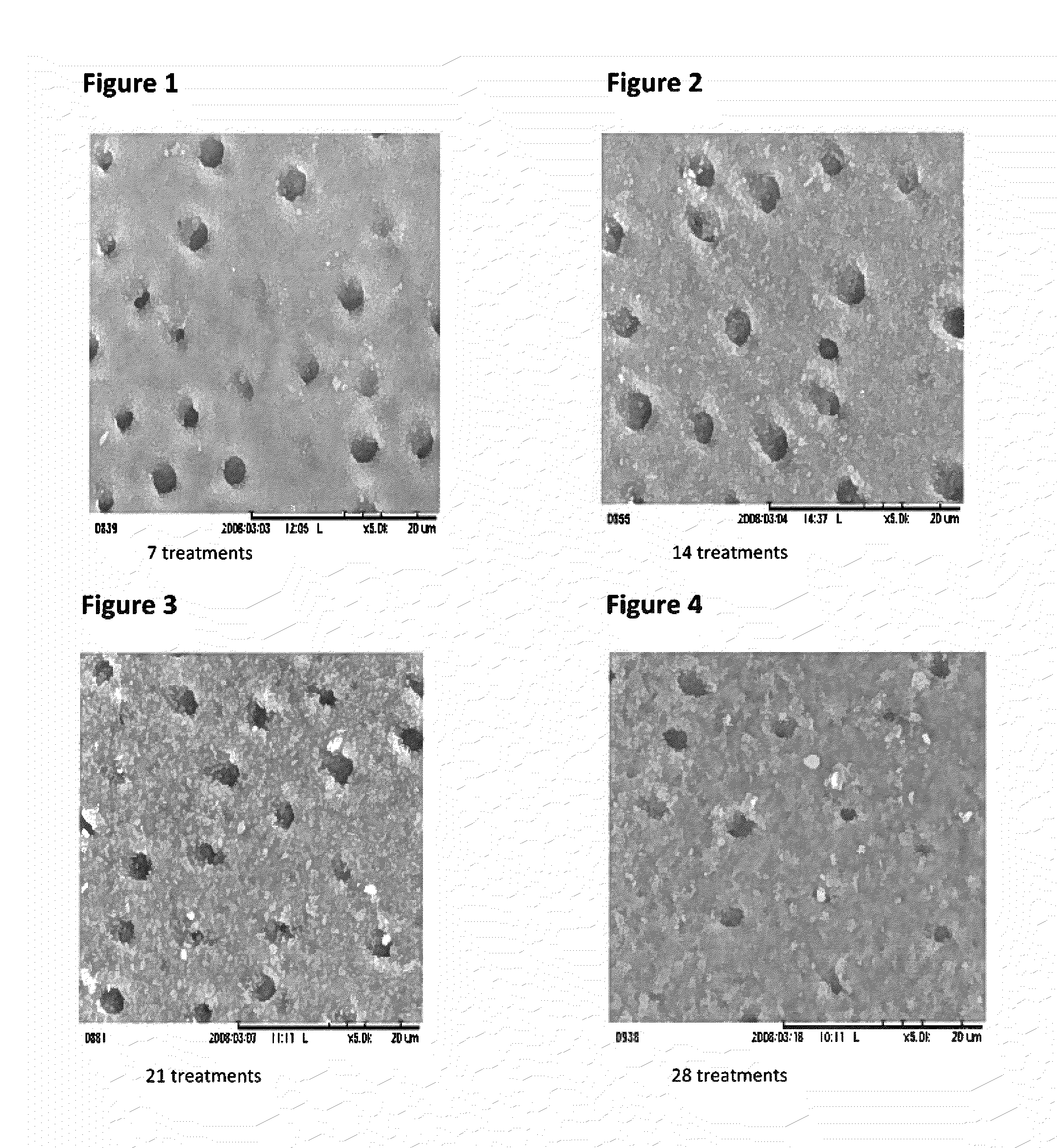
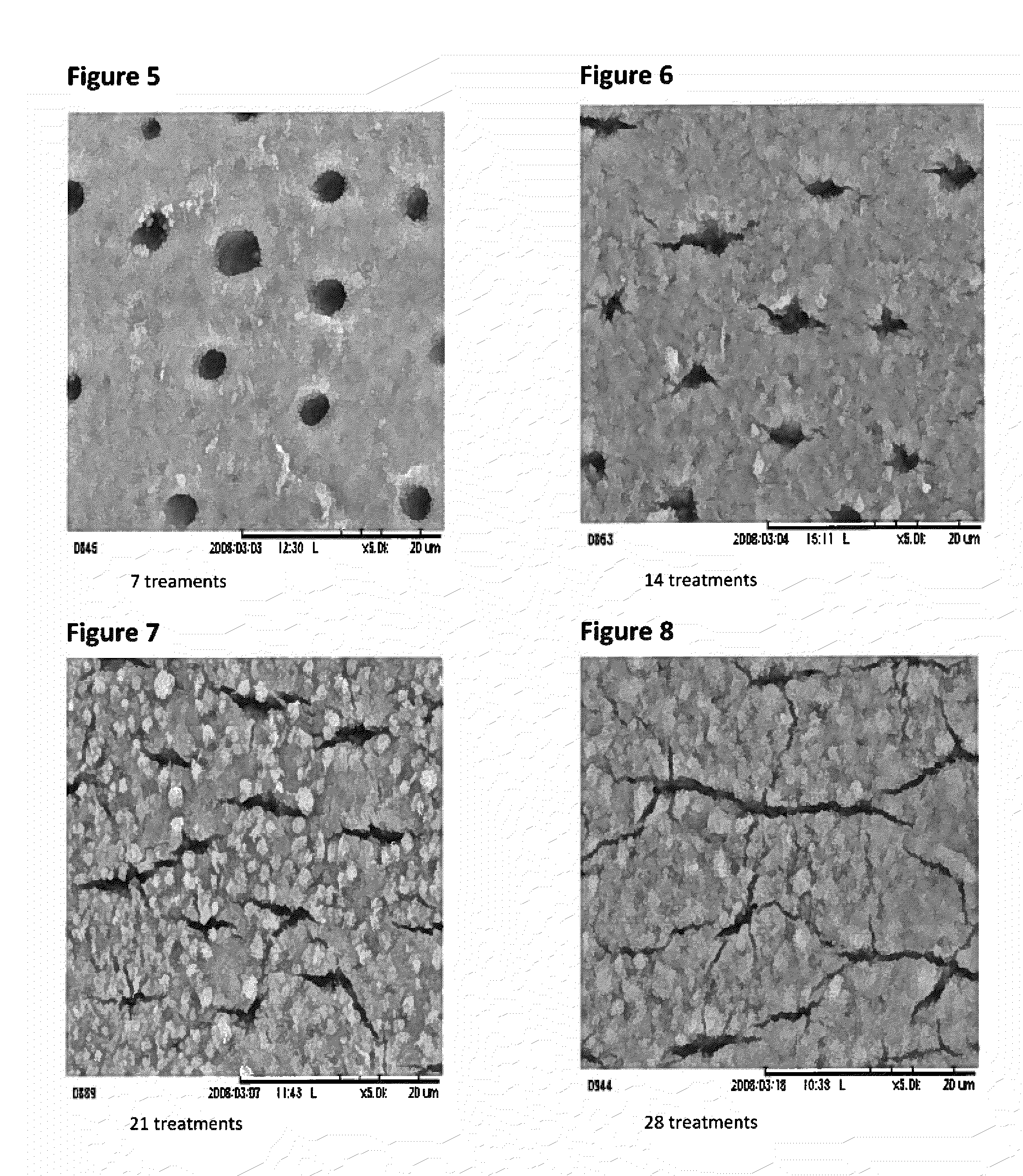
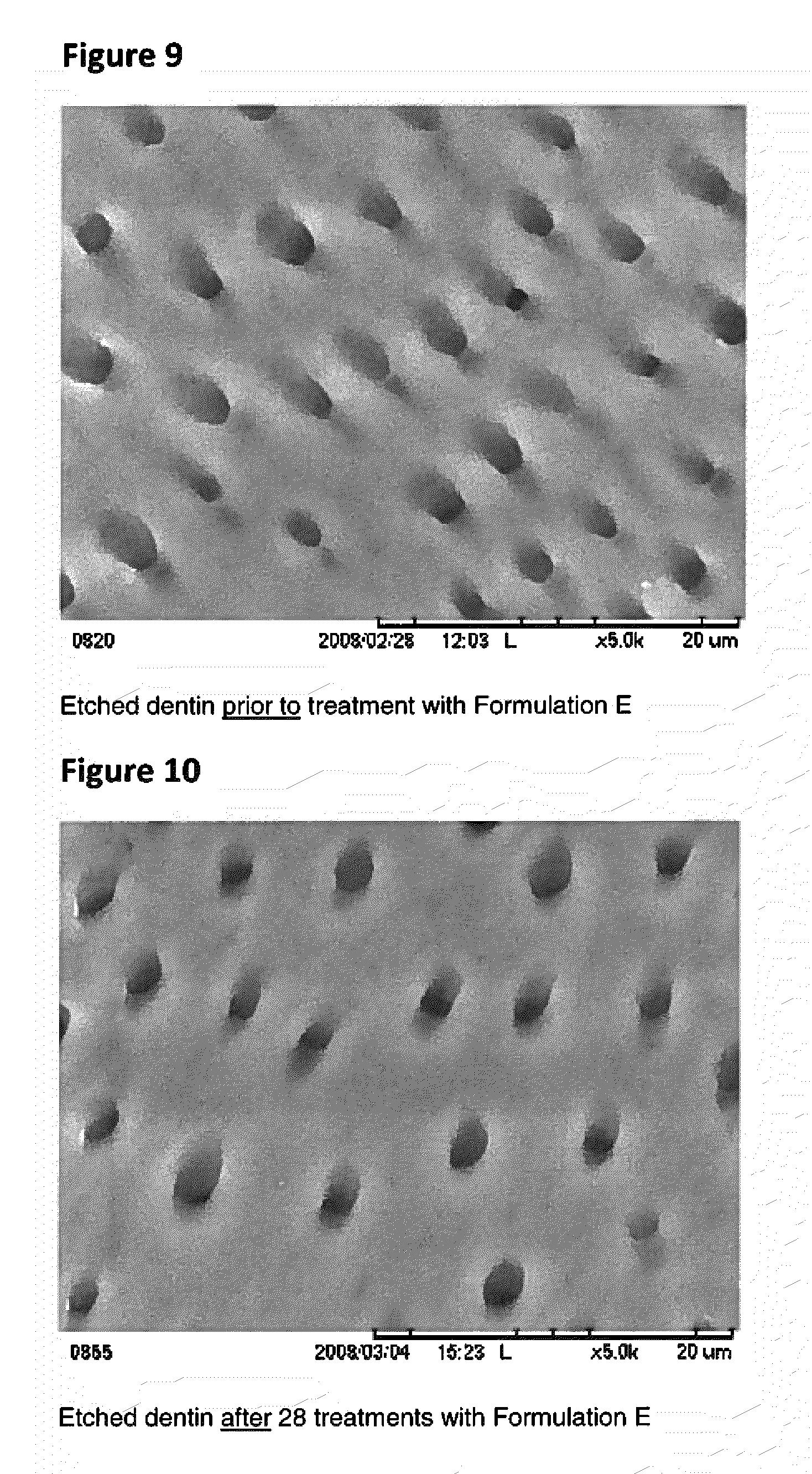
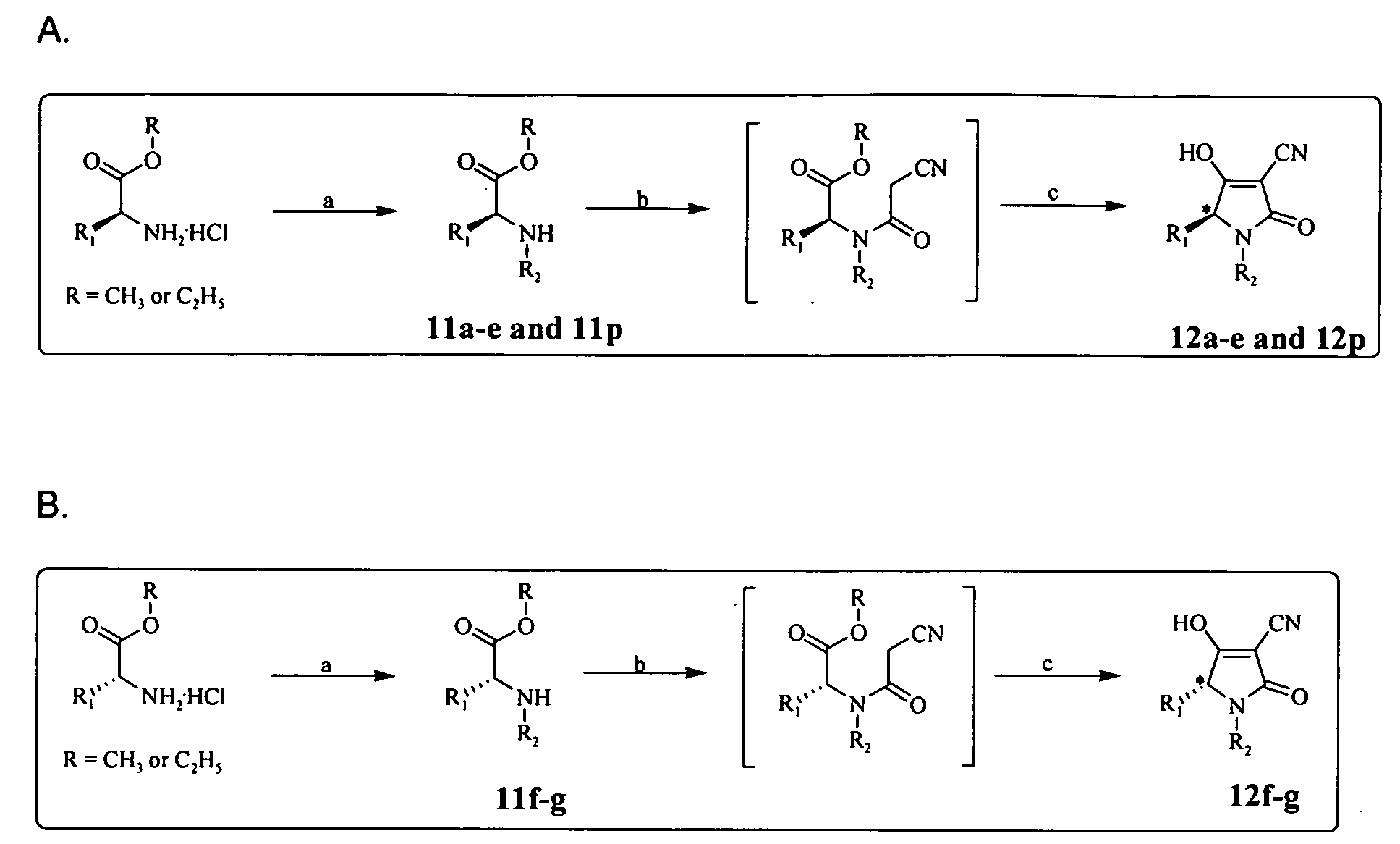
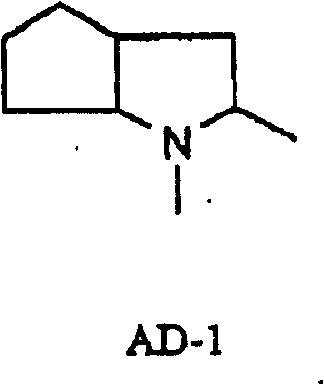
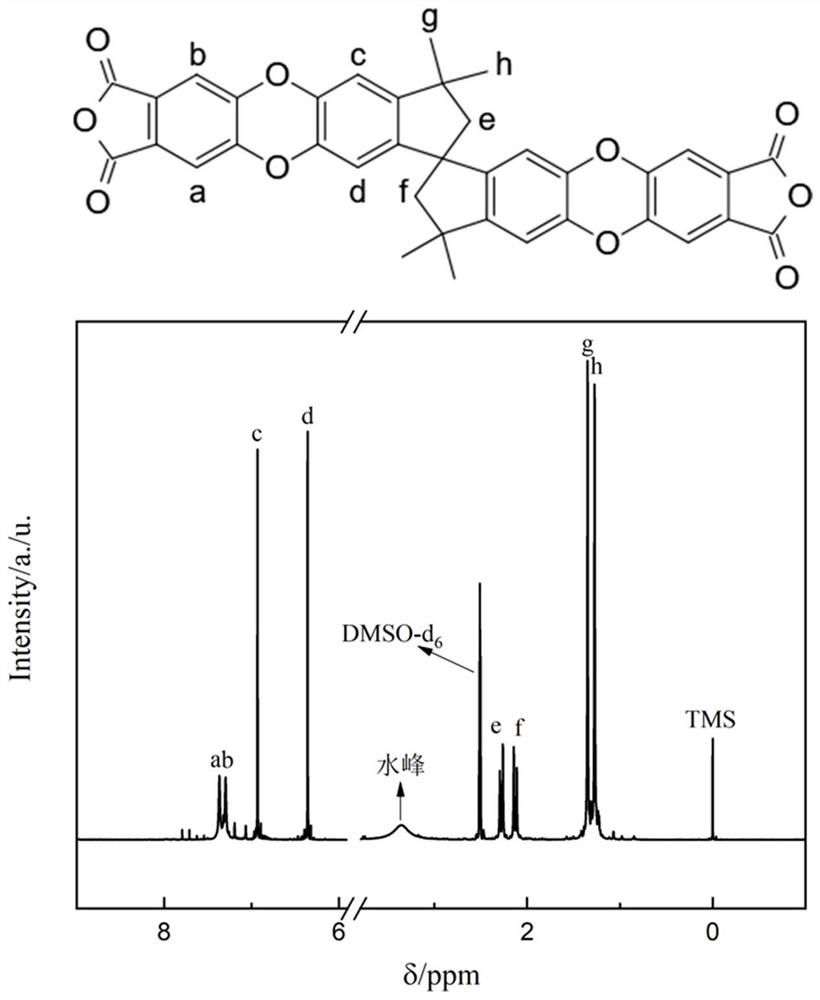
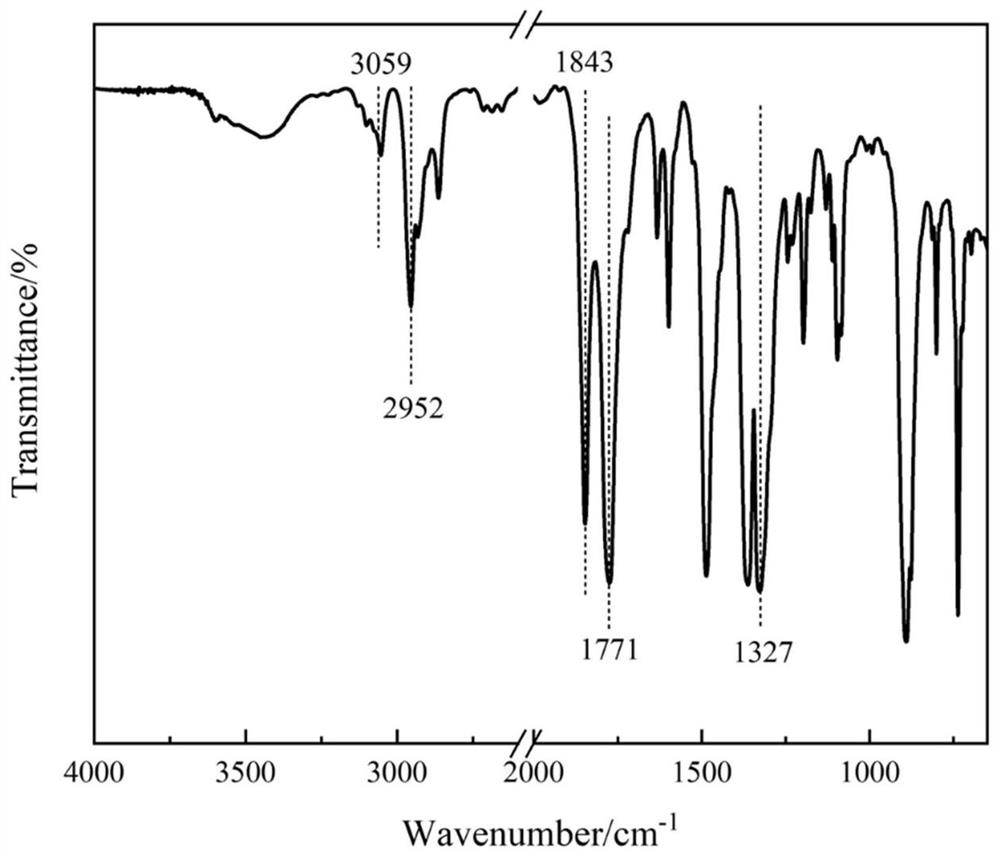
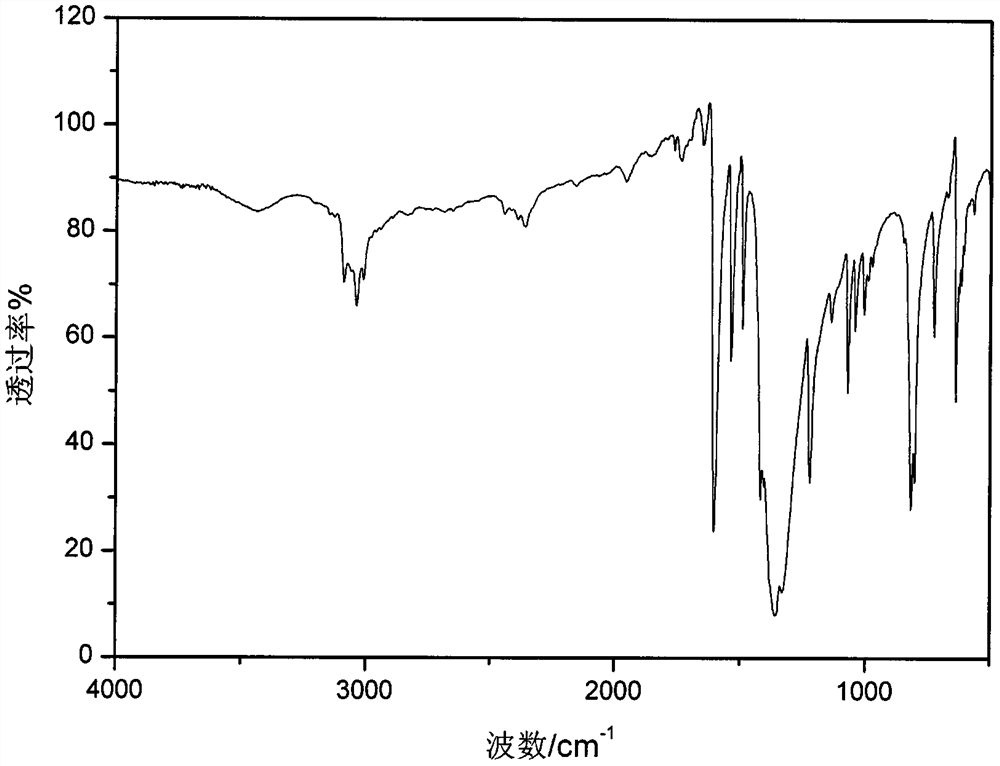
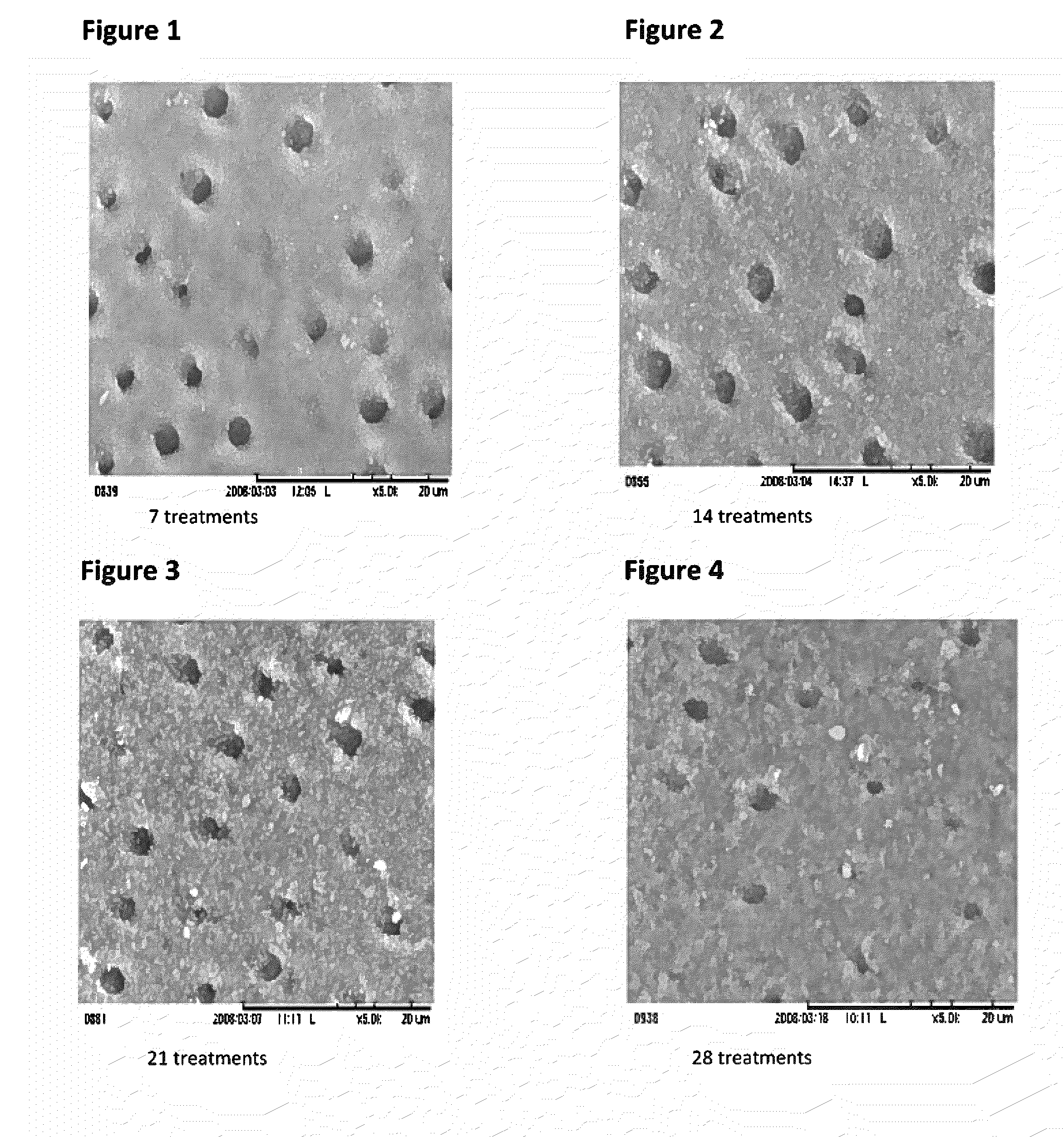
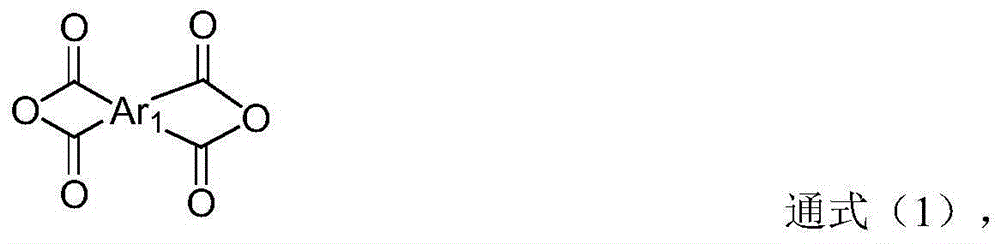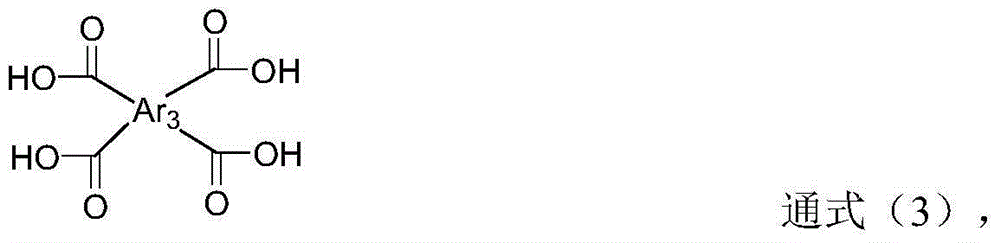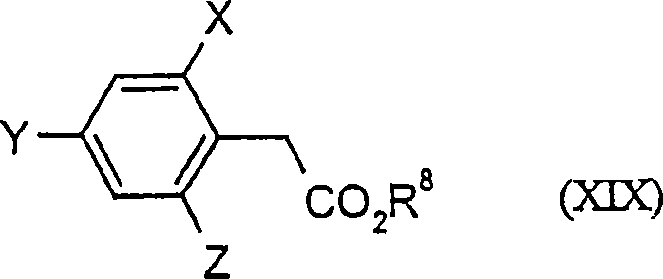