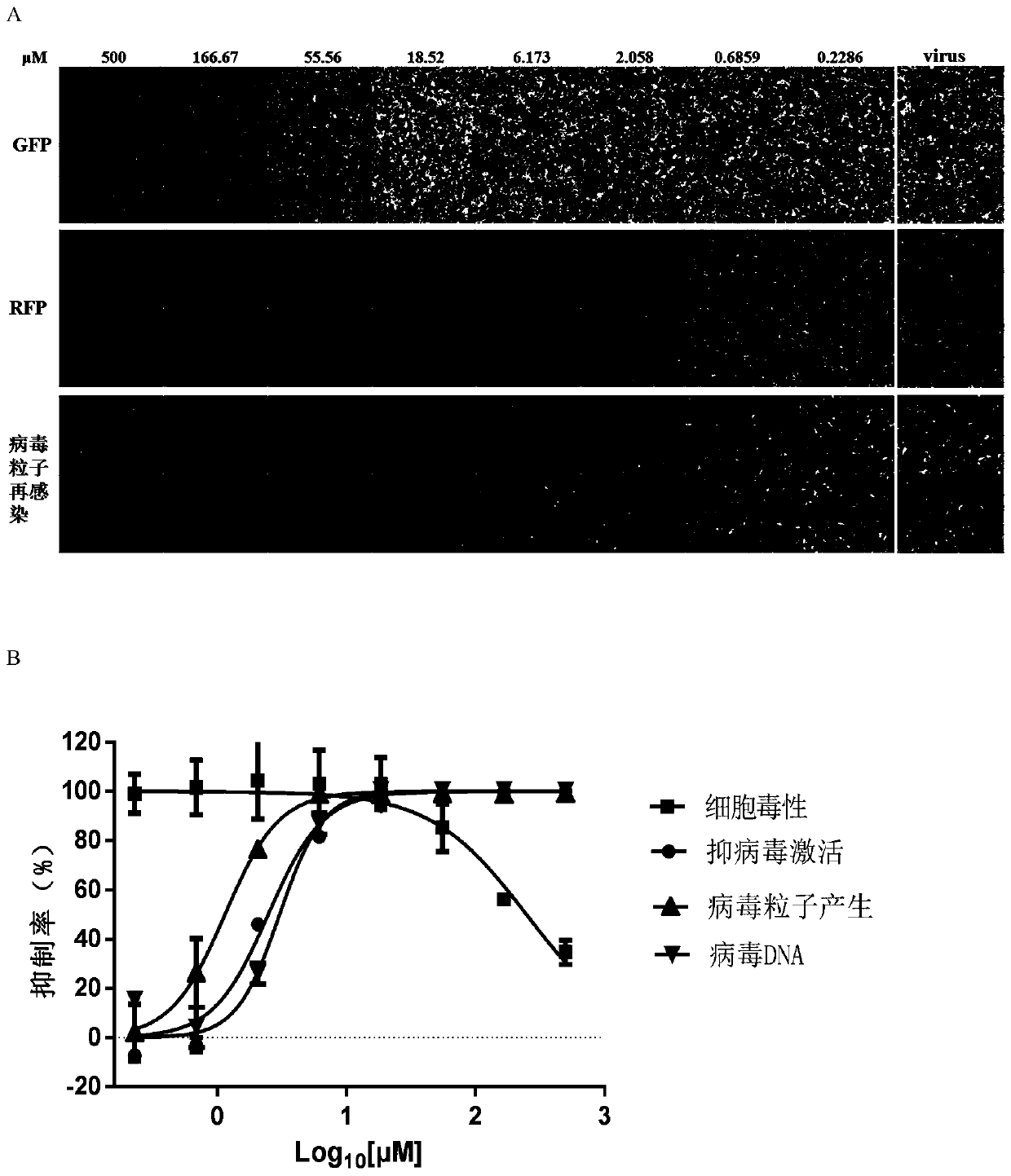
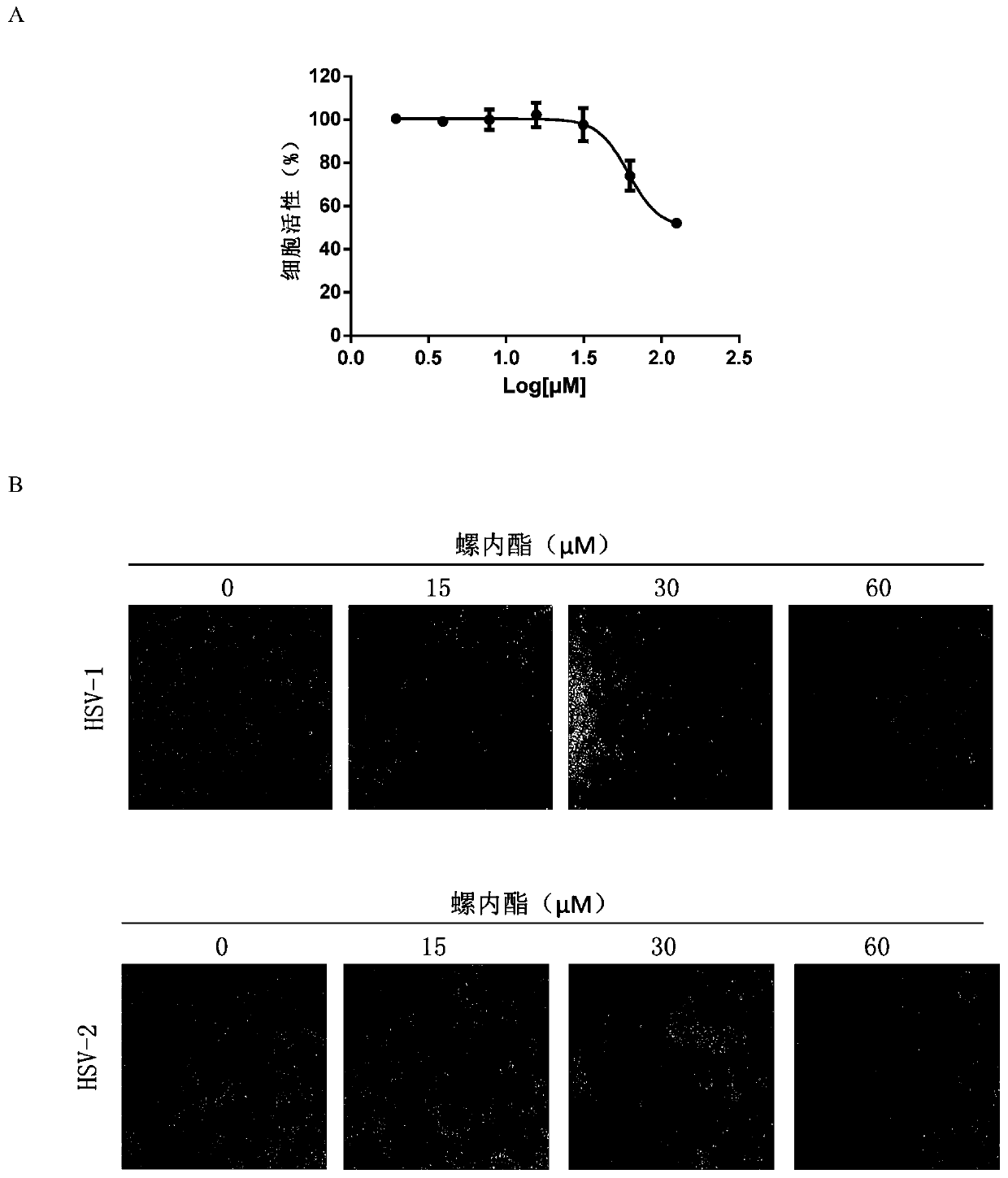
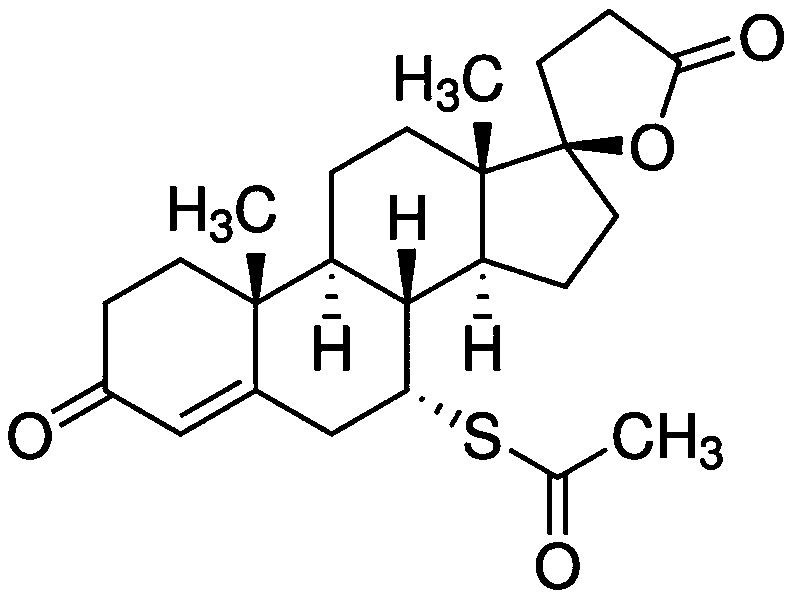
Application of spironolactone in preparation of drug for treating human herpesvirus infection
InactiveCN109925317AActive Broad SpectrumHigh activityOrganic active ingredientsAntiviralsSide effectCytopathic effect
The invention discloses application of spironolactone in preparation of a drug for treating human herpesvirus infection. We evaluate the effect of resisting Kaposi's sarcoma-associated herpes virus KSHV and herpes simplex viruses HSV-1 and HSV-2 of the spironolactone by measuring the level of infectious virion production, the level of DNA replication during lysis, or the degree of cytopathic effect caused by the virus. The spironolactone has significant antiviral activity and a dose-dependent effect, can inhibit the activation of replication during KSHV lysis with a half inhibitory concentration (IC50) of 1.145 microM to KSHV infectious virions. At non-toxic concentration, the spironolactone is effective in reducing cytopathic effect caused by HSV-1 and HSV-2. The spironolactone is a complete inhibitor of mineralocorticoids (such as aldosterone). The spironolactone is clinically used for inhibiting sodium reabsorption, potassium excretion, diuresis and the like of organisms and has thesmall toxic and side effects on the human body. The compound can inhibit replication of herpes virus in the lysis phase, and has a wide prospect of being developed into the drug for treating human herpes virus infection.