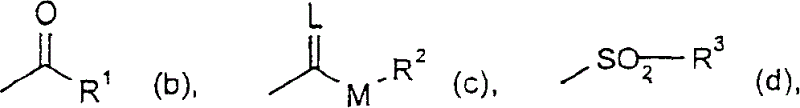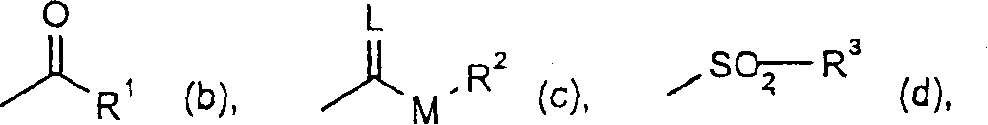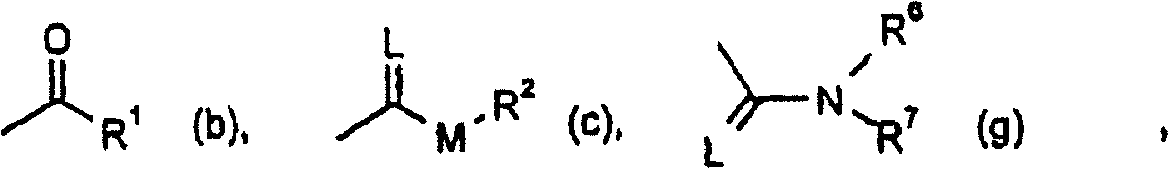Patents
Literature
35 results about "Tetramic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
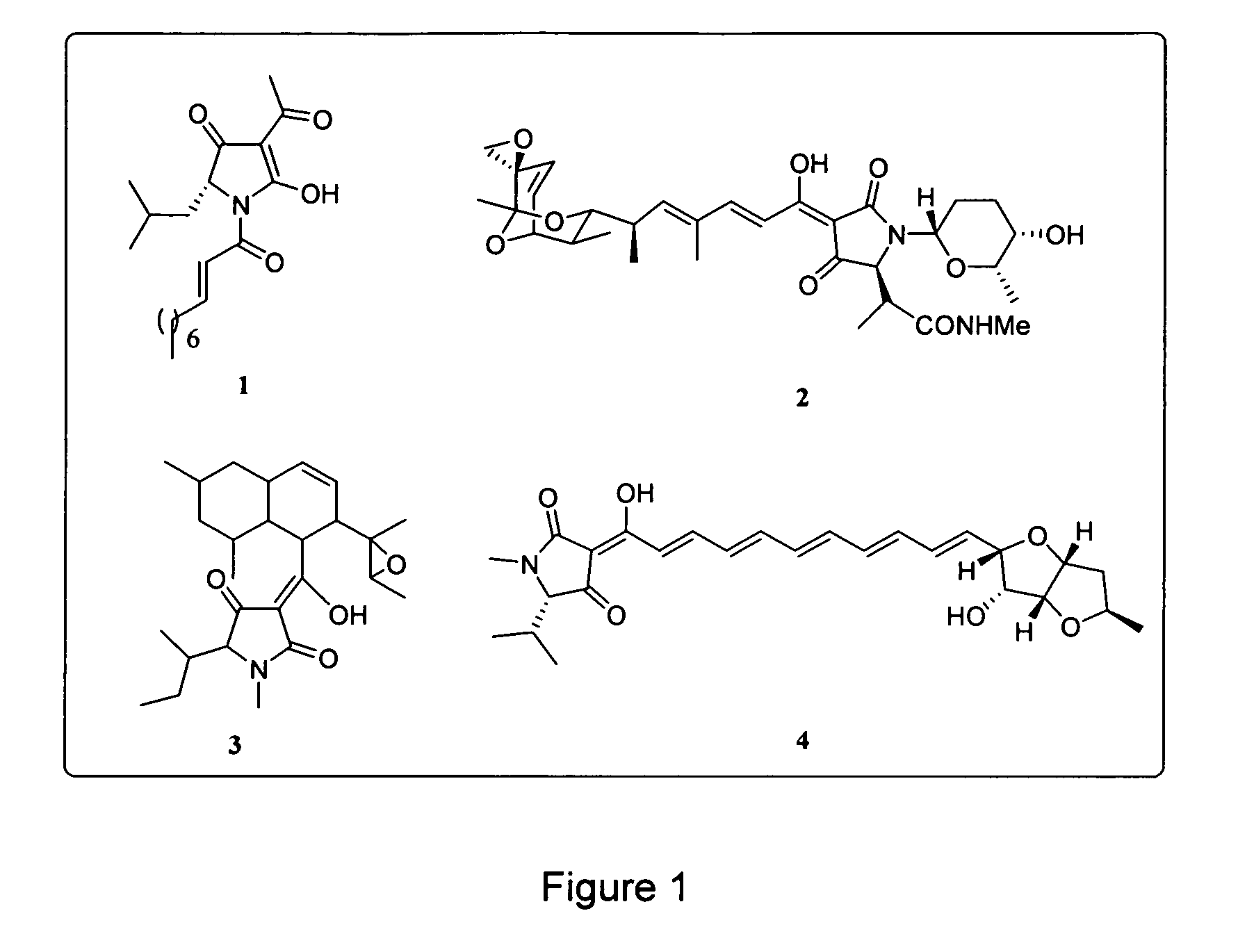
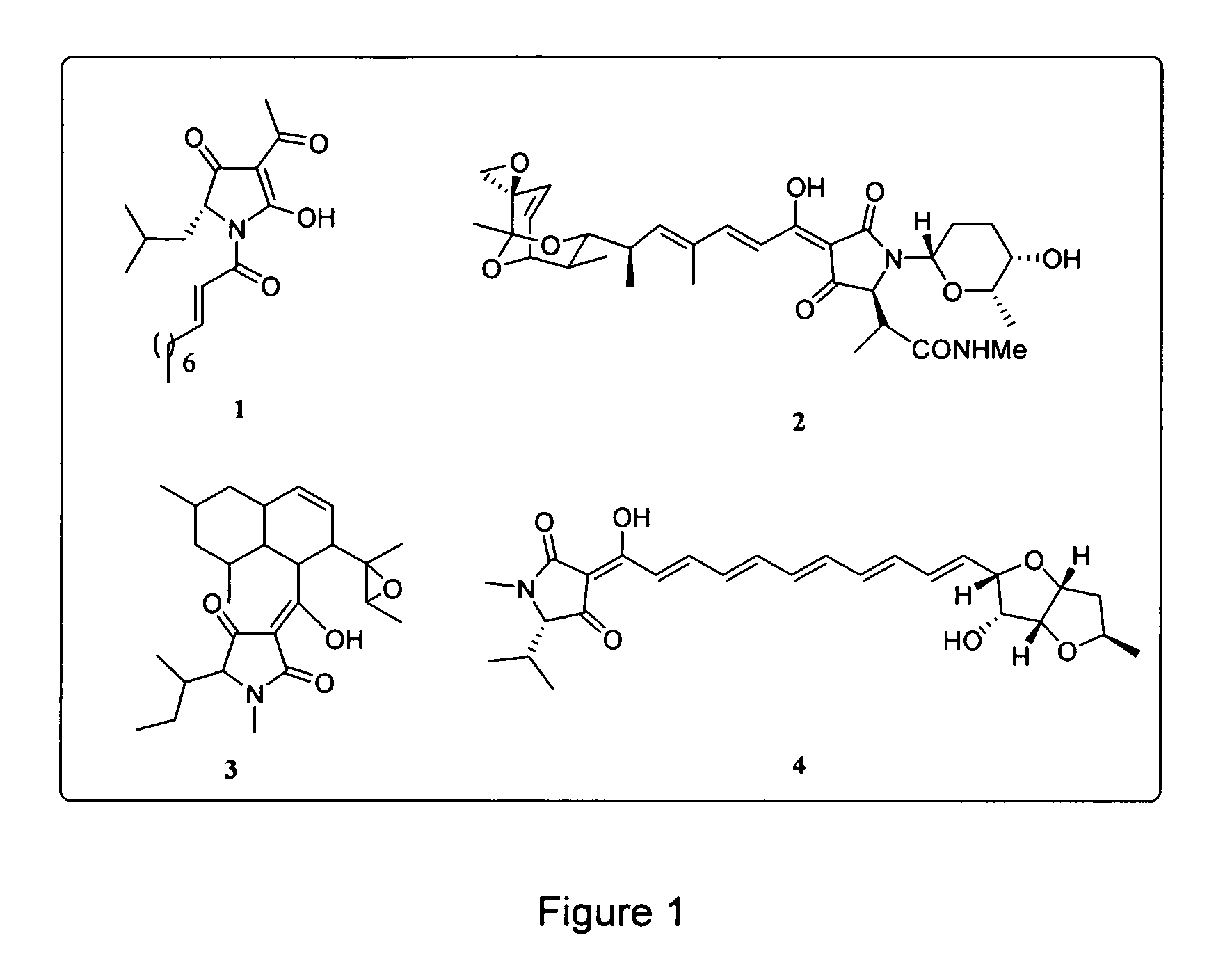
Tetramic acids with polyenyl substituents are an important class of compounds in medicinal chemistry. Both solid and solution phase syntheses of such molecules have been reported recently.
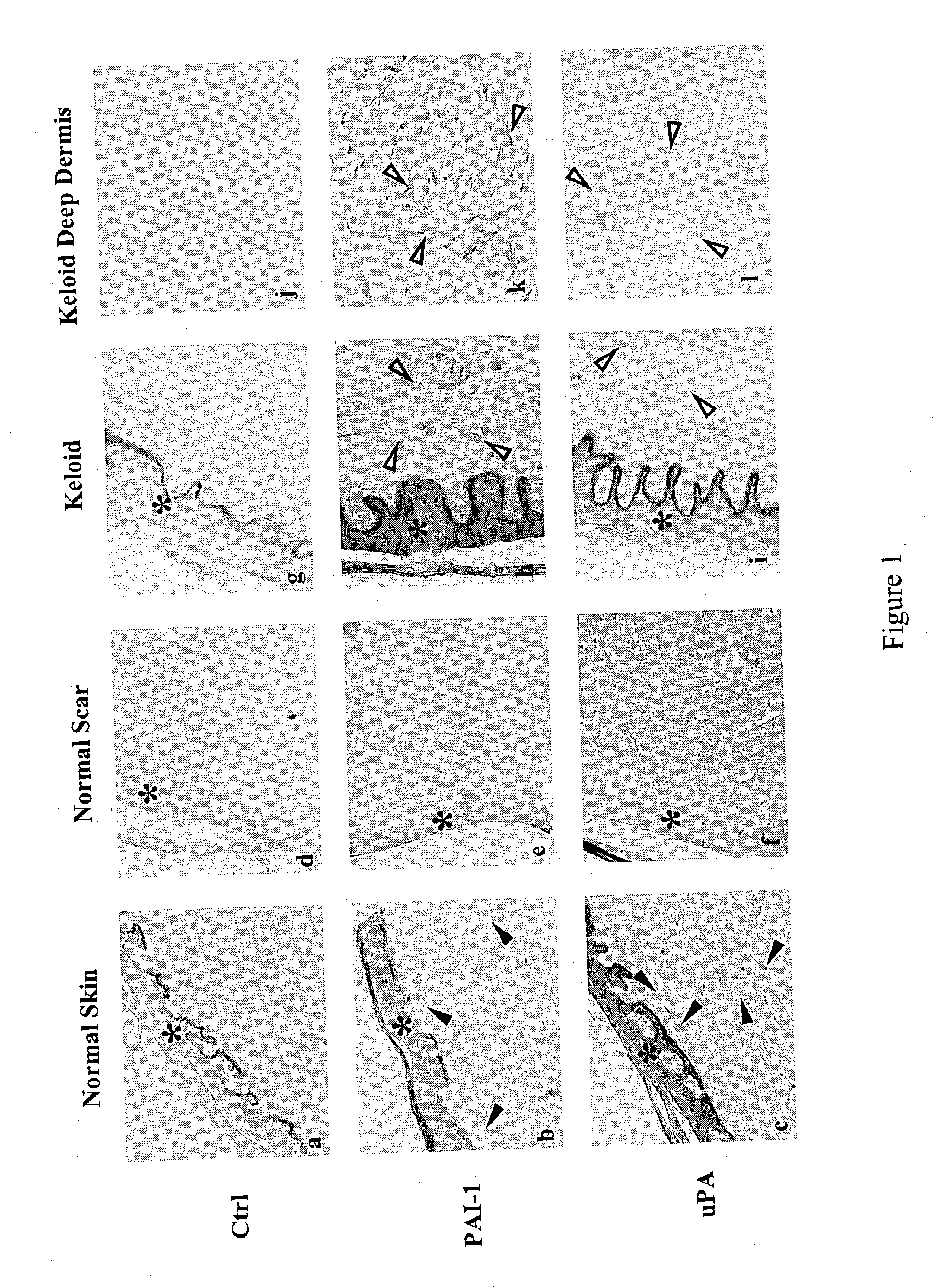
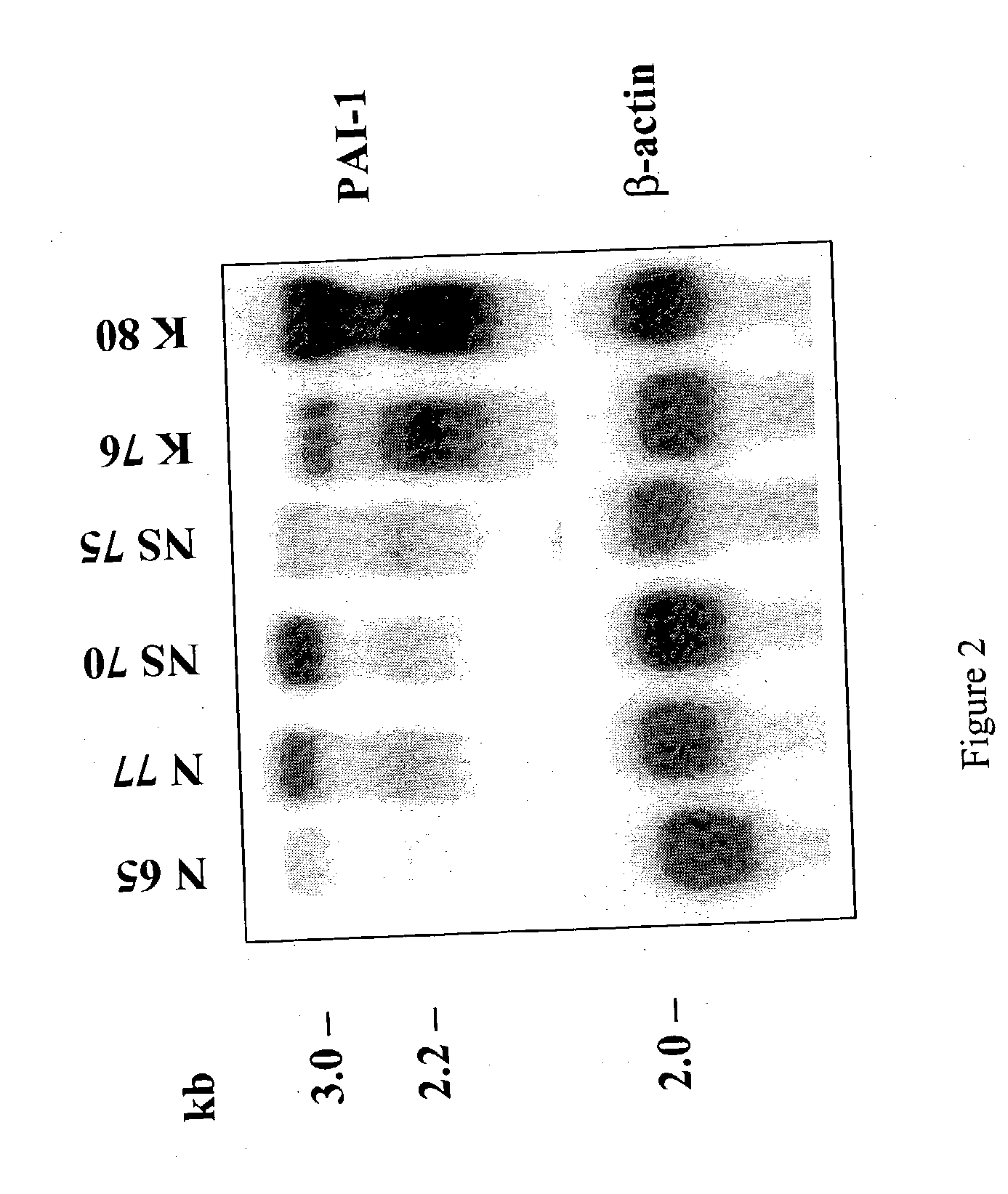
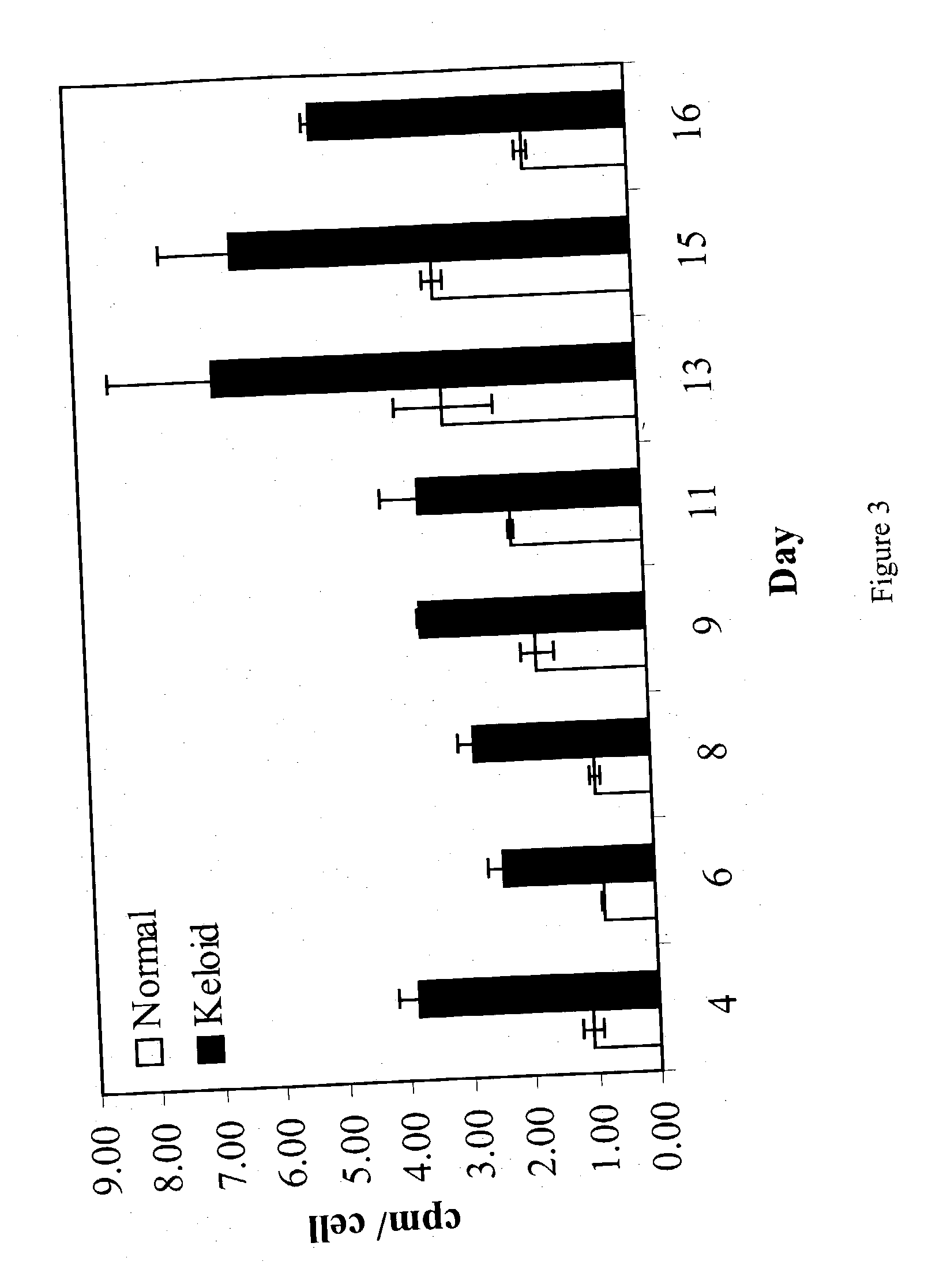
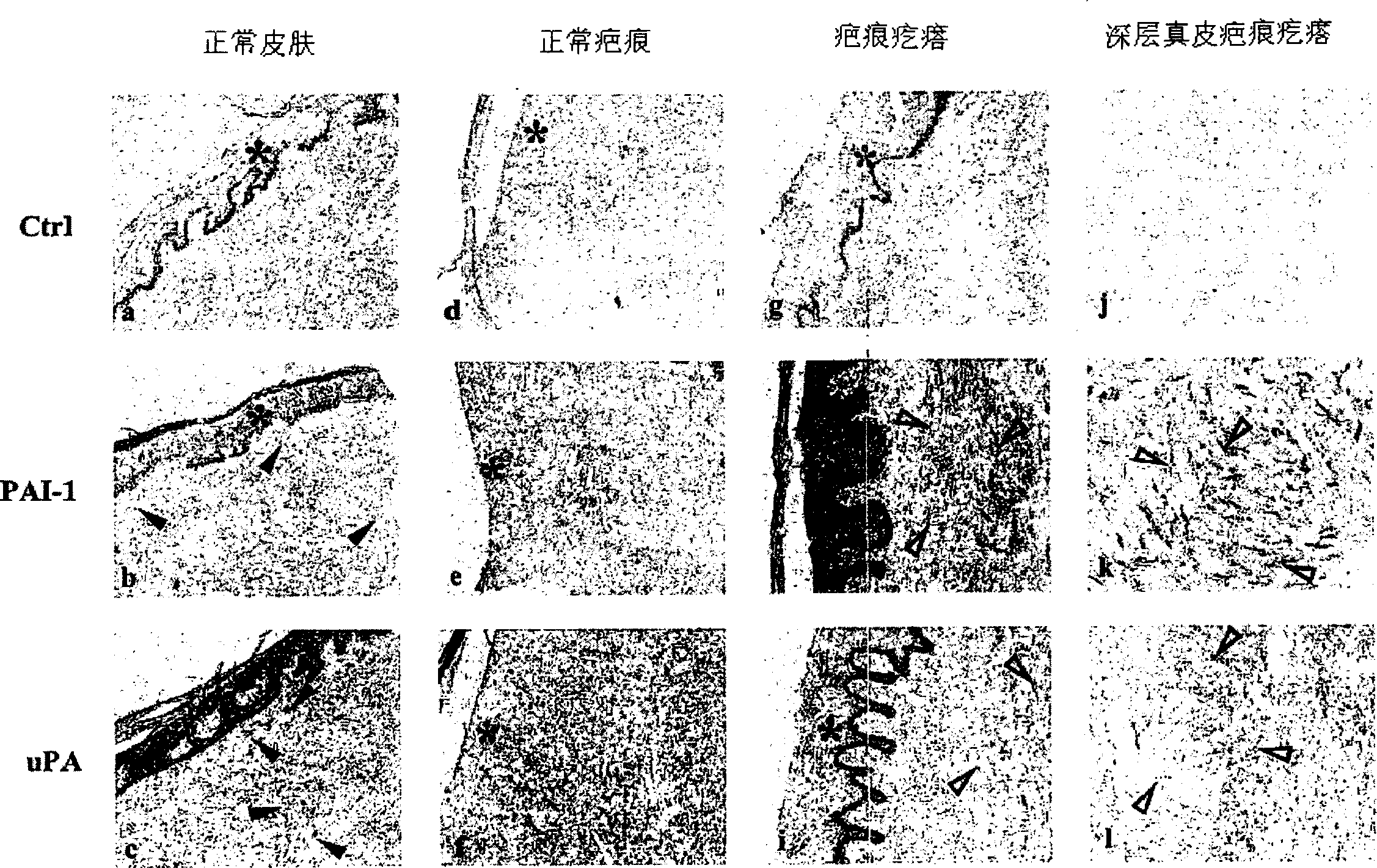
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
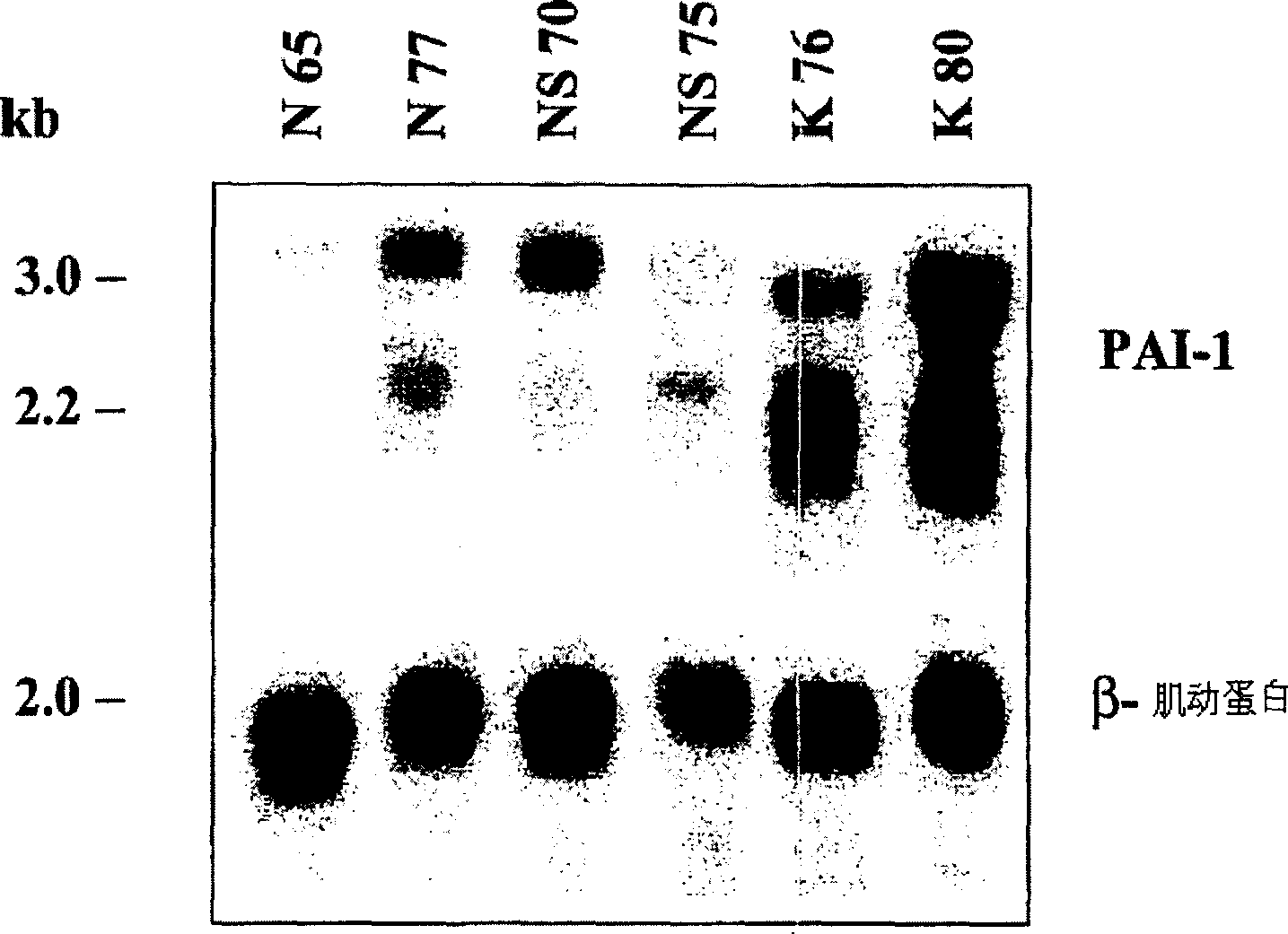
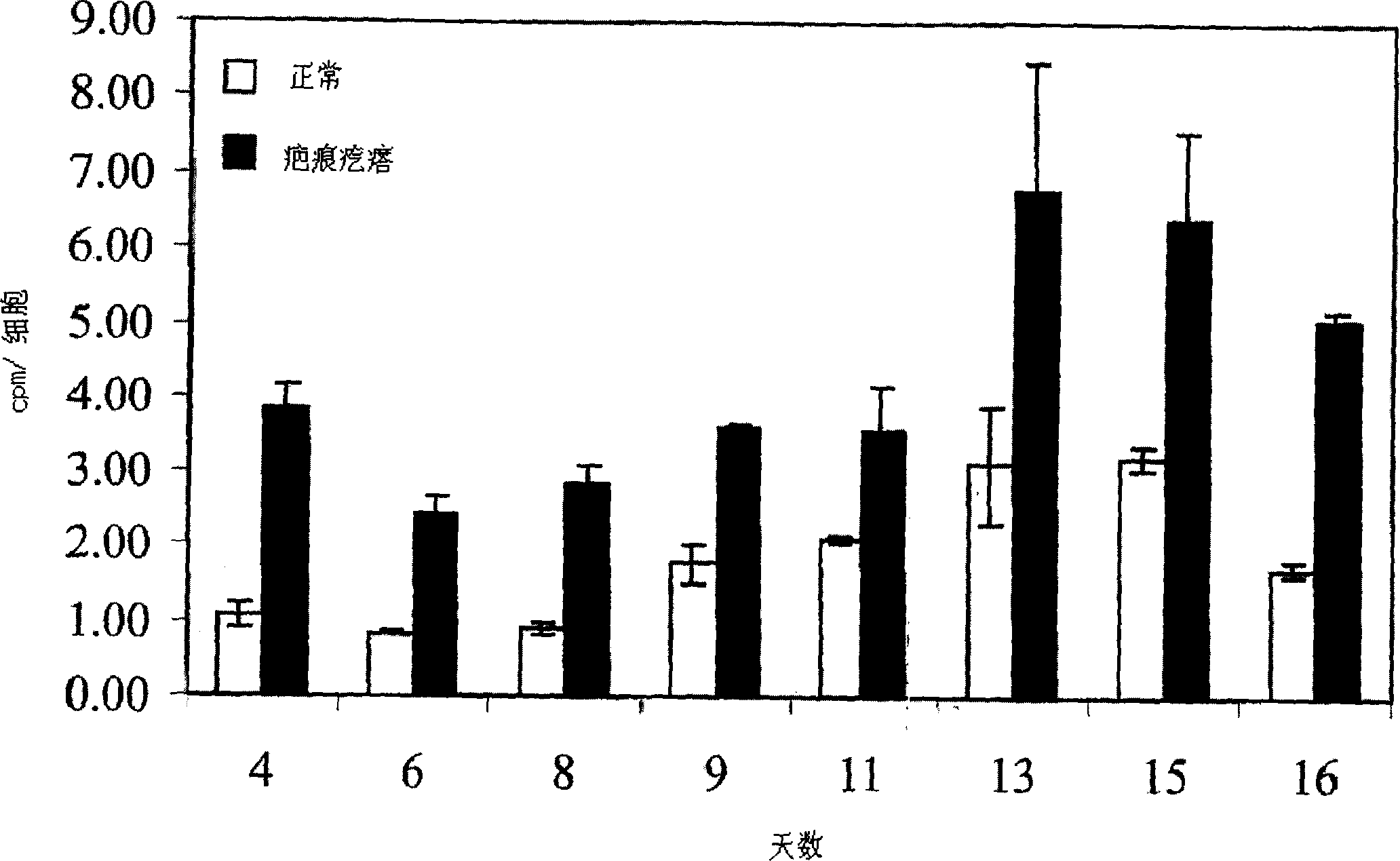
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
InactiveCN1668312AReduced uPA activityImmunoglobulins against animals/humansMuscular disorderVitamin CFibrosis
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
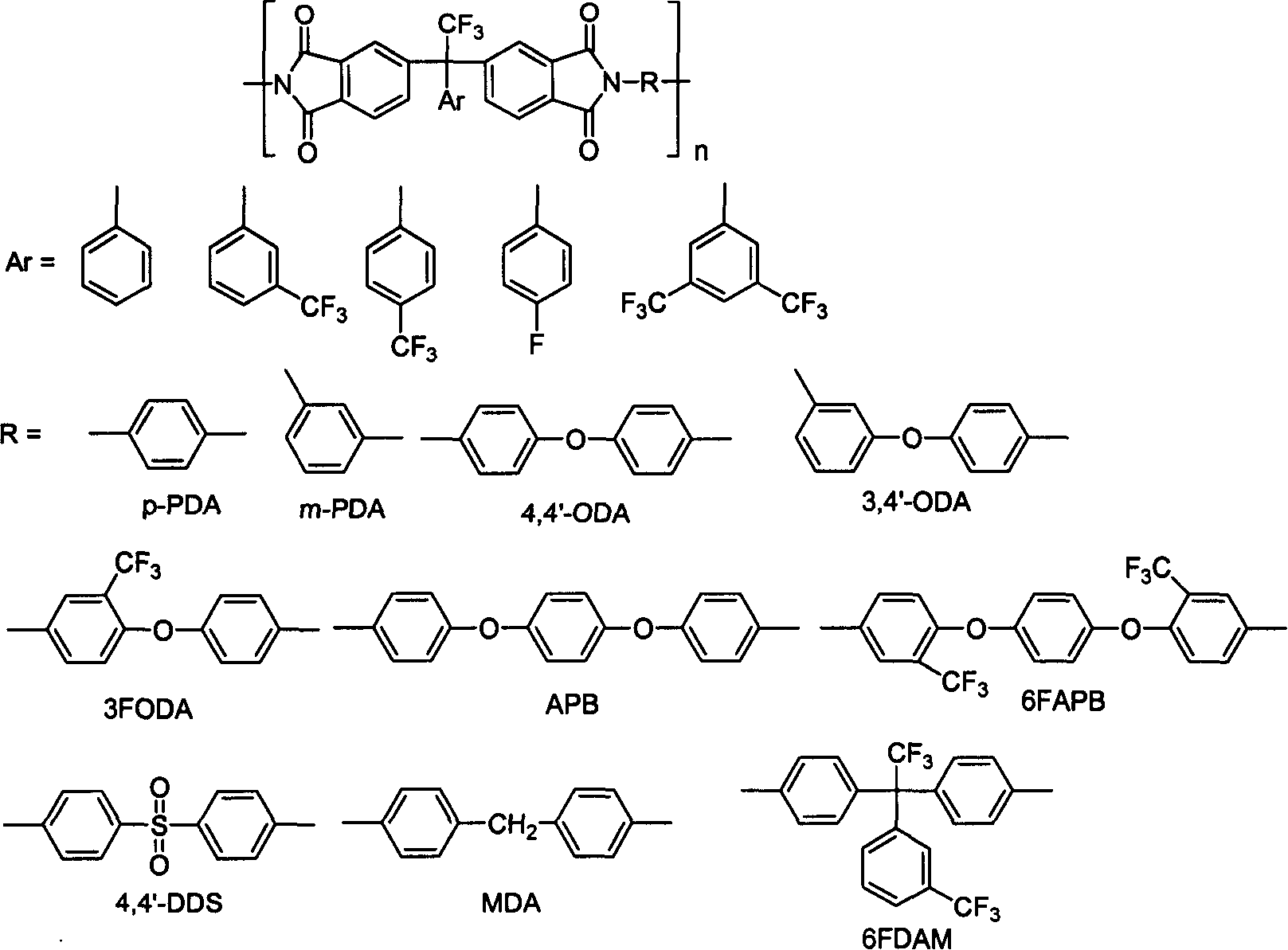
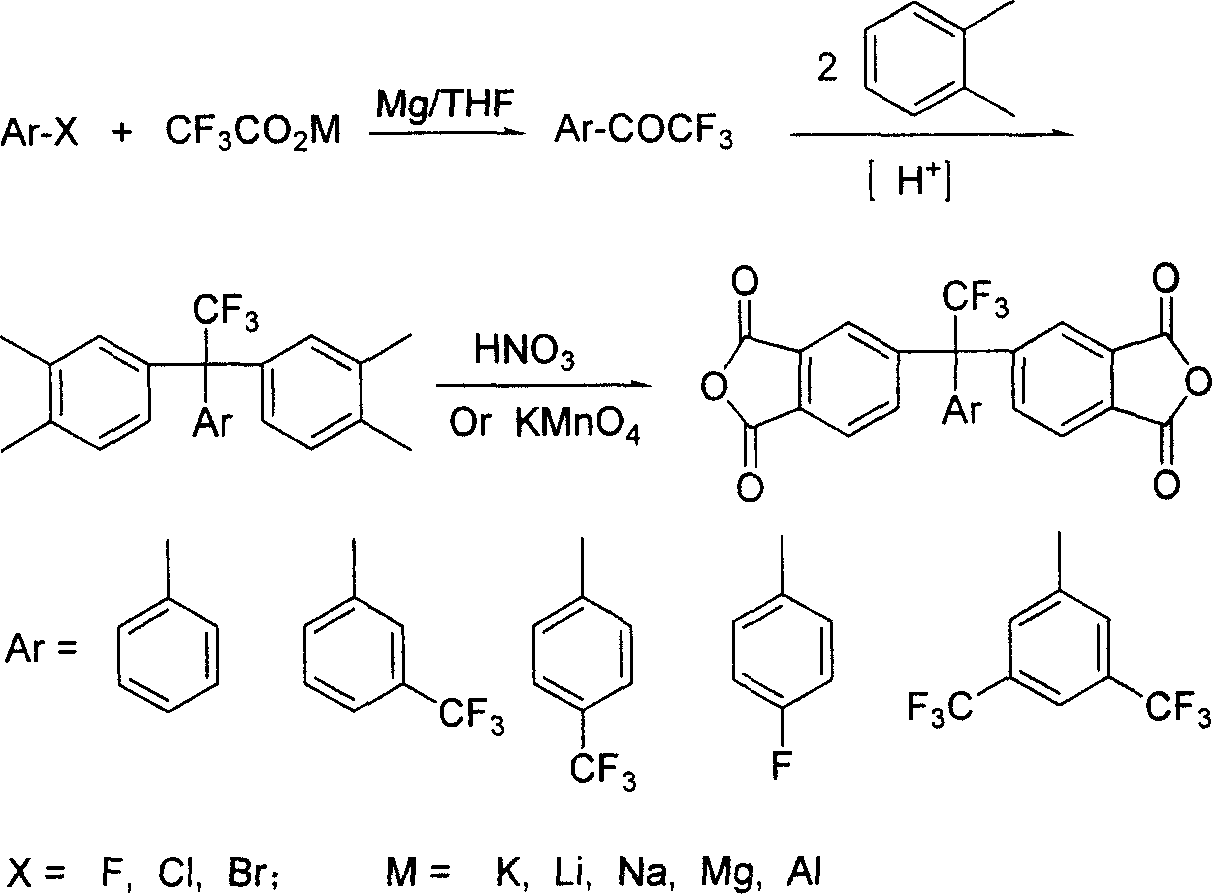
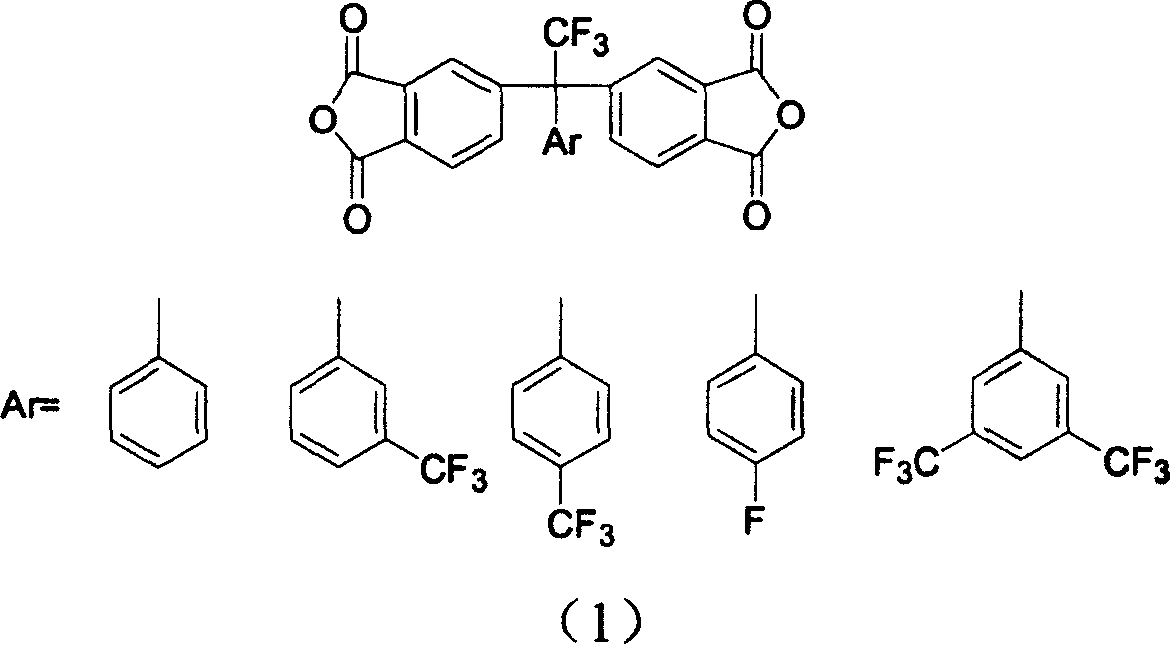
Fluoro-aromatic organic tetracarboxylic dianhydride and its preparation method and use
InactiveCN1605587AImprove performanceImprove heat stabilityLiquid crystal compositionsOrganic chemistryAcetic anhydrideStrong acids
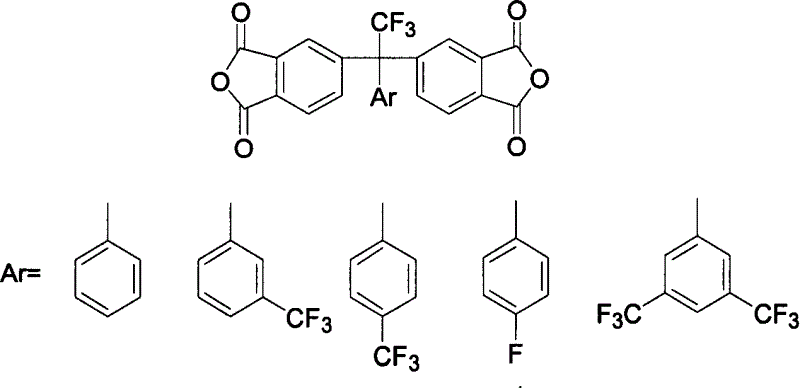
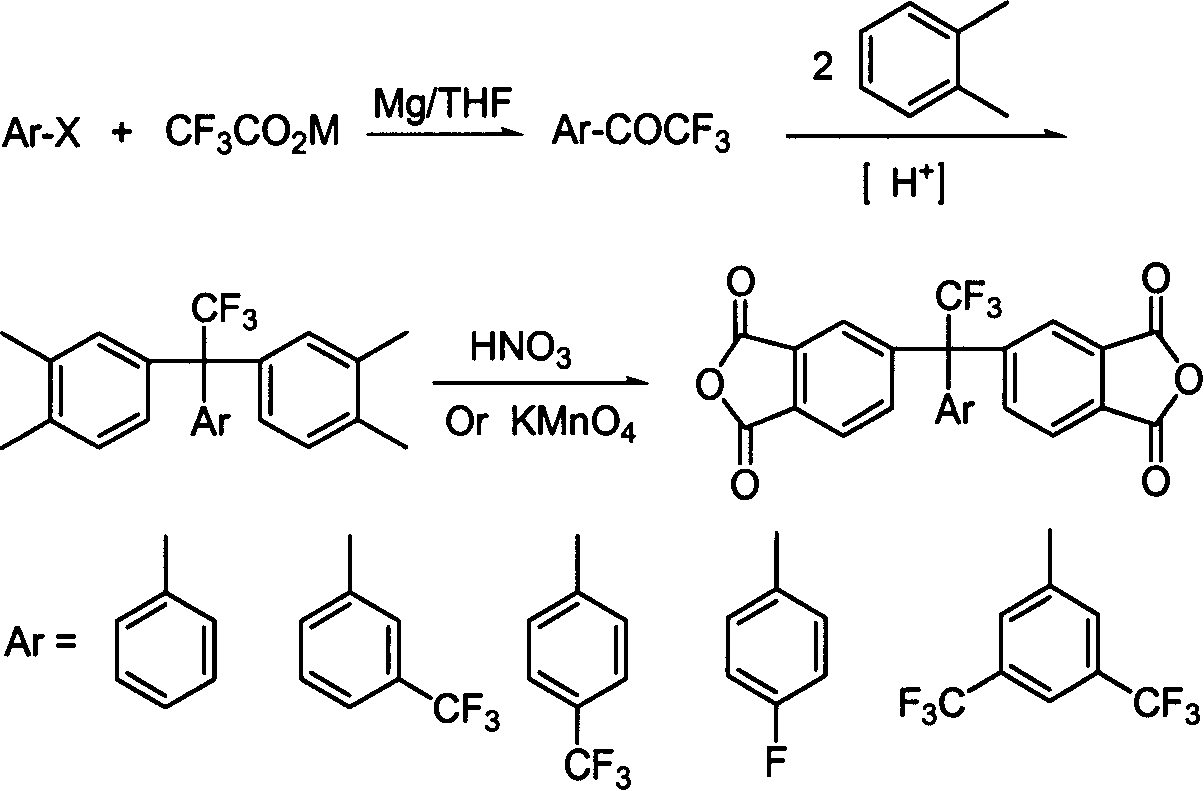
The present invention relates to the preparation process and use of fluoro aromatic organic tetraacid dianhydride and its derivative. The present invention prepares fluoro aromatic organic tetrahydric dianhydride and its derivative through the Grignard reaction between metal trifluoroacetate and substituted halogeno benzene to produce alpha, alpha, alpha-trifluoromethyl phenyil ketone; the condensation reaction of the alpha, alpha, alpha-trifluoromethyl phenyil ketone and one-xylene under the action of strong acid to obtain fluoro aromatic tetramethyl compound; the high temperature nitric acid oxidation or KMnO4 oxidation of the fluoro aromatic tetramethyl compound to obtain fluoro aromatic organic tetracid; and the final dewatering. Fluoro aromatic organic tetrahydric dianhydride and its derivative may be prepared into fluoro aromatic polyimide with excellent comprehensive performance through condensation with aromatic organic diamine and subsequent imination.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Polyamide acid composition and preparation method and application thereof
The invention relates to a polyamide acid composition with the viscosity capable of being controlled and a preparation method and application thereof. The problems that in the prior art, controlling of the viscosity is unstable in the preparation process of a polyamic acid solution, and further lengthening of a polymer molecular chain is difficult in the subsequent imidization process are solved. The preparation method for the polyamide acid composition comprises the following steps that (a) tetracarboxylic acid and diamine are stirred in an organic solvent at the low temperature of minus 10-40 DEG C and evenly mixed, and thus a composition solution of tetracid and the diamine is obtained; and (b) tetracarboxylic acid dianhydride is added into the composition solution of the tetracid and the diamine and stirred for reaction at the low temperature of minus 10-40 DEG C. The problems are solved through the technical scheme for obtaining the polyamide acid composition, controlling of the viscosity of the polyamic acid solution and further lengthening of the polymer molecular chain in the subsequent imidization process are achieved, and the polyamide acid composition with the viscosity capable of being controlled and the preparation method can be applied to industrial production of polyimide materials.