Owner:武汉威立得生物医药有限公司
Spirolactone and hydrochlorothiazide pharmaceutical composition solid preparation
InactiveCN102188434AGood effectOvercome deficienciesOrganic active ingredientsPharmaceutical non-active ingredientsSide effectHydrochlorothiazide
The invention discloses a spirolactone and hydrochlorothiazide liposome preparation which is prepared mainly from the following the ingredients in parts by weight: 1 part of spirolactone, 1 part of hydrochlorothiazide, 3-15 parts of egg phosphatidylcholine, 1-8 parts of cholesterol, and 0.5-5 parts of sodium glycyl-cholate. The invention further discloses a spirolactone and hydrochlorothiazide pharmaceutical composition solid preparation which is prepared by adding other excipient commonly used on pharmacy with the spirolactone and hydrochlorothiazide liposome preparation. The invention improves the product quality of the preparation and reduces the toxic and side effects.
Owner:HAINAN SHU ER PHARMA RES
(Heteroarylmethyl) Thiohydantoins as anticancer drugs
The invention refers to the use of androgen receptor antagonists for the treatment and / or prevention of fibroids, also known as uterine leiomyoma, leiomyomata. Particularly, the invention refers to the use of an androgen receptor antagonist being any one of the compounds according to the following list: cyproterone acetate, oxendolone, chlormadinone acetate, spironolactone, osaterone acetate, dienogest, flutamide, hydroxyflutamide, nilutamide, bicalutamide, RU 58841, LGD-2226, MDV3100, BMS-641988, BMS-779333, or 4-(3-{[6-(2-hydroxy-2-methylpropoxy)pyridin-3-yl]methyl}-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (thioxoimidazolidine derivative) for the treatment of fibroids.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Antihypertensive medicine synergist
InactiveCN103565813AImprove complianceLower doseOrganic active ingredientsCardiovascular disorderHypertension medicationsPatient compliance
Owner:刘超
Compound spirolactone nanoemulsion drug
InactiveCN102697900AEvenly distributedSystem transparencyOrganic active ingredientsEmulsion deliveryActive agentSurface-active agents