Owner:CHINA PETROLEUM & CHEM CORP +1
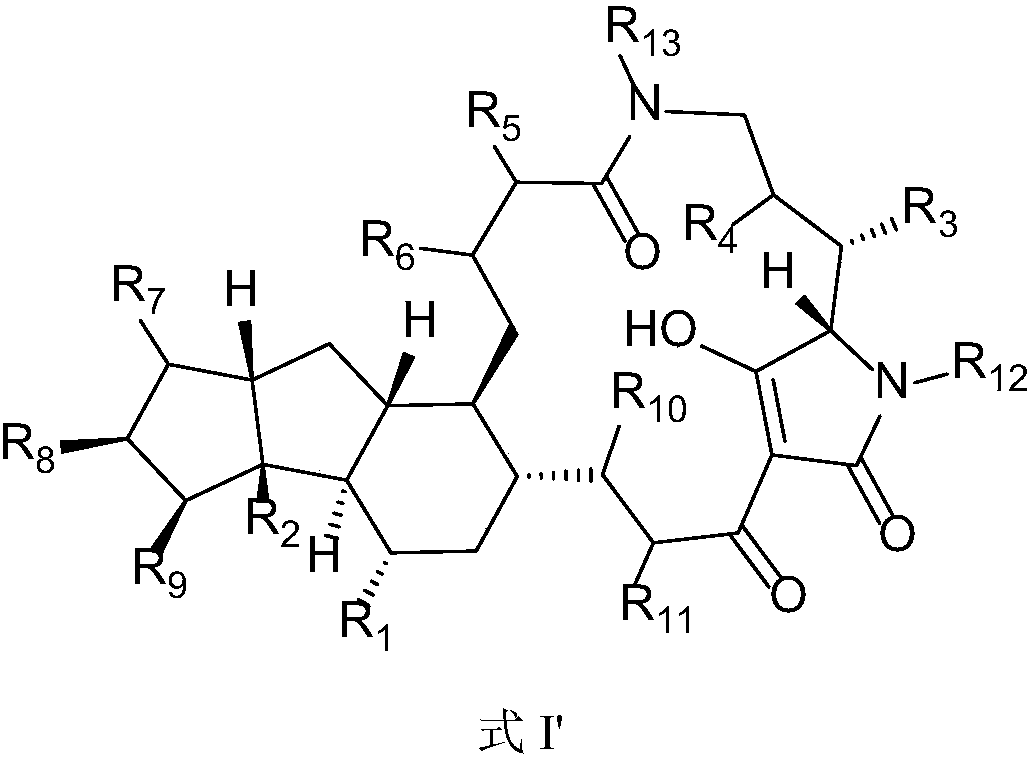
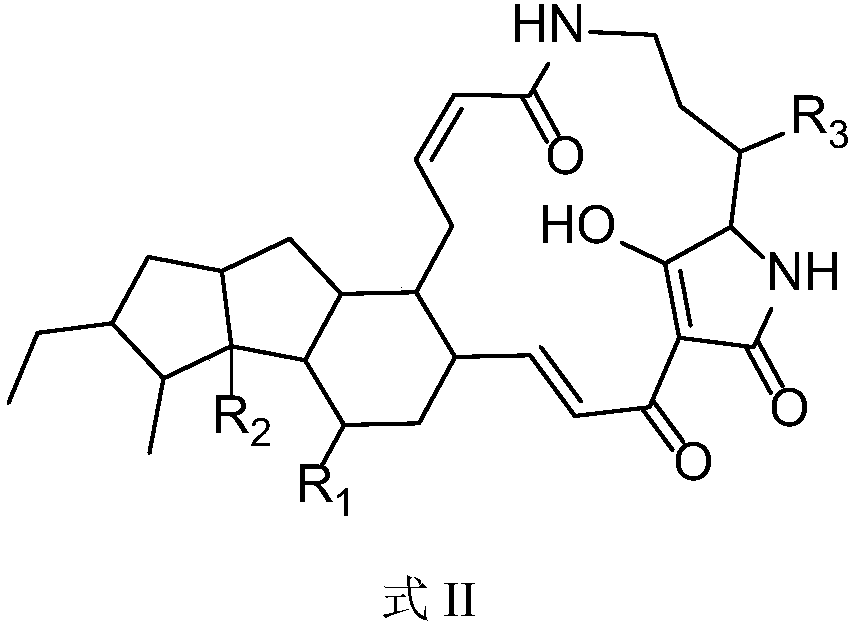
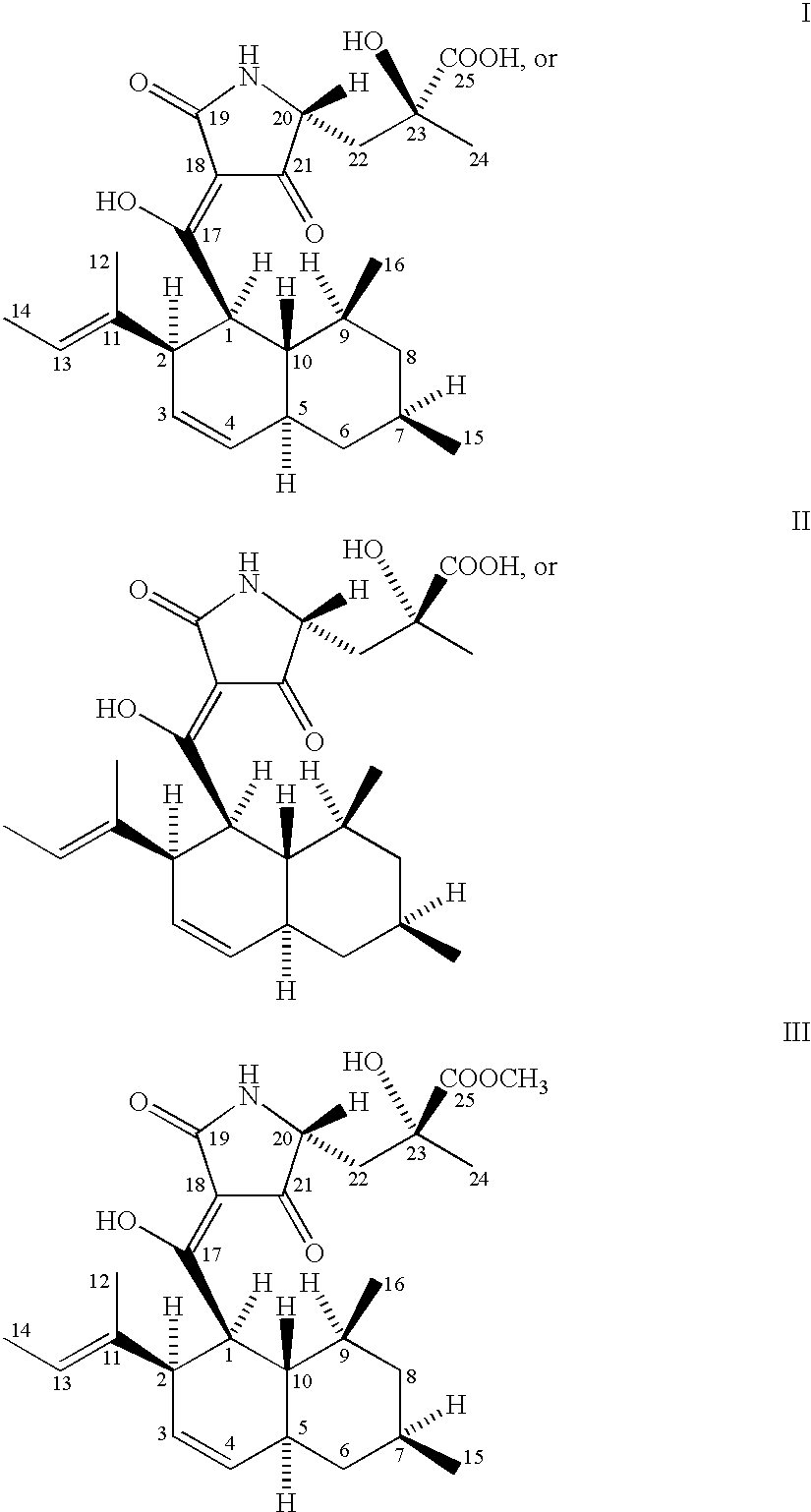
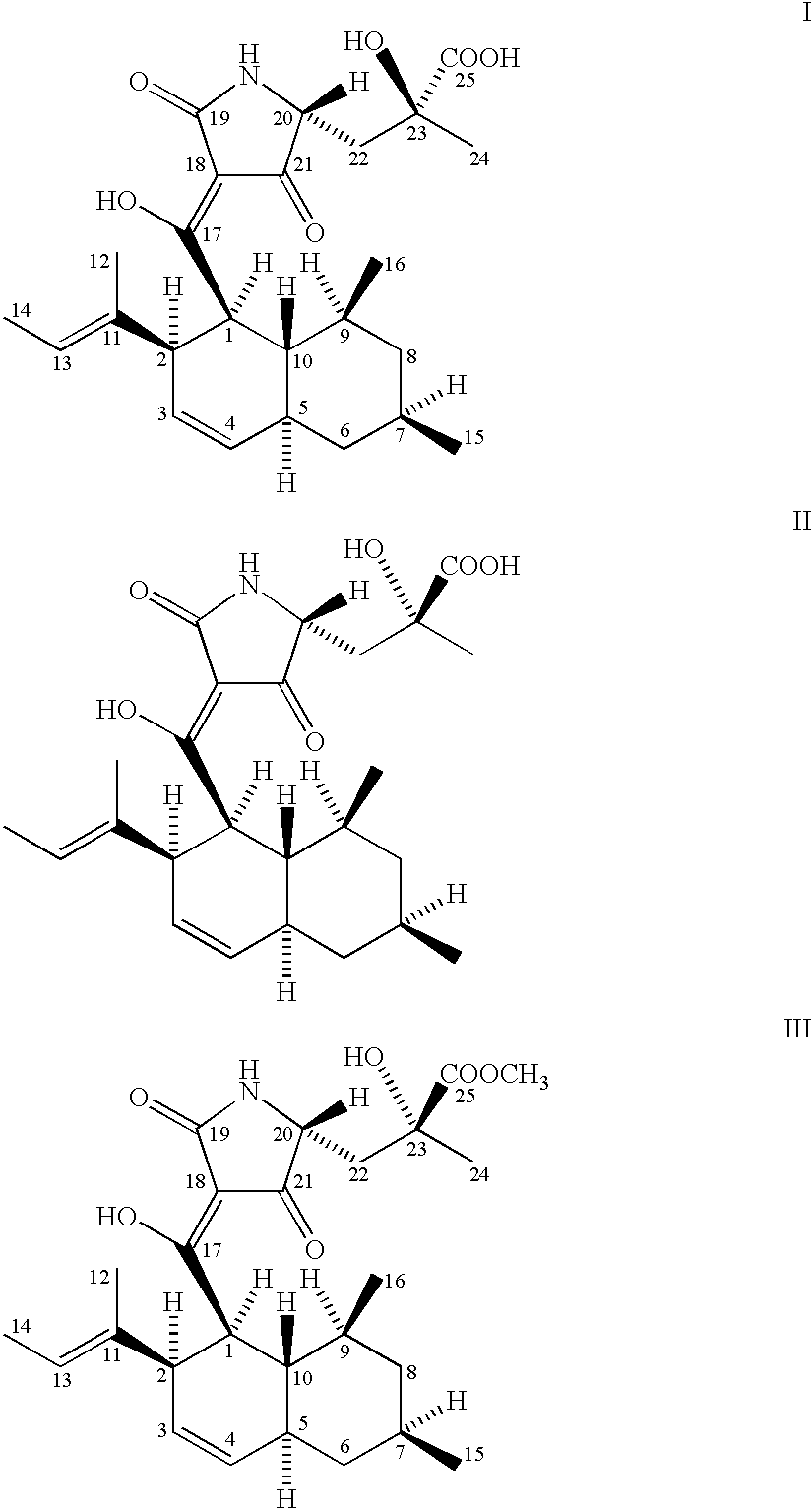
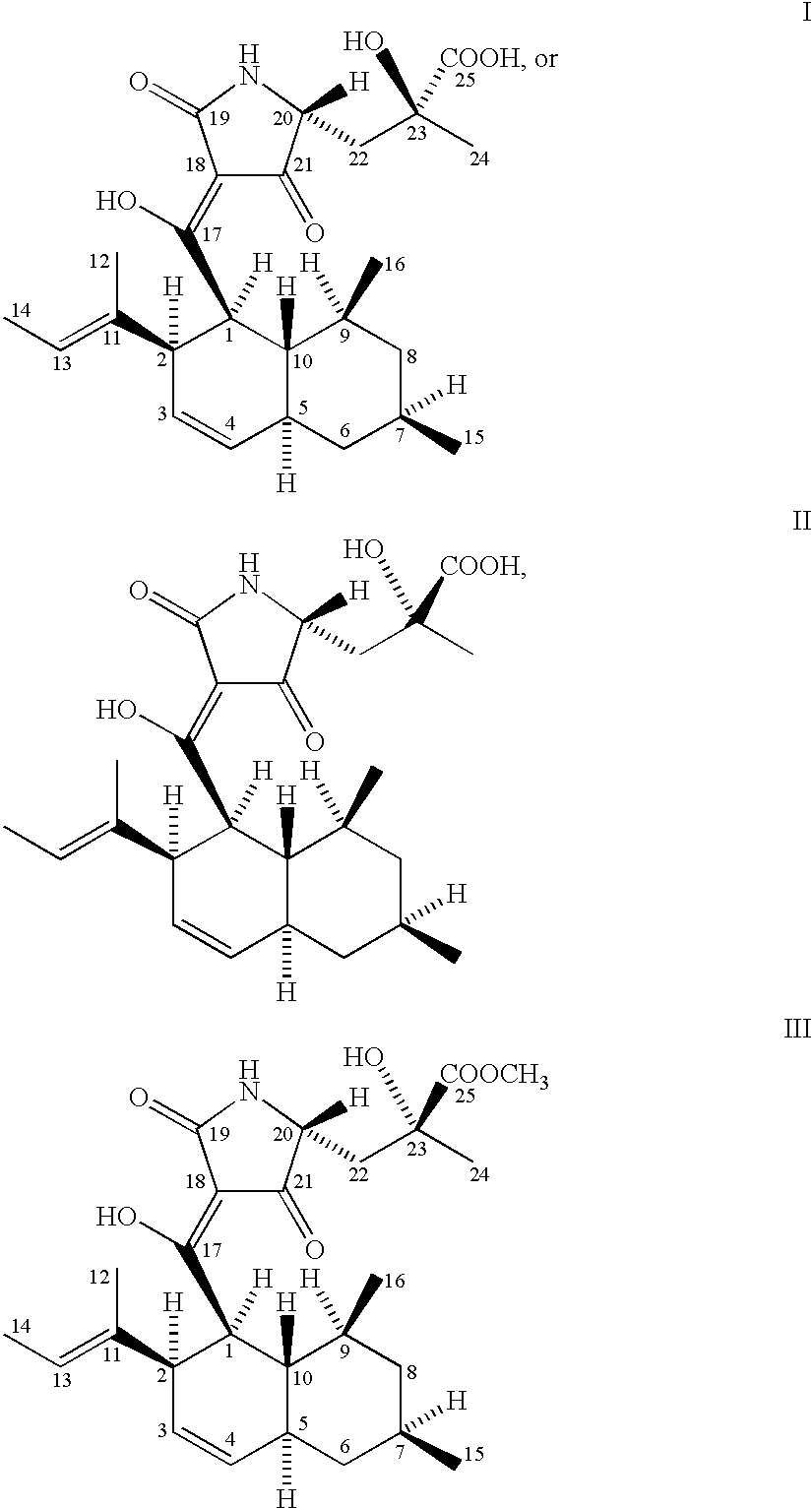
5,5,6-polycyclic tetramic-acid containing macrocyclic lactam compound and preparation method and application thereof
ActiveCN108623607AEnhanced inhibitory effectOrganic active ingredientsAntimycoticsTetramic acidAspergillus fumigatus
The invention discloses a 5,5,6-polycyclic tetramic-acid containing macrocyclic lactam compound and a preparation method and the application of the 5,5,6-polycyclic tetramic-acid containing macrocyclic lactam compound. The 5,5,6-polycyclic tetramic-acid containing macrocyclic lactam compound can be used as a tumor cell proliferation inhibitor or a tumor cell killer or an aspergillus fumigatus growth inhibitor.
Owner:OCEAN UNIV OF CHINA
Chemokine receptor antagonists
Novel tetramic acid-type compounds isolated from a CCR-5 active complex produced by fermentation under controlled conditions of a biologically pure culture of the microorganism, Chaetomium globosum Kunze SCH 1705, ATCC 74489., pharmaceutical compositions containing the compounds and the use of the CCR-5 antagonist compounds and compositions to treat HIV-1 infections in humans are disclosed.
Owner:MERCK SHARP & DOHME CORP
Insecticidal acaricidal composition and application thereof
ActiveCN103518729ASynergistic effect is obviousHigh activityBiocideAnimal repellantsTetramic acidActive component
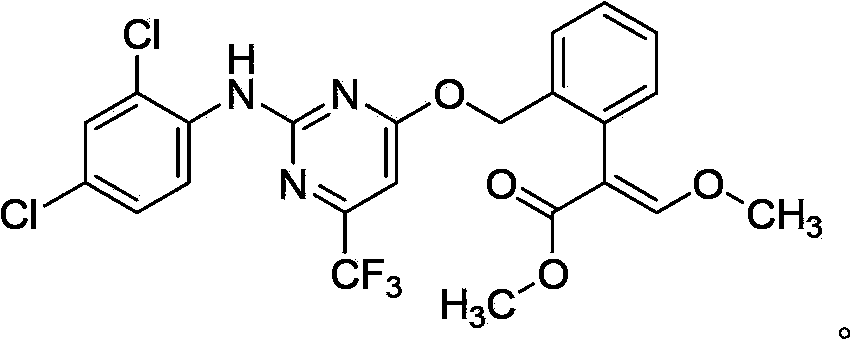
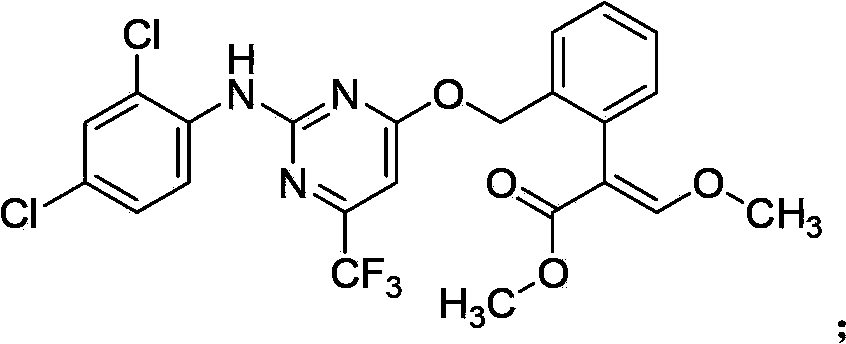
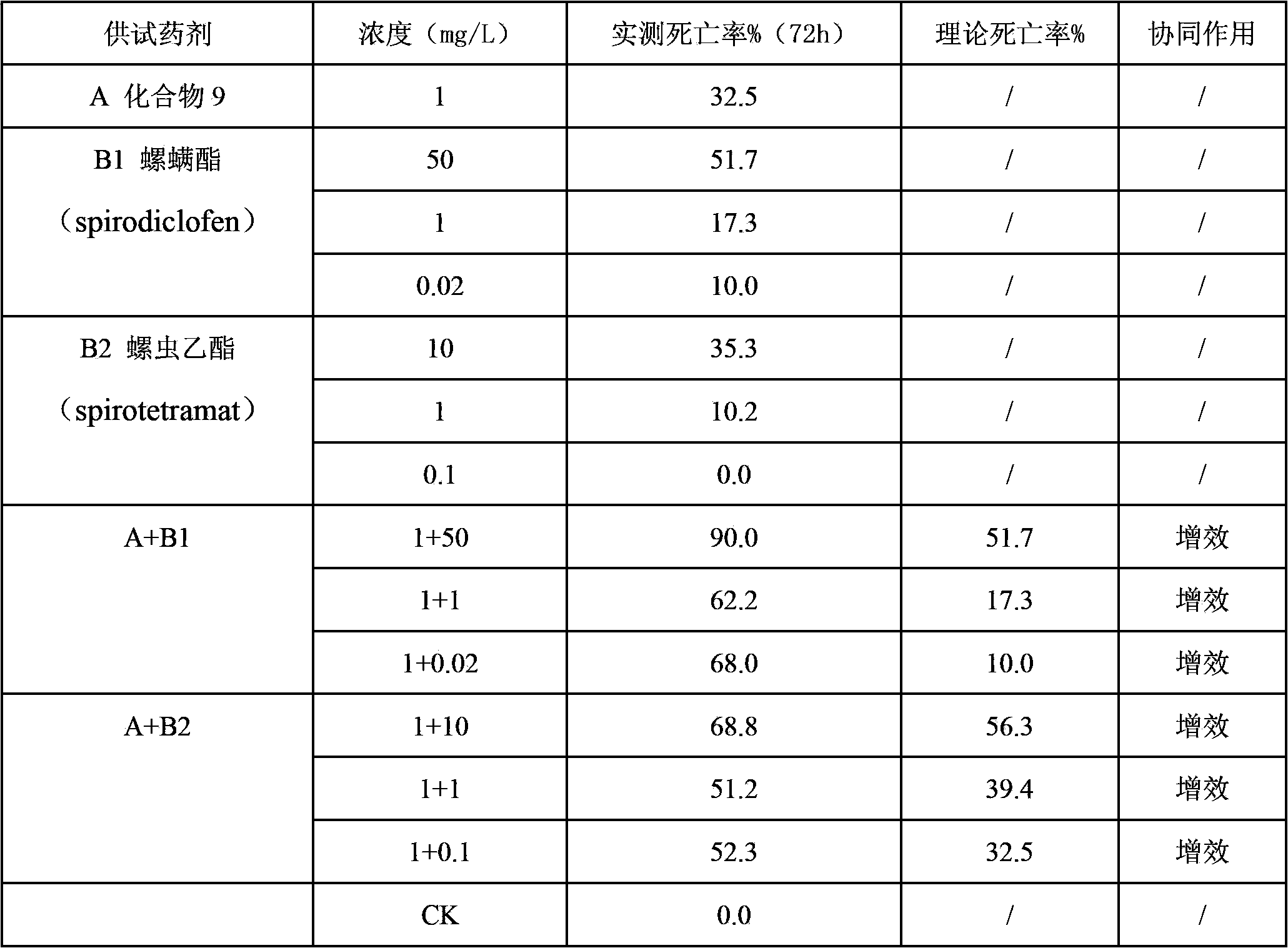
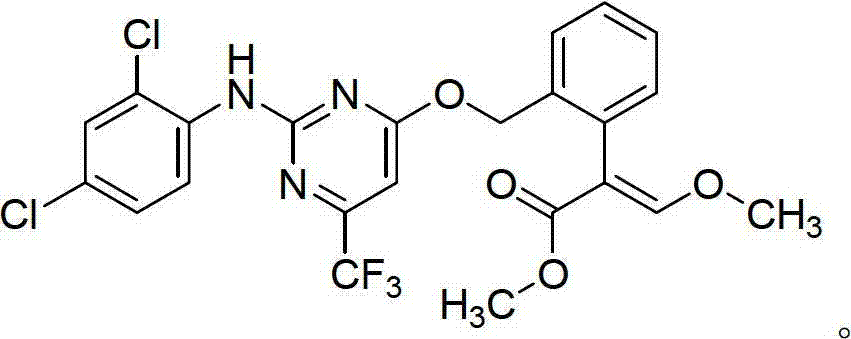
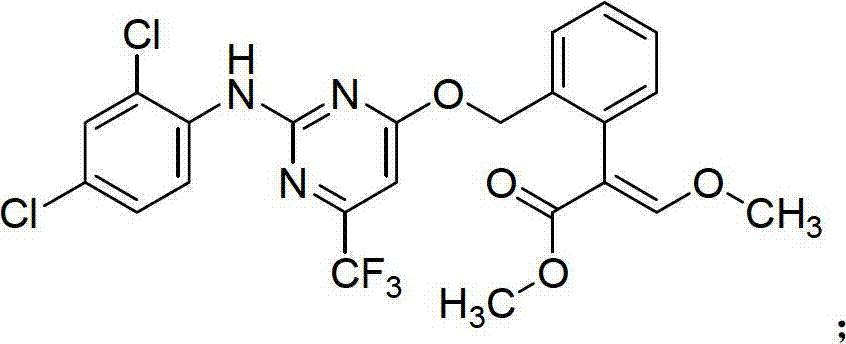
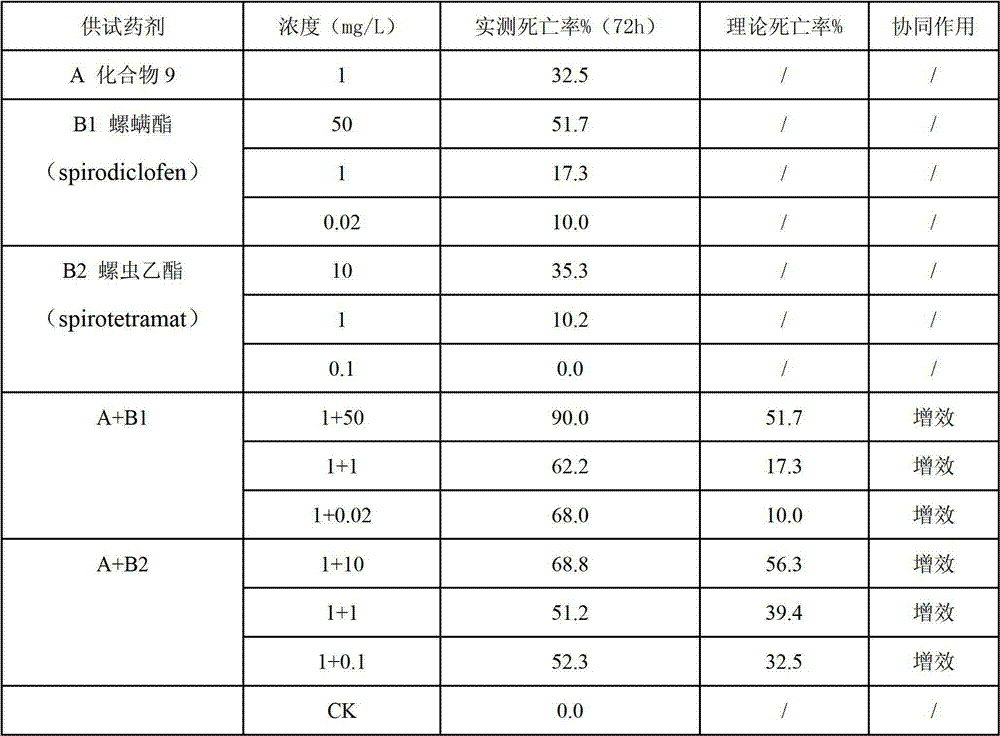
The invention belongs to the field of pesticides, and discloses an insecticidal acaricidal composition containing an active component A and an active component B, wherein the active component A is selected from the following compounds disclosed in the specification; the active component B is selected from tetronic acid (tetronic acid / tetramic acid derivative) insecticides and acaricides; the tetronic acid insecticides and acaricides comprise spirodiclofen, spiromesifen, spirotetramat or the like; and the weight ratio of the active component A to the active component B is 1:99-99:1. The composition has obvious synergistic action, widens the insecticidal spectrum, and reduces the pesticide consumption; and the pests can not easily generate drug resistance, and thus, the composition can be used for preventing and treating multiple pests and acarids on multiple crops.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
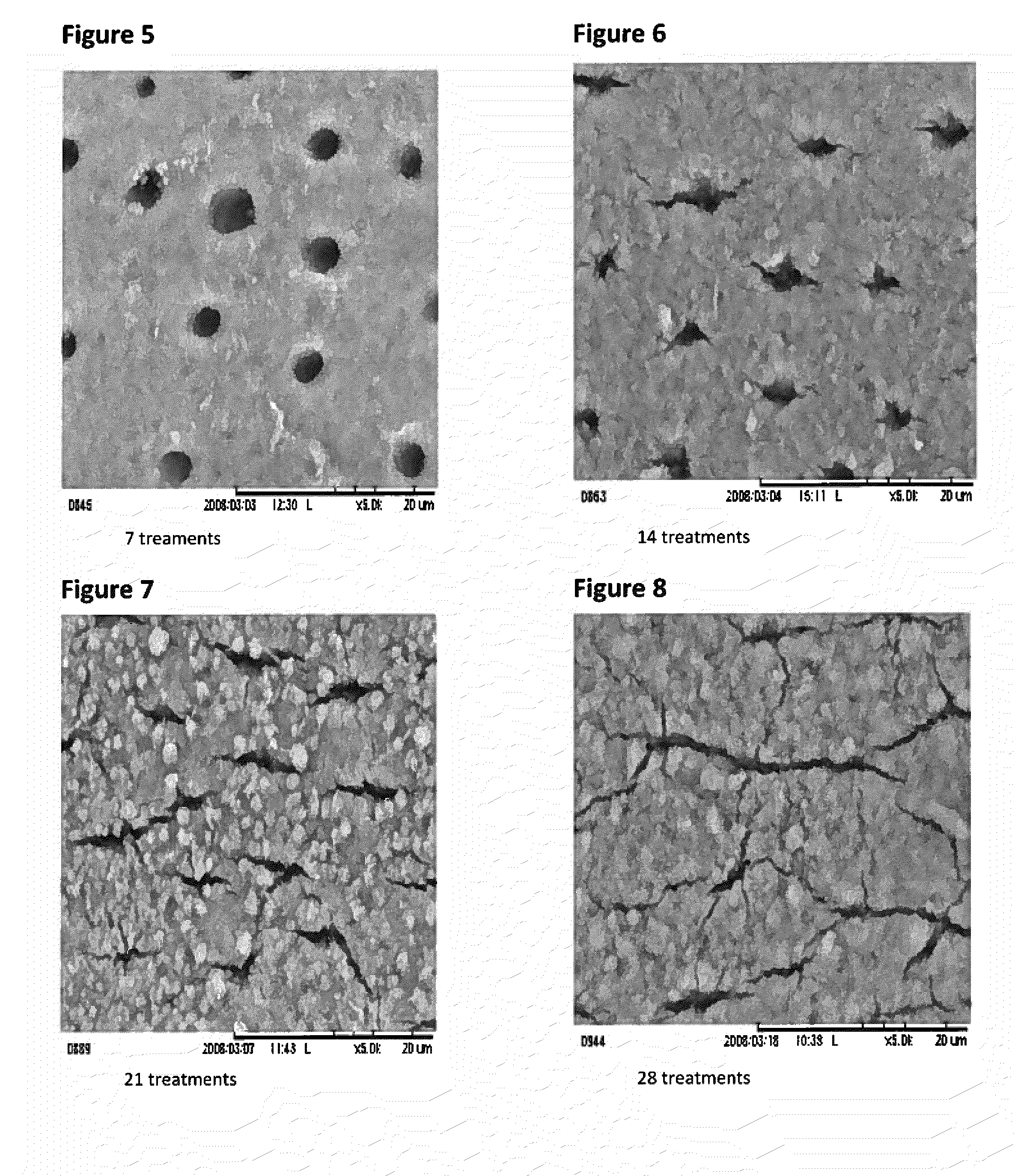
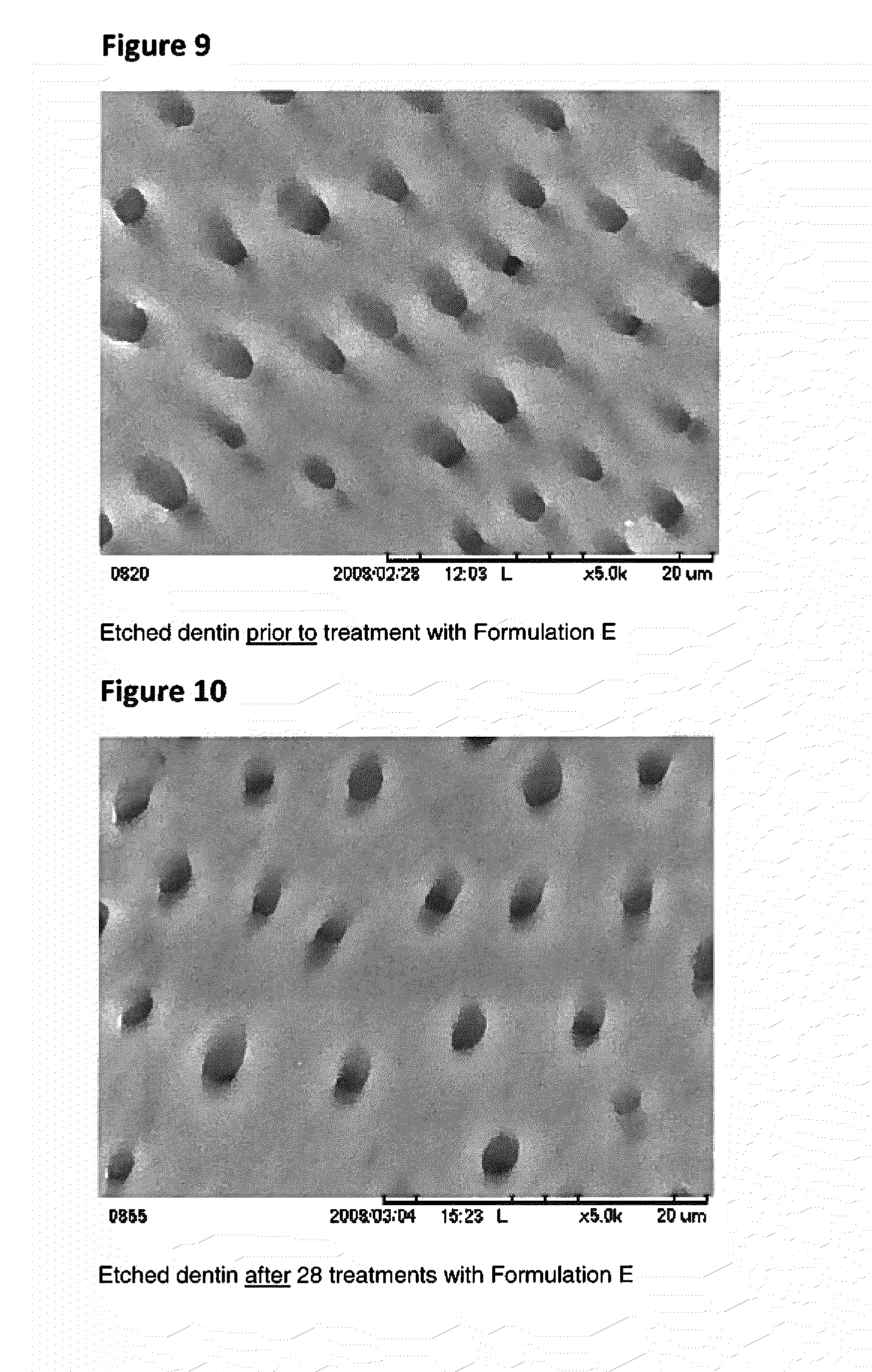
Tooth sensitivity treatment compositions
A tooth sensitivity treatment composition is disclosed. The composition includes a compound of formula IM1-A-M2-B-M1 (I)wherein: M1 is a monovalent or divalent metal; M2 is a polyvalent metal or metal oxide; and A and B are, independently, selected from the group consisting of C2-C6 diacids, triacids, and tetraacids. Methods for treating tooth sensitivity are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Analogs of tetramic acid
Tetramic acid analogues of Formula I and Formula II have antibacterial activity, primarily against gram-positive bacteria, and are iron chelators.
Owner:UNIV OF TENNESSEE RES FOUND
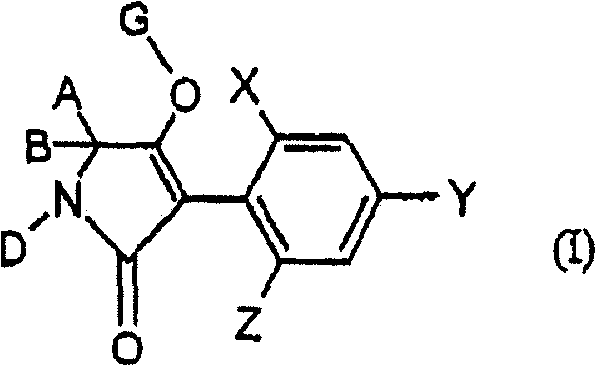
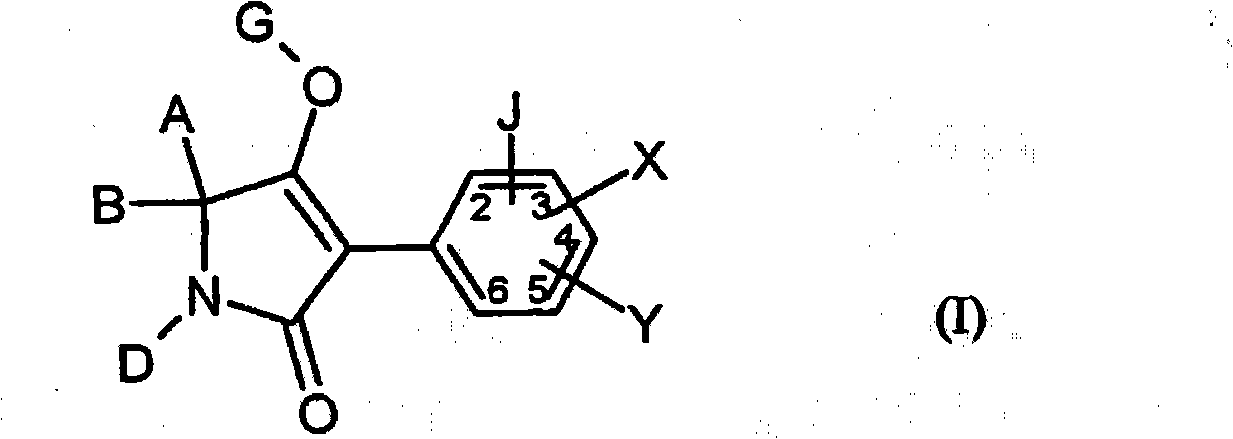
2.4-dihalogen-6-(C2-C3-alkyl)-phenyl substituted tetramic acid derivatives
Owner:BAYER CROPSCIENCE AG
Preparation method of aromatic diether dianhydride
ActiveCN112321550AShort reaction cycleSimple processOrganic chemistryBulk chemical productionTetramic acidPolymer science
The invention discloses a preparation method of aromatic diether dianhydride, which comprises the following steps of: by using isobutene or tert-butyl alcohol as a protecting group of carboxyl of chlorinated or nitrophthalic acid, carrying out etherification reaction with disodium bisphenol salt to obtain an intermediate, removing the tert-butyl ester protecting group in the intermediate by usinga small amount of acid without strong alkali treatment to obtain diether tetracarboxylic acid, and finally, enabling the solid tetracarboxylic acid to directly form anhydride at a proper temperature,wherein acetic anhydride dehydration is not needed, and the reaction time of the whole process is only about 21 hours. The preparation method of the aromatic diether dianhydride is short in reaction period, simple and safe in process, high in production efficiency and low in production cost.
Owner:吉林省亚安新材料有限公司
Terbium metal complex constructed by furantetracarboxylic acid and preparation method thereof
The invention relates to a terbium metal complex constructed by furantetracarboxylic acid and a preparation method thereof. The chemical composition of the complex contains a furantetracarboxylic acid (H4fa) ligand. The synthesis scheme comprises the following steps of: respectively dissolving potassium salt of furantetracarboxylic acid and TbCl3.6H2O in distilled water according to a stoichiometric ratio, mixing, and adding 1d HCl; and putting the obtained solution into a polytetrafluoroethylene lining, then putting the polytetrafluoroethylene lining into a reaction kettle, putting the reaction kettle into a drying oven, and carrying out heating reaction for 3 days to obtain a target product. The mass ratio of the potassium salt of the furantetracarboxylic acid to the TbCl3.6H2O is 1: 0.93.
Owner:TIANJIN POLYTECHNIC UNIV
Tooth sensitivity treatment compositions
A tooth sensitivity treatment composition is disclosed. The composition includes a compound of formula IM1-A-M2-B-M1 (I)wherein: M1 is a monovalent or divalent metal; M2 is a polyvalent metal or metal oxide; and A and B are, independently, selected from the group consisting of C2-C6 diacids, triacids, and tetraacids. Methods for treating tooth sensitivity are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Analogs of tetramic acid
Tetramic acid analogues of Formula I and Formula II have antibacterial activity, primarily against gram-positive bacteria, and are iron chelators.
Owner:UNIV OF TENNESSEE RES FOUND
2,4-dihalogen-6-(c2-c3-alkyl)-phenyl substituted tetramic acid derivatives
The invention relates to novel 2.4-dihalogen-6-(C2-C3-alkyl)-phenyl substituted tetramic acid derivatives of formula (I), wherein A, B, D, G, X, Y and Z have the above mentioned meaning. The invention also relates to several methods and intermediate products for the production and use thereof as pesticides and / or herbicides, in addition to selective herbicidal agents which contain 2.4-dihalogen-6-(C2-C3-alkyl)-phenyl substituted tetramic acid derivatives and at least one compound which improves the compatibility of cultivated plants.
Owner:BAYER CROPSCIENCE AG
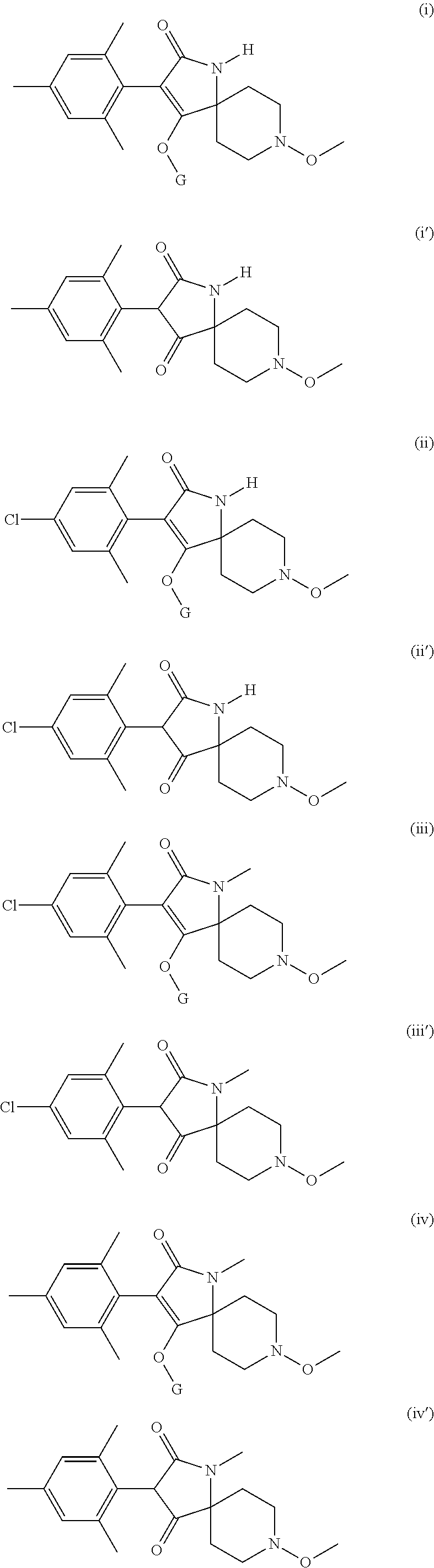
Use of tetramic acid derivatives as nematicides
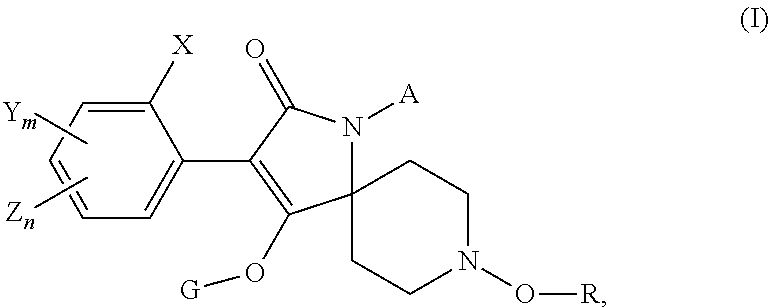
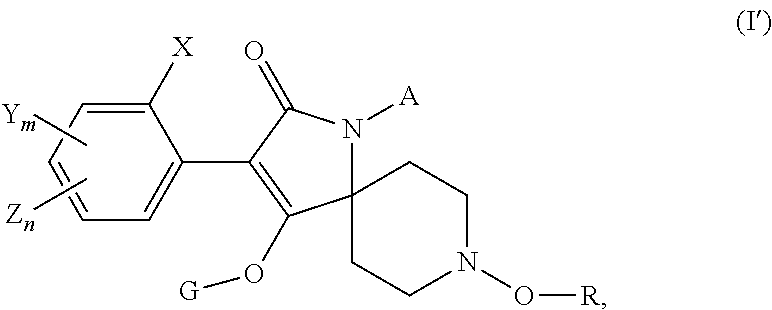
Use of a tetramic acid compound according to formula (I) or (I′) with a second nematicide as a treatment for crop plants to combat and control nematodes in the soil of said crop plants.