The invention discloses a compound spirolactone nanoemulsion drug. The nano drug is prepared from the following materials in mass percentage: 1%-15% of spirolactone, 15%-35% of surfactant, 5%-20% of cosurfactant, 5%-20% of oil, 0.5%-10% of hawthorn extract, 0.5%-10% of asarum extract and the balance of distilled water, wherein the sum of the mass percentages of the ingredients is 100%. According to the invention, after the spirolactone is prepared into nanoemulsion dosage form, the ability of penetrating blood brain barrier is obviously increased, the bioavailability of active compound is obviously improved, the half-life period of the drug is prolonged, and the administration times are reduced. The dissolving and penetrating abilities of the drug of the spirolactone are improved by the nanoemulsion, and the stability of the spirolactone is increased. The spirolactone nanoemulsion drug has obvious synergistic effect after being combined with aqueous extract of the hawthorn and asarum, and the curative effect of the drug is brought into full play.
Owner:NORTHWEST A & F UNIV
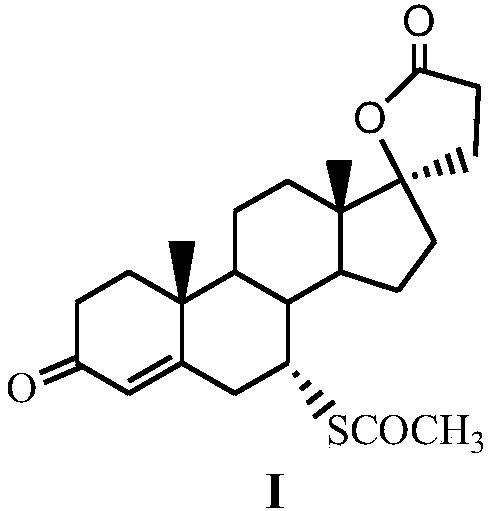
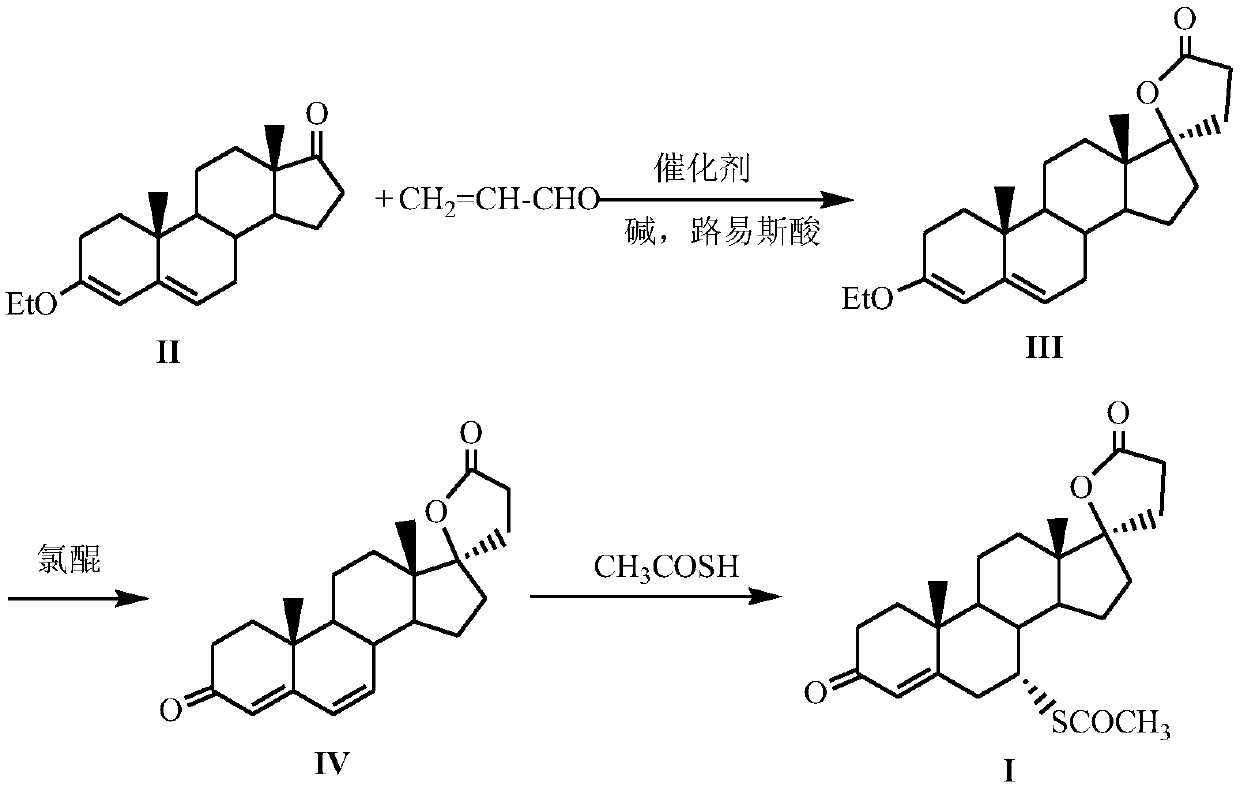
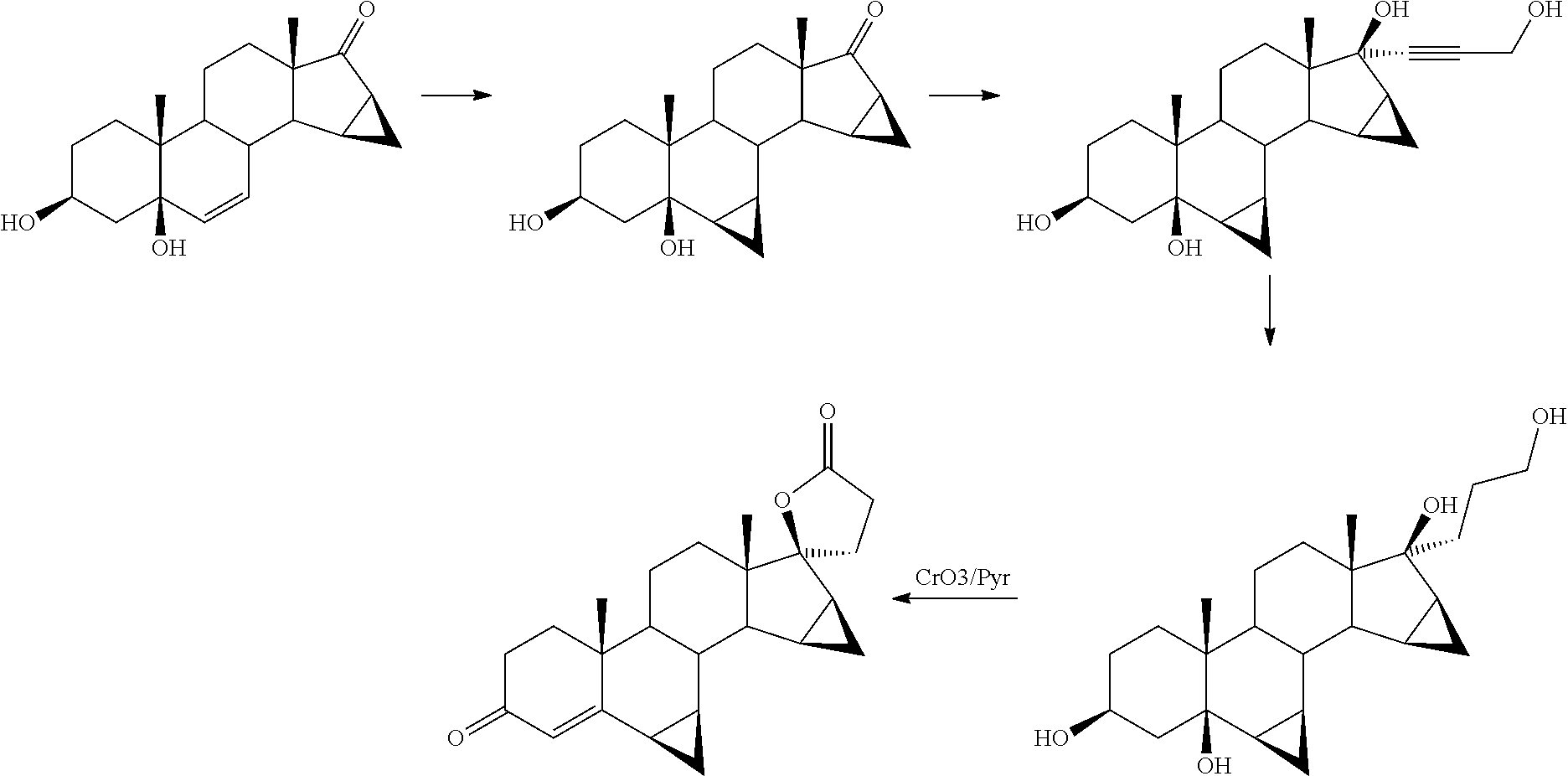
A kind of method for preparing spironolactone
The invention discloses a method for preparing spirolactone. The method comprises the following steps: 1, performing a reaction on a compound shown as a formula (II) and acrolein under the action of a catalyst, alkali and Lewis acid to obtain a compound shown as a formula (III); 2, performing a reaction on the compound shown as the formula (III) and chloranil to obtain a compound shown as a formula (IV); 3, performing an addition reaction on the compound shown as the formula (IV) and thioacetic acid to obtain the spirolactone. The preparation method of the spirolactone, provided by the invention, has the advantages of a short synthetic route, cheapness and easy obtainment of used reagents, simple operation, high total yield rate, and suitability for industrial production; a novel way for preparing the spirolactone is provided.