Owner:SYNGENTA PARTICIPATIONS AG
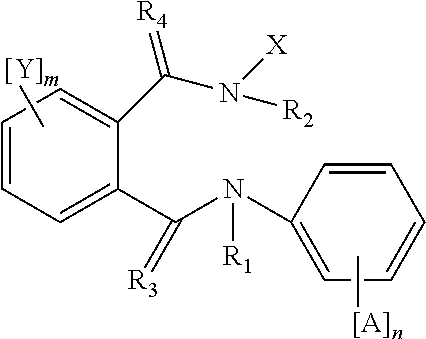
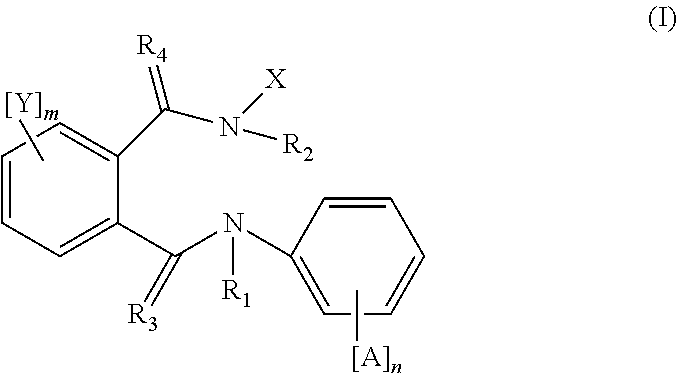
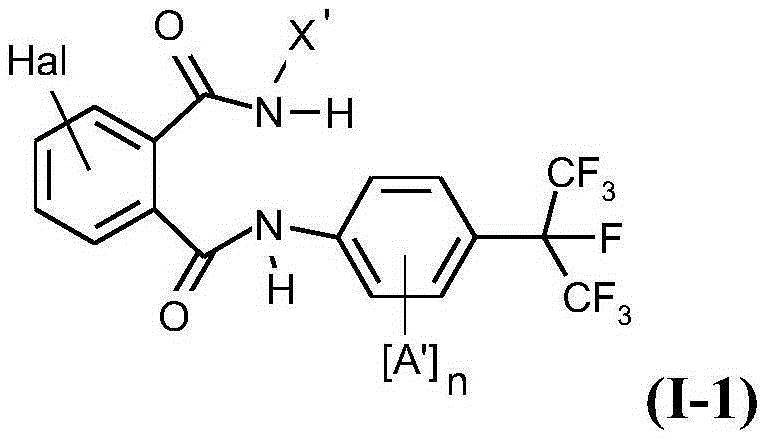
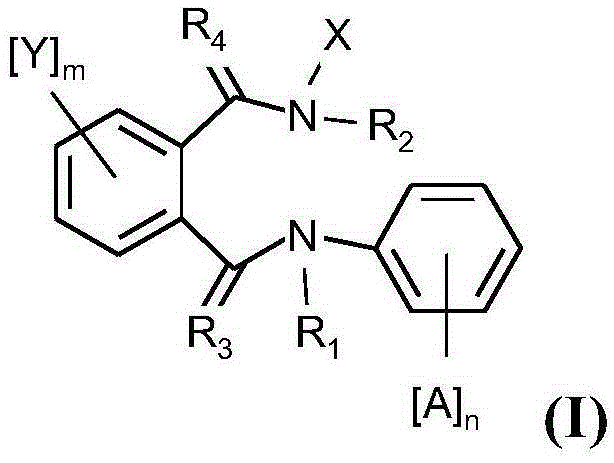
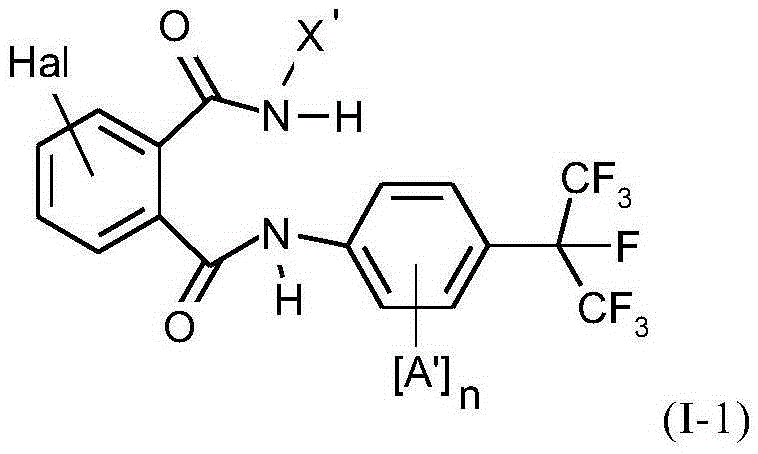
Active compound combinations having insecticidal properties
The present invention relates to novel active compound combinations comprising at least one known compound of the formula (I)in which X, R1 to R4, A, n, Y, and m are as defined in the description, and at least one further known active compound from the class of the of tetronic and tetramic acid derivatives, diacylhydrazines, benzoylureas and further classes, which combinations are highly suitable for controlling animal pests such as insects and unwanted acarids.
Owner:BAYER CROPSCIENCE AG
Active compound combinations having insecticidal properties
Owner:BAYER CROPSCIENCE AG
Fluoro-aromatic organic tetracarboxylic dianhydride and its preparation method and use
InactiveCN100400529CImprove performanceLiquid crystal compositionsOrganic chemistryPolymer scienceAcetic anhydride
The present invention relates to the preparation process and use of fluoro aromatic organic tetraacid dianhydride and its derivative. The present invention prepares fluoro aromatic organic tetrahydric dianhydride and its derivative through the Grignard reaction between metal trifluoroacetate and substituted halogeno benzene to produce alpha, alpha, alpha-trifluoromethyl phenyil ketone; the condensation reaction of the alpha, alpha, alpha-trifluoromethyl phenyil ketone and one-xylene under the action of strong acid to obtain fluoro aromatic tetramethyl compound; the high temperature nitric acid oxidation or KMnO4 oxidation of the fluoro aromatic tetramethyl compound to obtain fluoro aromatic organic tetracid; and the final dewatering. Fluoro aromatic organic tetrahydric dianhydride and its derivative may be prepared into fluoro aromatic polyimide with excellent comprehensive performance through condensation with aromatic organic diamine and subsequent imination.
Owner:INST OF CHEM CHINESE ACAD OF SCI
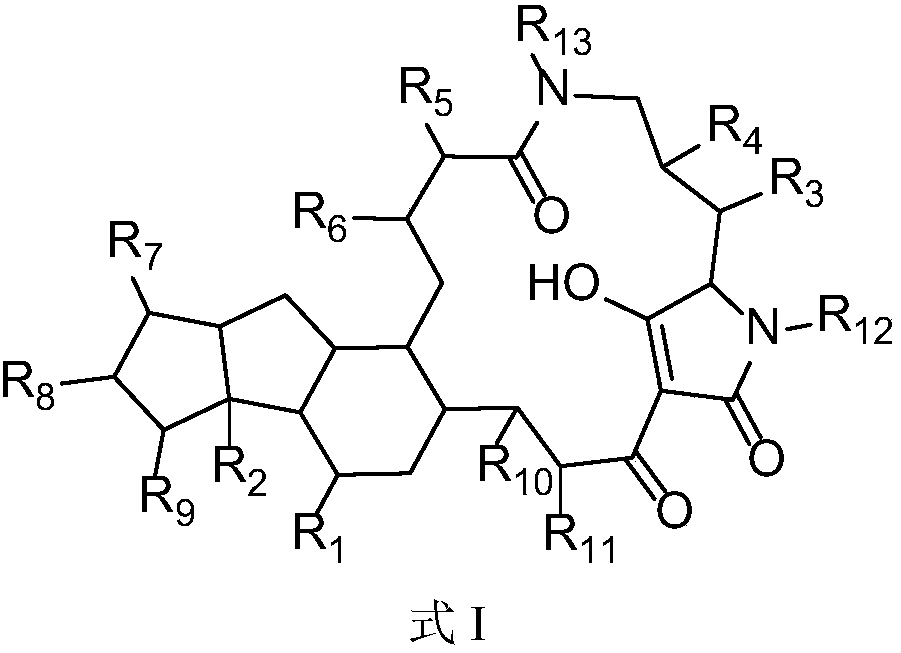
4-OXO-alkylated tetramic acid compound, preparation method and use thereof
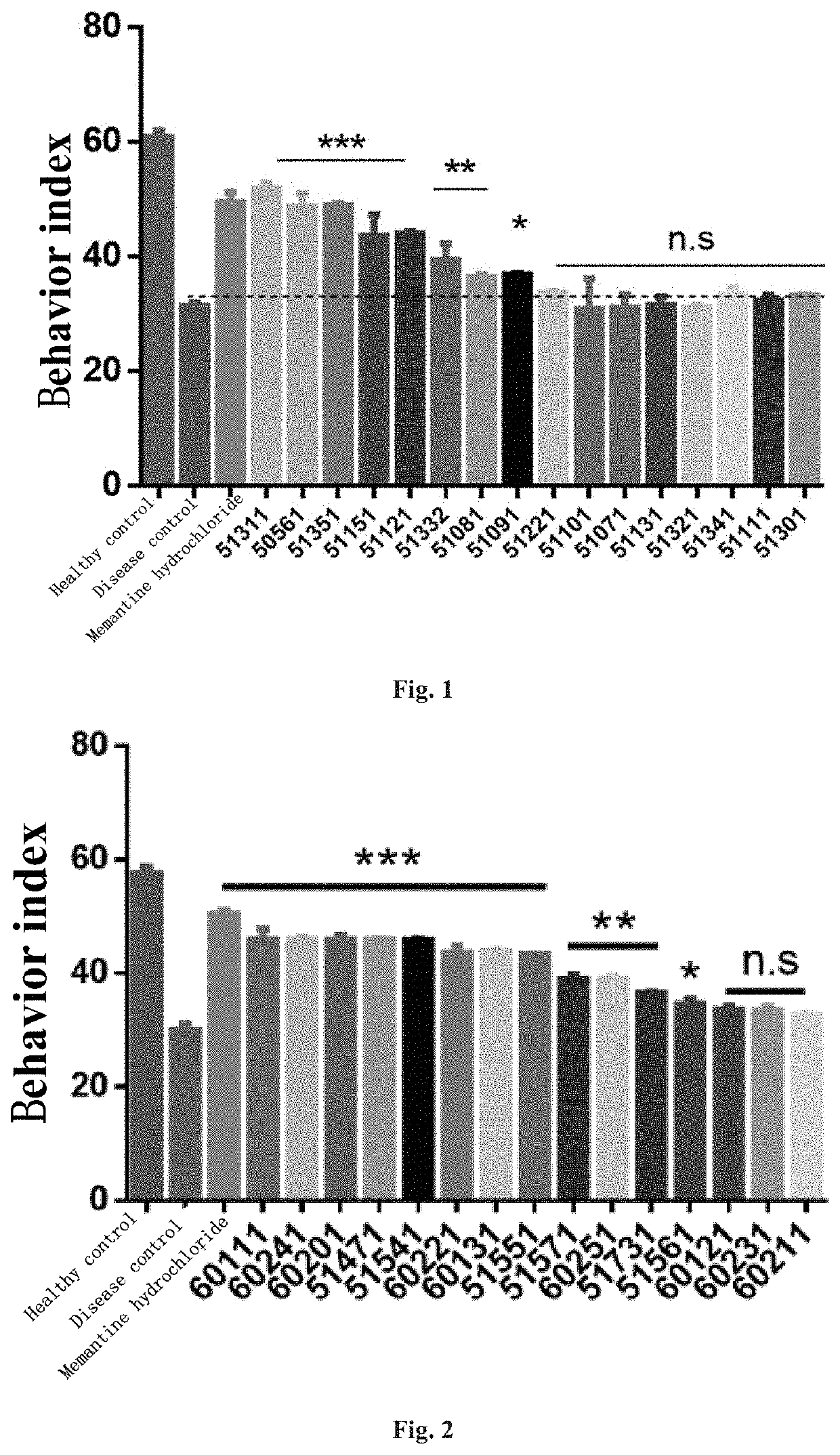
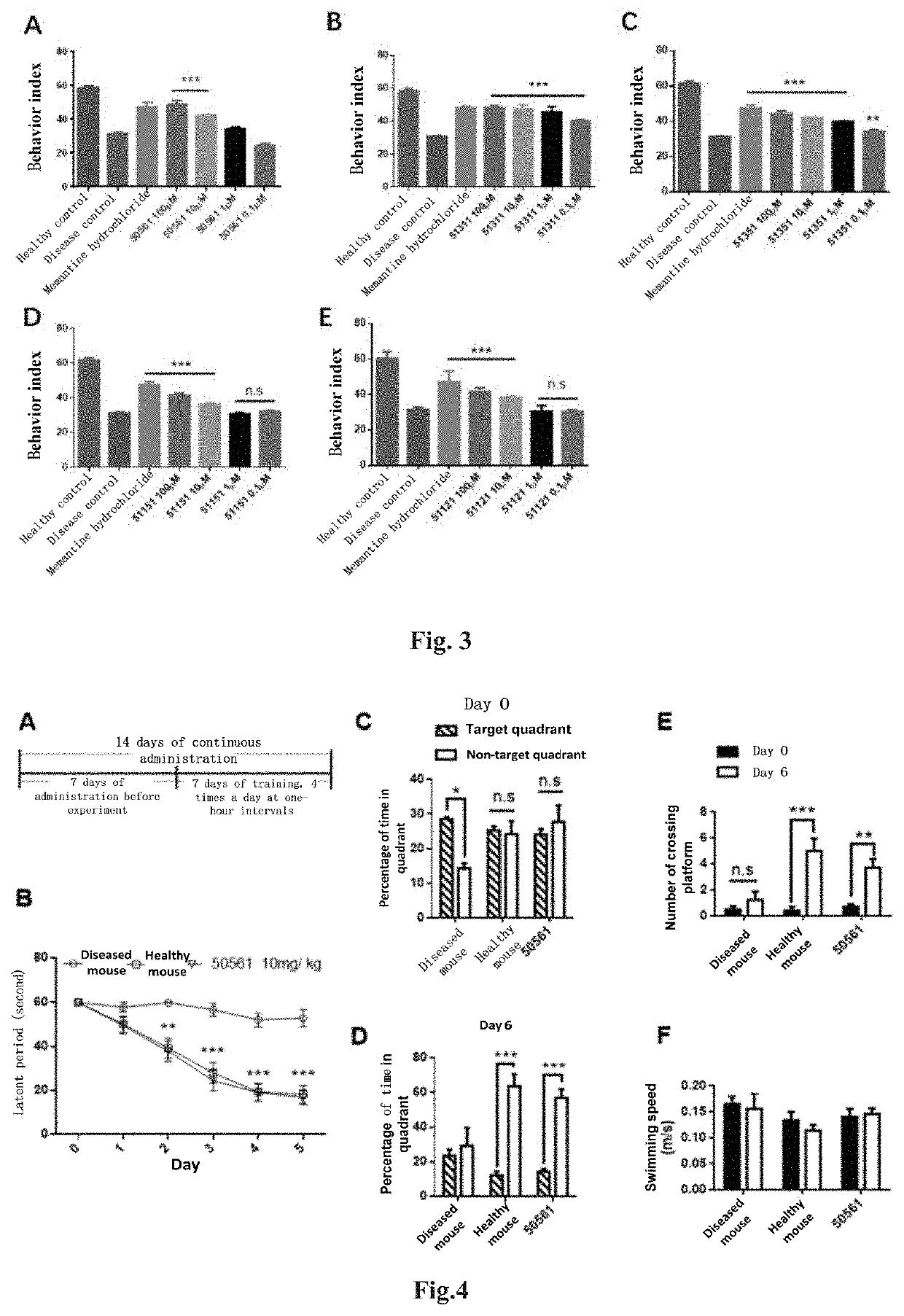
ActiveUS11130733B2Save memoryImprove securityOrganic active ingredientsNervous disorderHuntingtons choreaPsychiatry
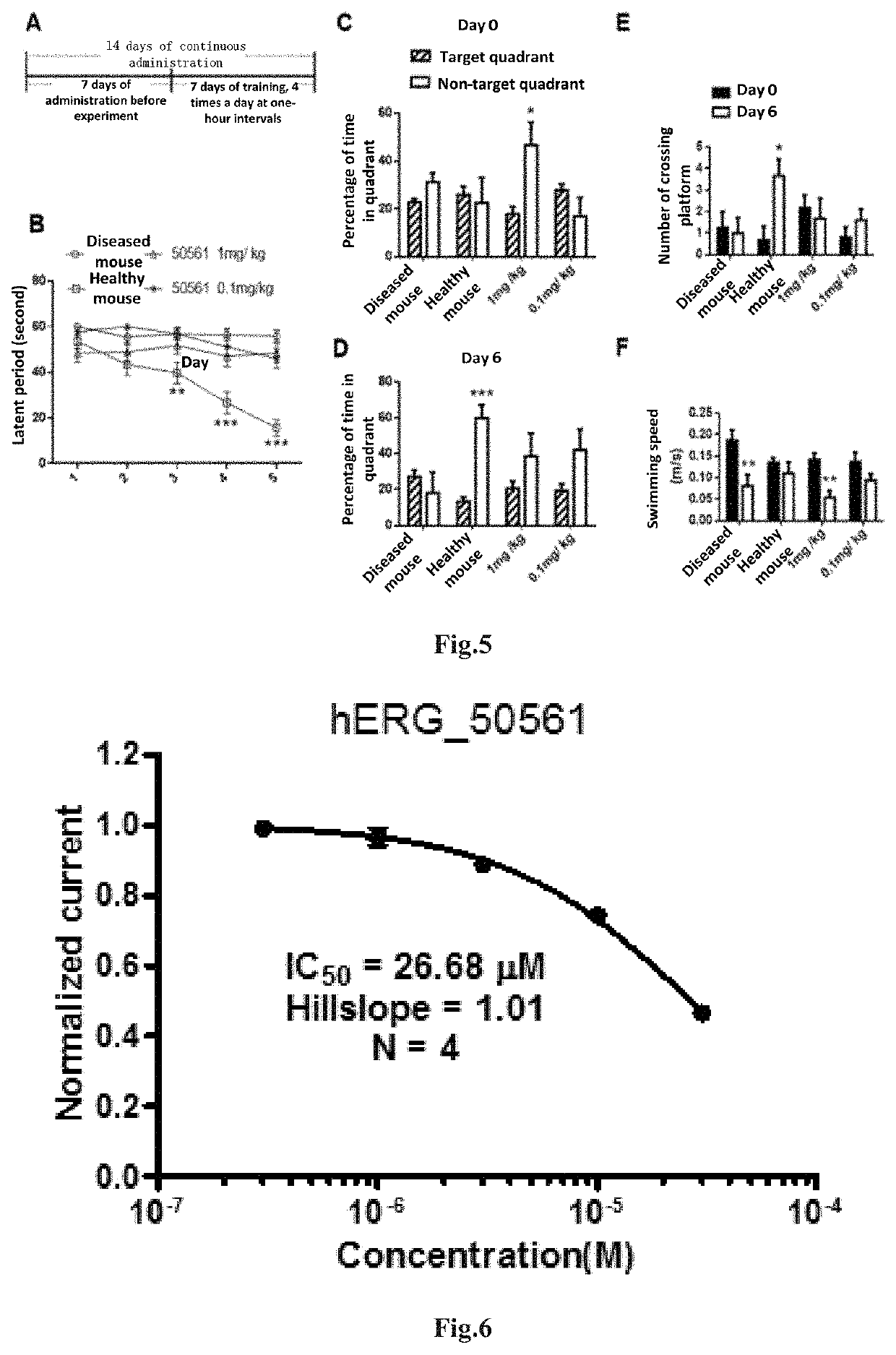
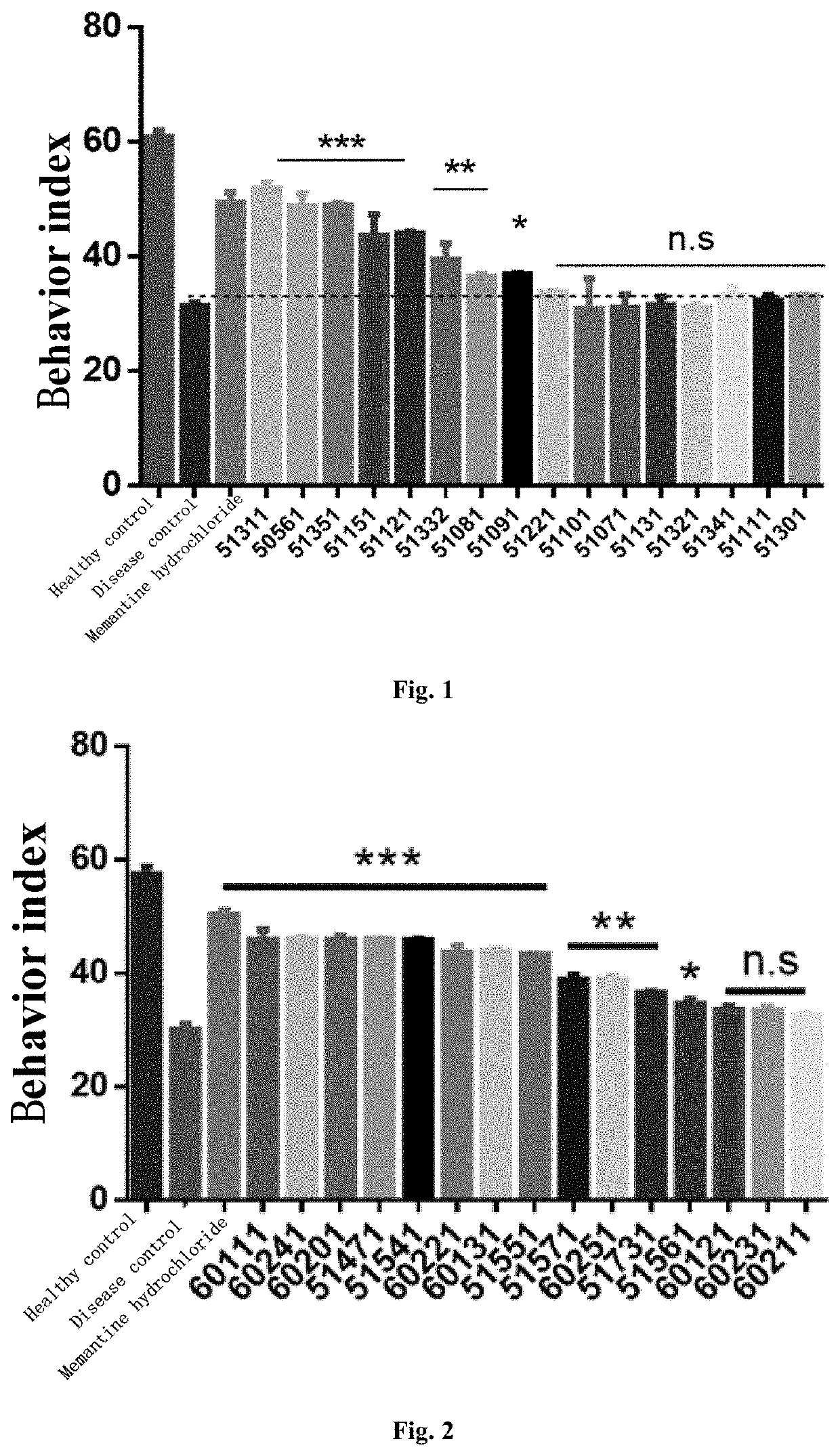
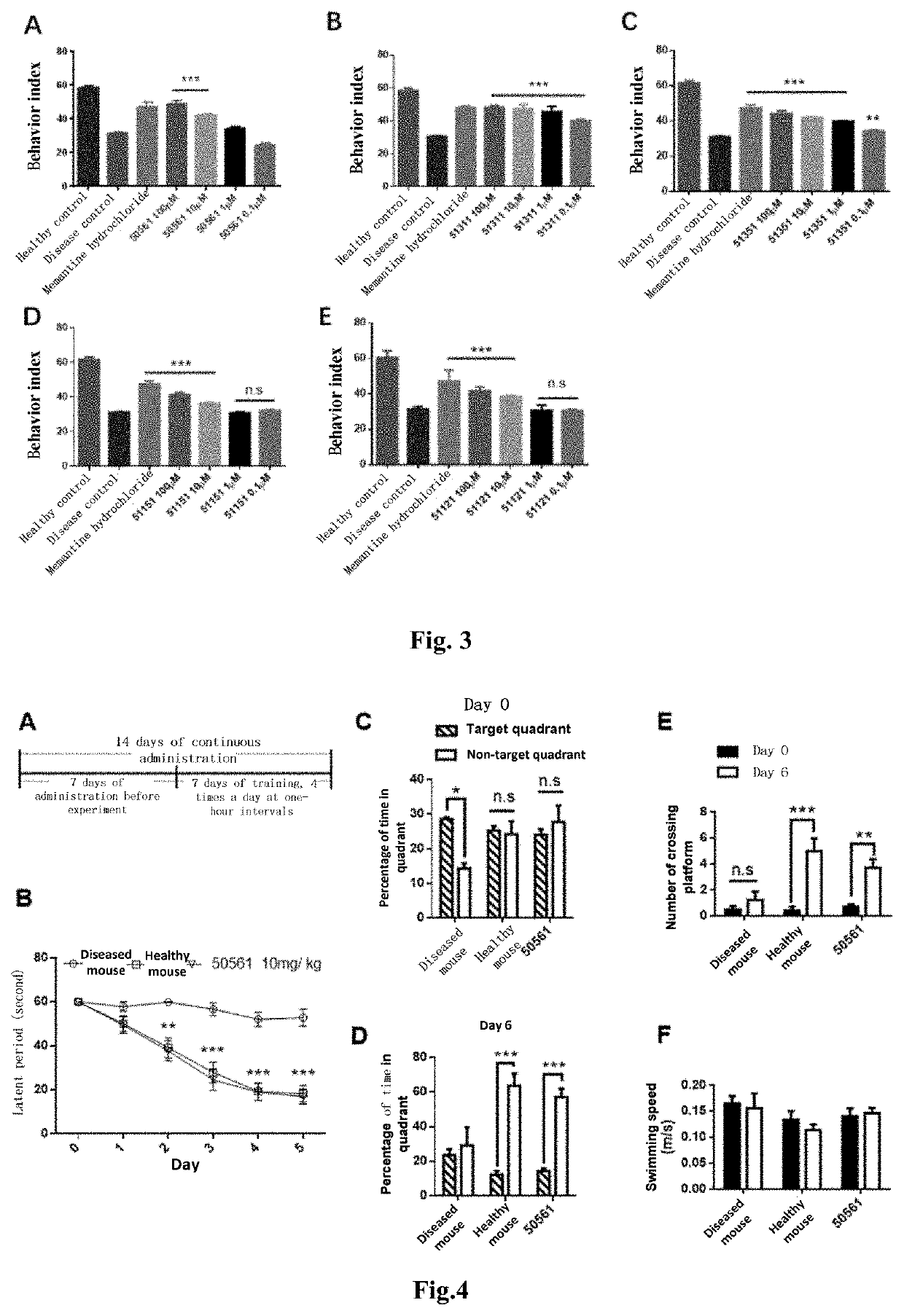
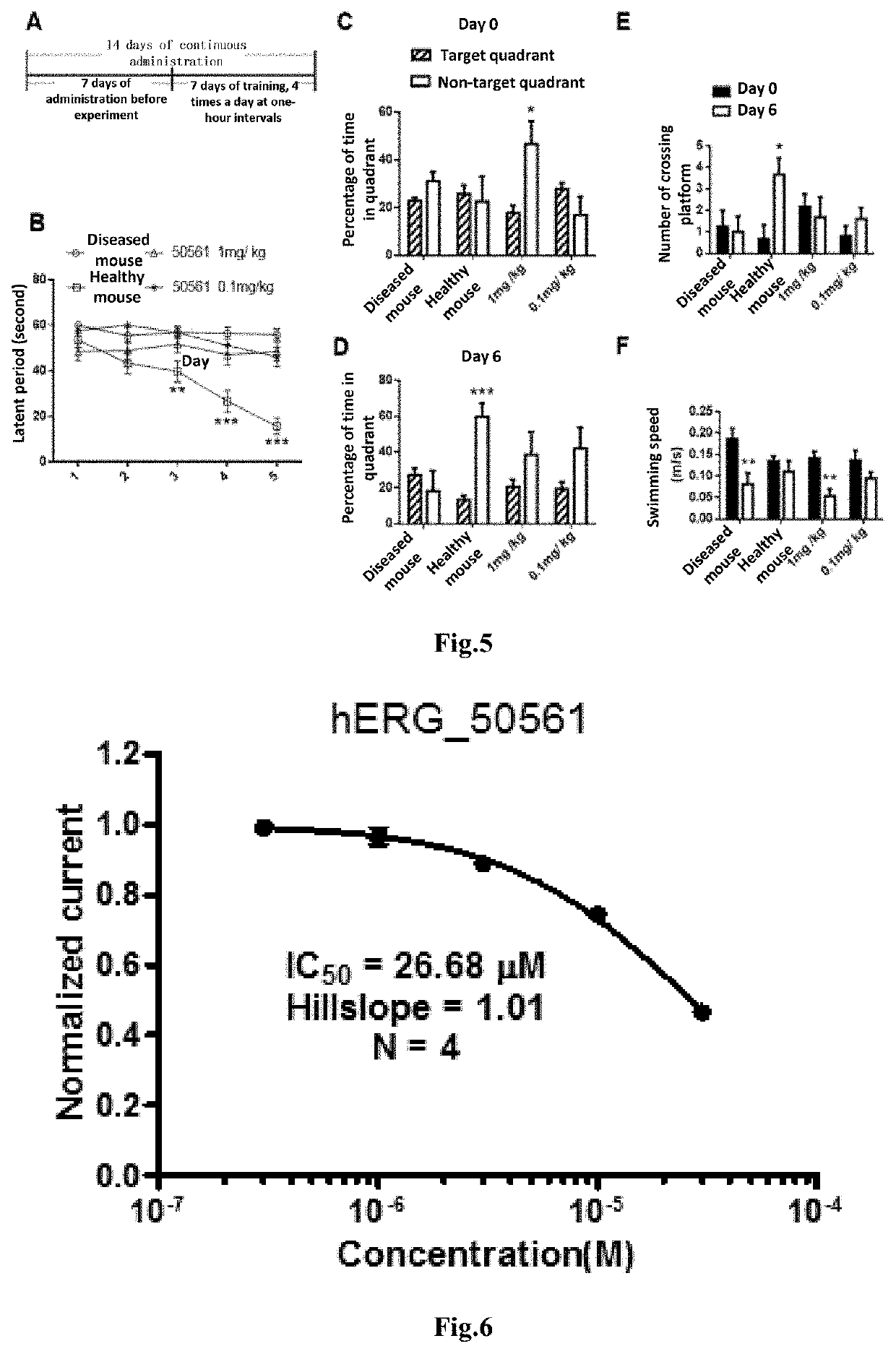
The present invention relates to a new compound and a preparation method and use thereof. The general structural formula of the compound is shown in Formula I. Animal experiments show that the compound has the effect of saving the memory of animal models. It is of high safety, has no mutagenicity, can remain in blood for several hours after oral or intravenous injection, and can enter the brain. The compound can be used to prepare a medicament for treating Alzheimer's disease, Parkinson's disease, Huntington's disease, vascular dementia, schizophrenia, and autism among other diseases.
Owner:BEIJING JOEKAI BIOTECH
Treatments for gastrointestinal conditions
InactiveUS10011589B2Reduce absorptionReduce compoundingAntibacterial agentsOrganic chemistryDiseaseNitroimidazole
Compounds for the treatment of bacterial and parasitic infections which are hybrid compounds of compounds having antibacterial or antiparasitic activity and compounds that decrease the absorption of the hybrid compound from the gastrointestinal tract. The compounds are preferably for use against C. difficile infections and comprise a hybrid molecule of an anti-C. difficile compound such as a nitroimidazole and a tetramic acid derivative.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
Insecticidal acaricidal composition and application thereof
ActiveCN103518729BReduce usageResidue reductionBiocideAnimal repellantsTetramic acidActive component
The invention belongs to the field of pesticides, and discloses an insecticidal acaricidal composition containing an active component A and an active component B, wherein the active component A is selected from the following compounds disclosed in the specification; the active component B is selected from tetronic acid (tetronic acid / tetramic acid derivative) insecticides and acaricides; the tetronic acid insecticides and acaricides comprise spirodiclofen, spiromesifen, spirotetramat or the like; and the weight ratio of the active component A to the active component B is 1:99-99:1. The composition has obvious synergistic action, widens the insecticidal spectrum, and reduces the pesticide consumption; and the pests can not easily generate drug resistance, and thus, the composition can be used for preventing and treating multiple pests and acarids on multiple crops.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