Owner:HUAIHAI INST OF TECH
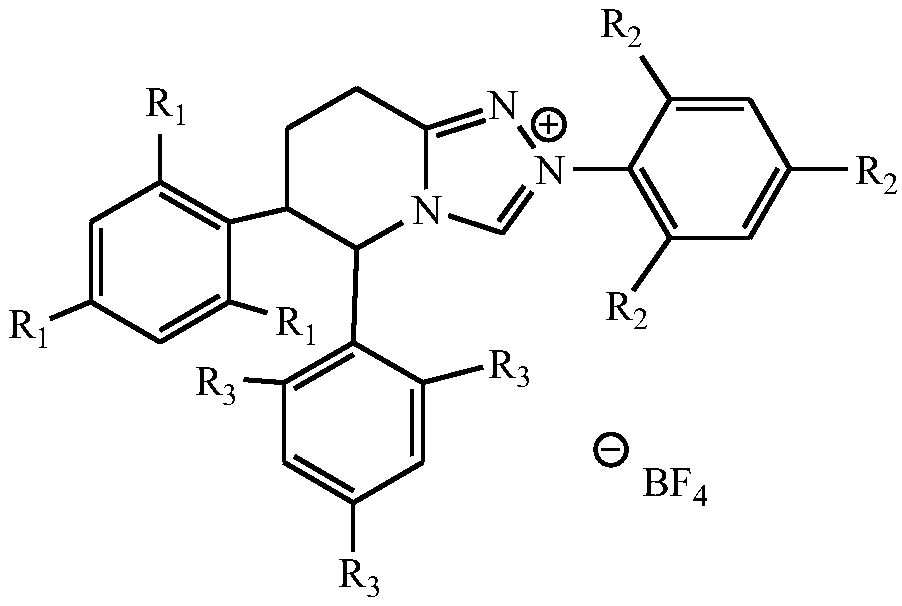
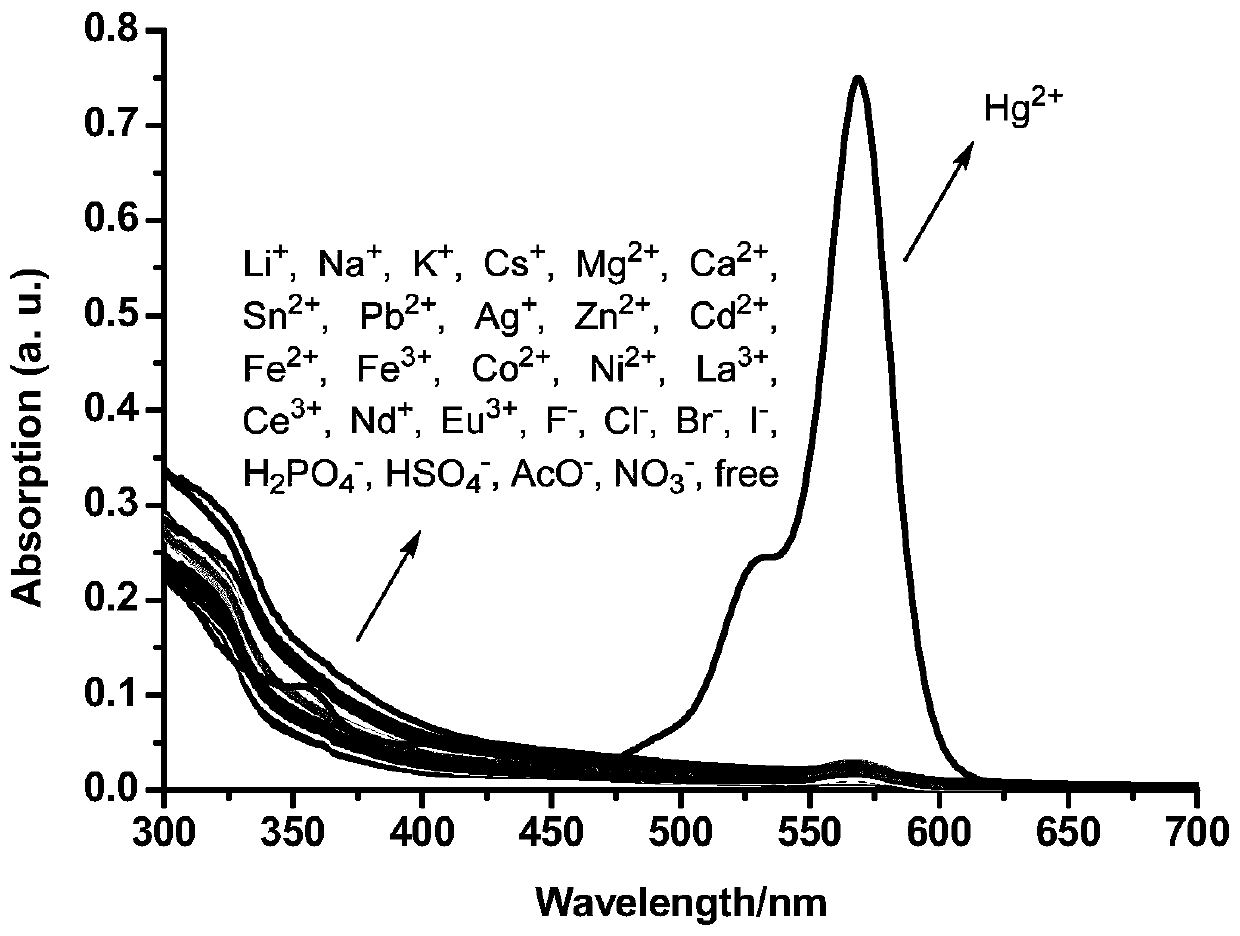
Formyl thiourea bridged ferrocene-rhodamine B spironolactam multi-channel response receptor molecule and synthesis method and application thereof
InactiveCN110437287AImprove detection efficiencyEfficient outputMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsThioureaSynthesis methods
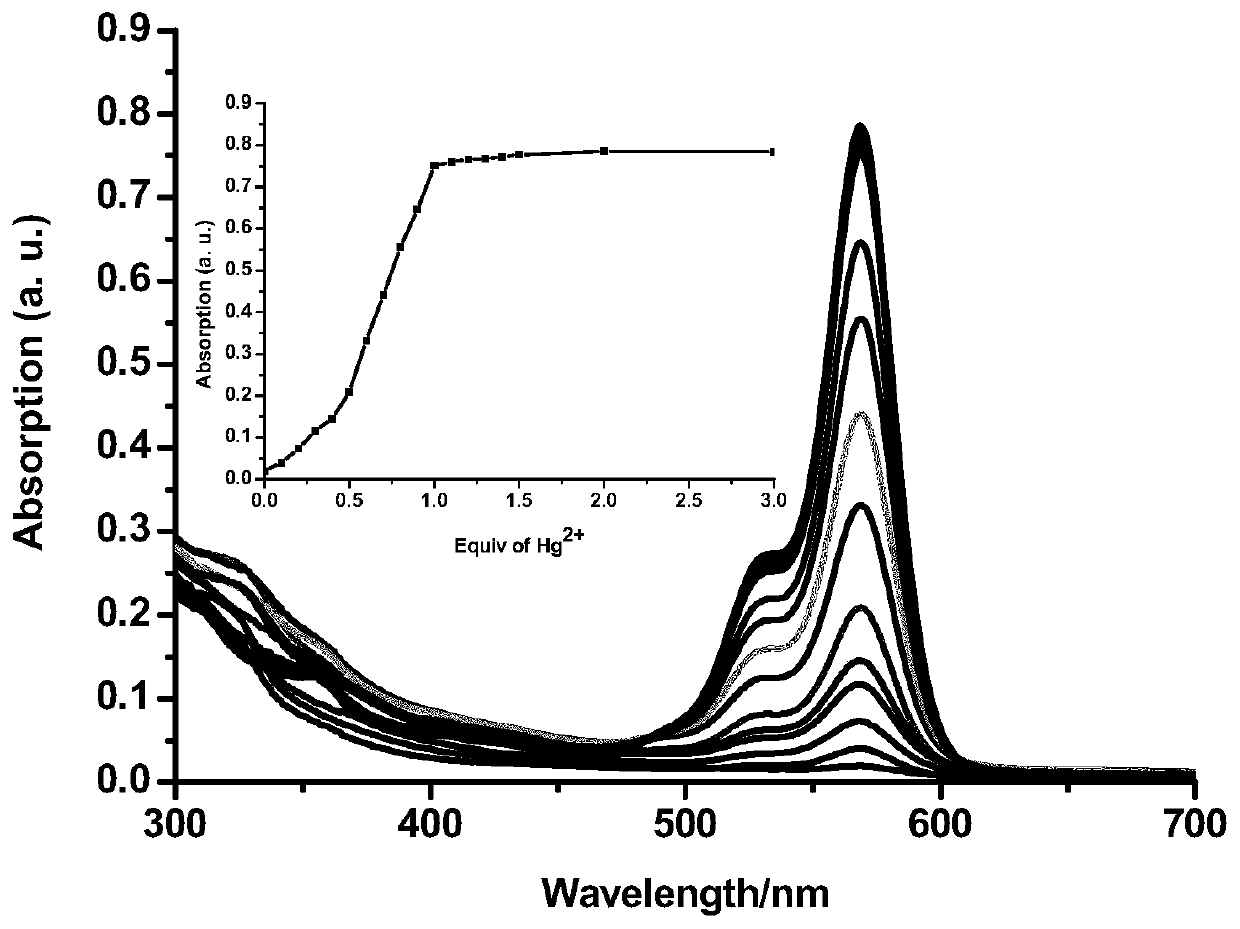
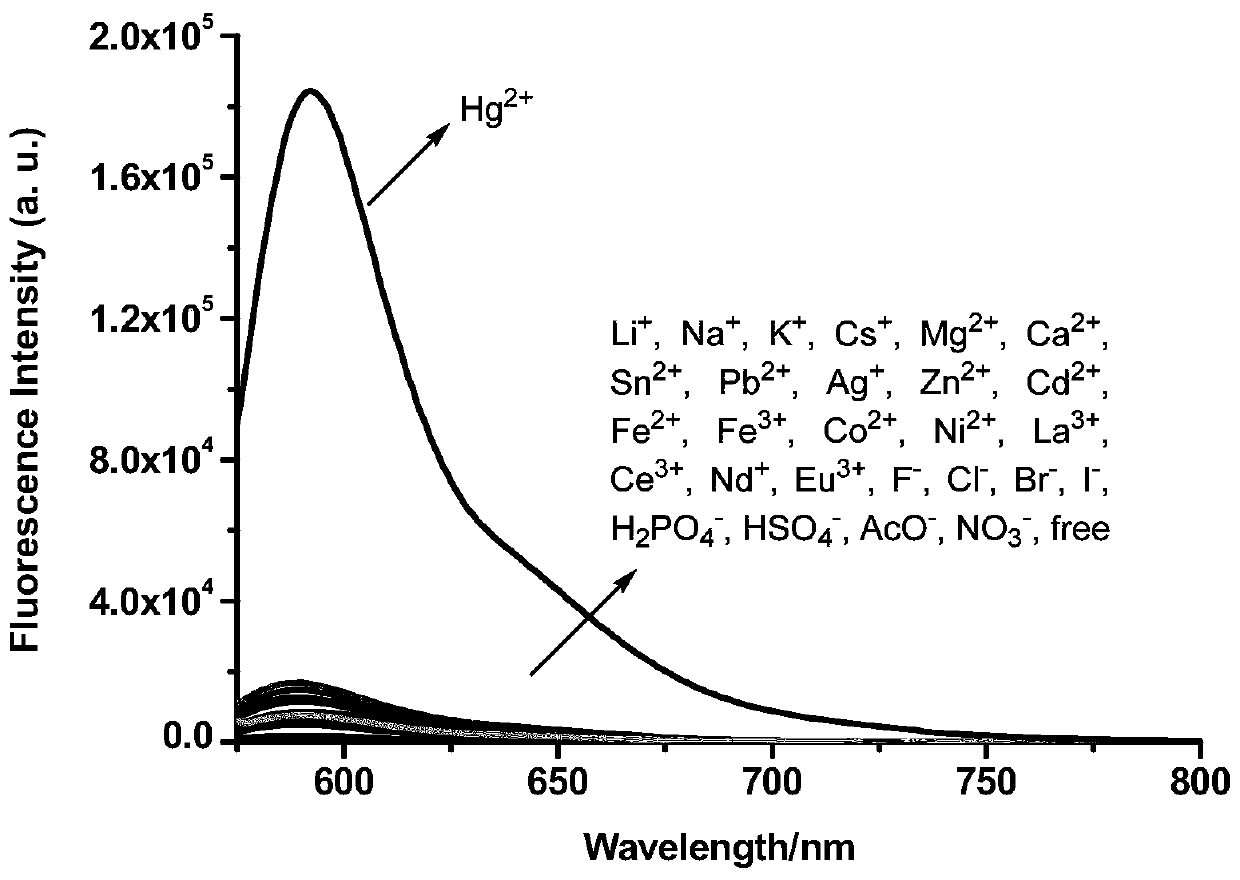
The invention discloses a formyl thiourea bridged ferrocene-rhodamine B spironolactam multi-channel response receptor molecule and a synthesis method and application thereof. Combining ferrocene and rhodamine B spironolactam into a reactive photoactive acceptor molecule with formyl thiourea as a bridge structure, the specificity of Hg<2+> ions are used for promoting desulfurization and ring closing reaction to form a 1,3,4-oxadiazole heterocyclic ring, a rhodamine B spironolactone ring is simultaneously opened, the efficient and quick detection of selectivity of Hg<2+> ions in aqueous phases or cells based on electrochemistry, ultraviolet visibility, fluorescence spectroscopy and other technologies is realized, and broad application prospects are achieved.
Owner:SHANDONG NORMAL UNIV
Process for obtaining 17-spirolactones in steriods
The invention relates to processes for obtaining steroids with a spirolactone group in position 17, particularly to industrially obtaining 6β,7β; 15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21,17-carbolactone, commonly known as Drospirenone, as well as to intermediates useful in said process.
Owner:CRYSTAL PHARMA SA
Oil-in-water compound spironolactone nano emulsion medicine
InactiveCN102716220AImprove solubilityReduce first pass effectOrganic active ingredientsEmulsion deliveryHalf-lifeAsarone
The invention discloses an oil-in-water compound spironolactone nano emulsion medicine. The nano medicine is prepared from the following raw materials in percentage by mass: 1 to 5 percent of spironolactone, 15 to 30 percent of surfactant, 5 to 20 percent of cosurfactant, 5 to 20 percent of oil, 0.5 to 10 percent of hawthorn extract, 0.5 to 1.0 percent of wild ginger extract and the balance of distilled water, and the sum of the mass percentages of the components is 100 percent. After spironolactone is prepared into the nano emulsion preparation, so that the blood brain barrier penetrating capacity is improved obviously, the bioavailability of raw medicines is improved obviously, the half-life period of the medicine is prolonged, and the administration times are reduced; the nano emulsion improves the medicine dissolution and infiltration capacity of spironolactone and the stability of spironolactone; and after the hawthorn water extract and the wild ginger water extract are compounded, the nano emulsion medicine is obvious in synergistic effect, and is full in curative effect.
Owner:NORTHWEST A & F UNIV
Spironolactone dripping pills
InactiveCN1634080AIncrease surface areaHas a wetting effectOrganic active ingredientsPill deliverySide effectAdditive ingredient
The invention discloses a pharmaceutical composition for treating edema diseases and cardiovascular and cerebrovascular diseases, in particular an oral drop pill preparation of the pharmaceutical composition with spironolactone as an active ingredient. The purpose of the present invention is to make up for the deficiencies of the prior art, to provide patients and medical workers with a medicine with large drug loading capacity, rapid drug release, rapid effect, high bioavailability, small toxic and side effects, low price and easy to take. Spironolactone drop pills. Take spironolactone as raw material, add surfactant polyethylene glycol and other substrates, heat until the pharmaceutical composition melt and / or emulsion and / or suspension containing spironolactone and substrate are obtained, put it into the dropping pill machine, drop into It is formed by shrinking in a condensing agent, taken out and wiped dry.
Owner:北京博智绿洲医药科技有限公司
Compound micro-cream for acne treatment and preparation method thereof
Disclosed is a compound microemulsion cream for treating acne which comprises the following constituents (by weight ratio): (1) stearic acid 8-10%, cetyl alcohol 2-4%, glyceryl monostearate 2-3%, fluid wax 2-8%, (2) triethanolamine 3-5%, 2-6% of at least one selected from glycerin, propylene glycol, 1,3-butylene glycol, 10% of ethyl-p-hydroxybenzoate alcoholic solution 1%, (3) 8-12% of at least one of n-propanol, n-butanol and n-pentanol, (4) medicaments including Azithromycin 0.25-0.75%, spironolactone 0.2-0.4%, vitamine-A acid 0.025-0.05%, and balancing purified water.
Owner:中国人民解放军济南军区第四0一医院
Compositions and methods for treatment of renal disease
InactiveUS20150258062A1Prevent kidney damageReduce riskOrganic active ingredientsBiocideDiseaseGlomerular diseases
The invention relates to methods, compositions, and kits for the treatment of proteinuria and / or hypertension (e.g., proteinuria and / or hypertension arising from primary renal disease (e.g., focal segmental glomerulosclerosis, glomerular disease) or secondary to other conditions (e.g., diabetes, diabetic nephropathy, liver disease). Specifically, the invention relates to methods involving combination therapy wherein an indoline (e.g., indapamide) is administered in combination with an anti-aldosterone agent (e.g., spironolactone and / or epleronone).
Owner:GUPTA AJAY
Immediate release formulation of a triple combination of active pharmaceutical ingredients useful in the treatment of polycystic ovary syndrome
InactiveUS20210290639A1Optimal flowability and compaction propertyMarked robustness and industrial scalabilityOrganic active ingredientsCoatingsOral medicationImmediate release
Immediate release formulation of a triple combination of active pharmaceutical ingredients useful in the treatment of polycystic ovarysyndrome It relates to an immediate release formulation for oral administration, comprising; a) a combination of three active pharmaceutical ingredients which are spironolactone; pioglitazone or a salt thereof; and metformin or a salt thereof; and b) solid polyethylene glycol having an average molecular weight from 3350 to 8000 g / mol; wherein: each of the active pharmaceutical ingredients are present in a therapeutically effective amount; and the polyethylene glycol is present in an amount such that decreases the dissolution profiles in vitro of each one of the active pharmaceutical ingredients without modifying the disintegration time of the formulation compared with the dissolution profiles of each one of the active pharmaceutical ingredients of a formulation having the same composition but without the solid polyethylene glycol, as well as, to its preparation process.
Owner:HOSPITAL SANT JOAN DE DEU +1
Oil-controlling, anti-blackening and skin-whitening cream
InactiveCN101185622BResist irritationPrevent photoagingCosmetic preparationsToilet preparationsTherapeutic effectHyperpigmentation
The invention discloses a cosmetic which can balance oil secreting, resist sunlight and skin aging and essentially consists of rennin, honey, titanium dioxide, carbocisteine, niacin amide, vitamin E, vitamin F, cod liver oil, spironolactone and substrate cream, which are prepared according to certain weight proportion and function coordinately to achieve the effect of whitening and skincare. The invention has the triple effects including skincare, sun block and aging resistance. Firstly, the cosmetics regulates the metabolism of skin oil secretion to balance the secretion of the oil secretion, smoothen pores and reduce the occurrence rate of acne; secondly, the product effectively prevents people from being hurt by ultraviolet and reduces the settlement of melanin, thereby having good sunblock and whitening effects; finally, the product slows down the aging of skin, smoothens the skin and provides delicate and fair skin to users. The invention has scientific components, simple fabrication and unique treatment effect and is suitable for large-batch production.
Owner:孙奕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com