2,4-dihalogen-6-(c2-c3-alkyl)-phenyl substituted tetramic acid derivatives
The present invention relates to novel 2,4-dihalogen-6-(C) of formula (I) 2 -C 3 -Alkyl)phenyl-substituted tetramic acid derivatives, wherein A, B, D, G, agents and / or herbicides, the invention also relates to an aspect comprising 2,4-dihalogen-6-(C 2 -C 3 -Alkyl)phenyl-substituted tetramic acid derivatives and in another aspect a selective herbicidal composition comprising at least one compound that improves crop compatibility.
Owner:BAYER CROPSCIENCE AG
Samarium metal complex constructed by furantetracarboxylic acid and preparation method thereof
The invention relates to a samarium metal complex constructed by furantetracarboxylic acid and a preparation method thereof. The chemical composition of the complex contains a furantetracarboxylic acid (H4fa) ligand. The synthesis scheme comprises the following steps of: respectively dissolving potassium salt of furantetracarboxylic acid and SmCl3.6H2O in distilled water according to a stoichiometric ratio, mixing, and adding 1d HCl; and putting the obtained solution into a polytetrafluoroethylene lining, then putting the polytetrafluoroethylene lining into a reaction kettle, putting the reaction kettle into a drying oven, and carrying out heating reaction for 3 days to obtain a target product. The mass ratio of the potassium salt of furantetracarboxylic acid to SmCl3.6H2O is 1: 0.91.
Owner:TIANJIN POLYTECHNIC UNIV
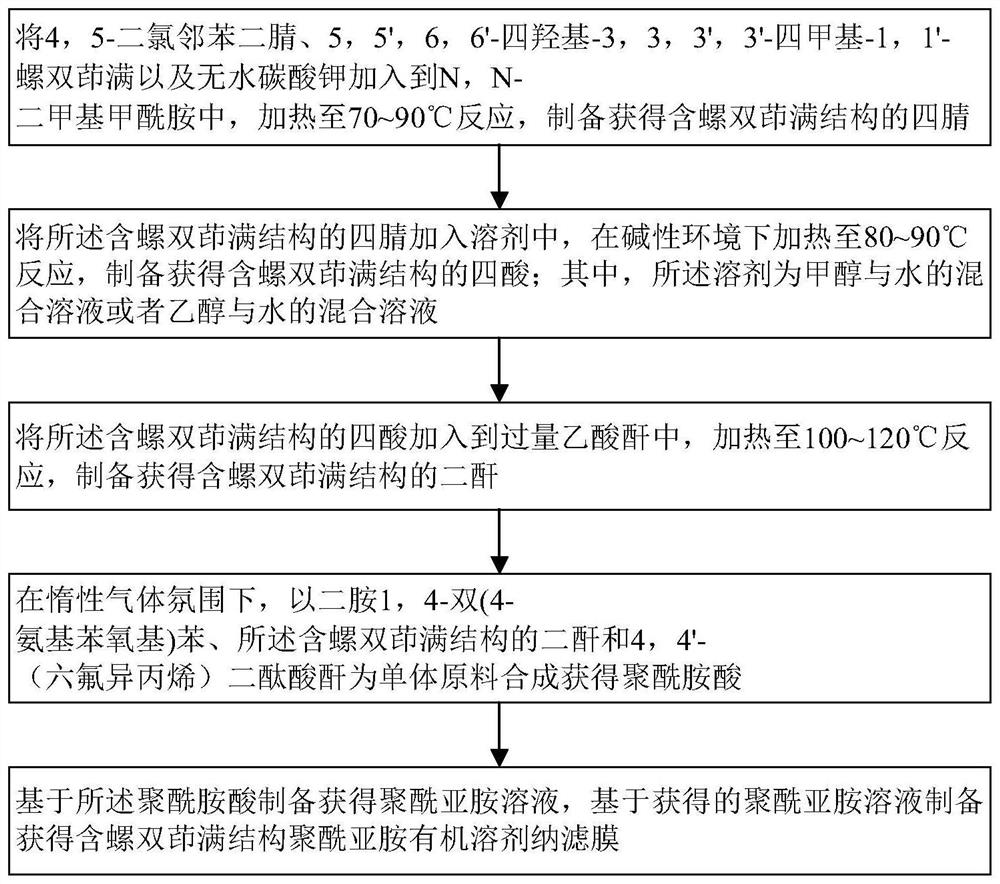
Preparation method of polyimide organic solvent nanofiltration membrane containing spirobiindane structure
ActiveCN114797487AImprove solubilityImprove stress resistanceSemi-permeable membranesGeneral water supply conservationTetramic acidPolymer science
The invention discloses a preparation method of a polyimide organic solvent nanofiltration membrane containing a spirobiindane structure, which comprises the following steps: adding 4, 5-dichlorophthalonitrile, 5, 5 ', 6, 6'-tetrahydroxy-3, 3, 3 ', 3'-tetramethyl-1, 1 '-spirobiindane and anhydrous potassium carbonate into N, N-dimethylformamide to prepare tetranitrile containing the spirobiindane structure; tetracid containing a spirobiindane structure is prepared; dianhydride containing a spirobiindane structure is prepared and obtained; in an inert gas atmosphere, synthesizing to obtain polyamide acid; and preparing to obtain the polyimide organic solvent nanofiltration membrane containing the spirobisindane structure. The preparation method provided by the invention is simple and easy to implement, the cast membrane forms an all-form spongy pore structure in the phase inversion process due to the introduction of a rigid distortion structure, and the compaction resistance and long-term operation stability of the nanofiltration membrane can be greatly enhanced.
Owner:XI AN JIAOTONG UNIV
Silver metal complex constructed by furantetracarboxylic acid and preparation method of silver metal complex
InactiveCN112694490AGroup 1/11 organic compounds without C-metal linkagesSilver organic compoundsFuranTetramic acid
The invention relates to a silver metal complex constructed by furantetracarboxylic acid and a preparation method of the silver metal complex. The chemical composition of the complex contains a furantetracarboxylic acid (H4fa) ligand and a bpy ligand. The synthesis scheme comprises the following steps: dissolving potassium salt of furantetracarboxylic acid and AgNO3 in distilled water according to a stoichiometric ratio, dissolving a bpy ligand in acetonitrile, mixing, and adding 1d HCl; and putting the obtained solution into a polytetrafluoroethylene lining, then putting the polytetrafluoroethylene lining into a reaction kettle, putting the reaction kettle into a drying oven, and carrying out heating reaction for 3 days to obtain a target product. The mass ratio of the potassium salt of furantetracarboxylic acid to AgNO3 to bpy is 1: 0.85: 0.39.
Owner:TIANJIN POLYTECHNIC UNIV
Tooth sensitivity treatment compositions
A tooth sensitivity treatment composition is disclosed. The composition includes a compound of formula IM1-A-M2-B-M1 (I)wherein: M1 is a monovalent or divalent metal; M2 is a polyvalent metal or metal oxide; and A and B are, independently, selected from the group consisting of C2-C6 diacids, triacids, and tetraacids. Methods for treating tooth sensitivity are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
New trifluoromethoxy-phenyl substituted tetramic acid-derivatives useful to combat parasites including insects, arachnid, helminth, nematode and mollusk and/or undesirable plant growth and in hygienic sectors
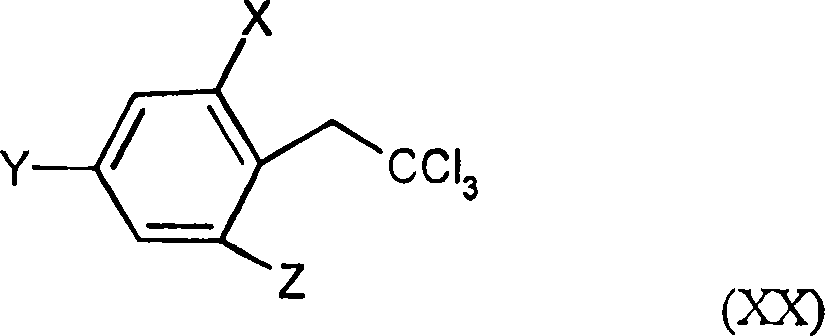
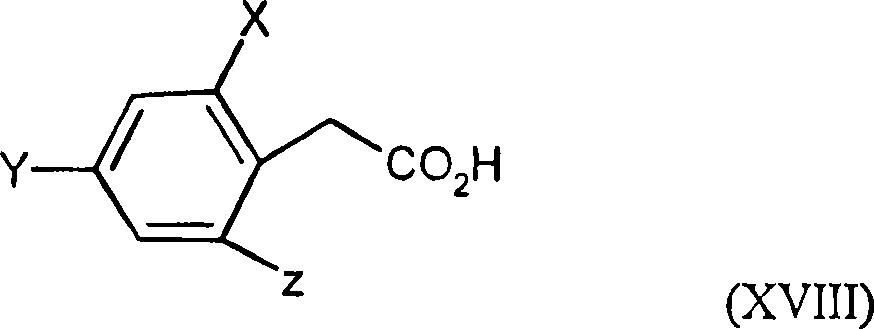
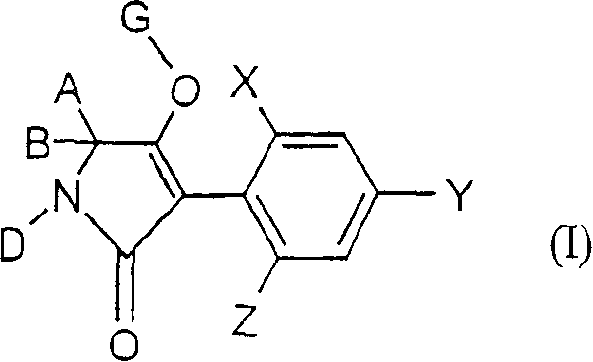
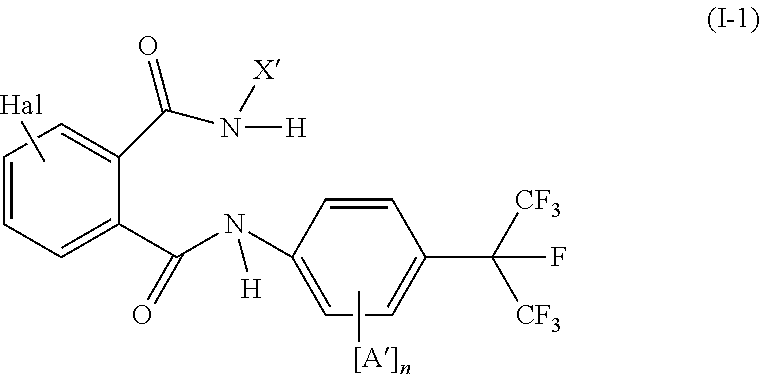
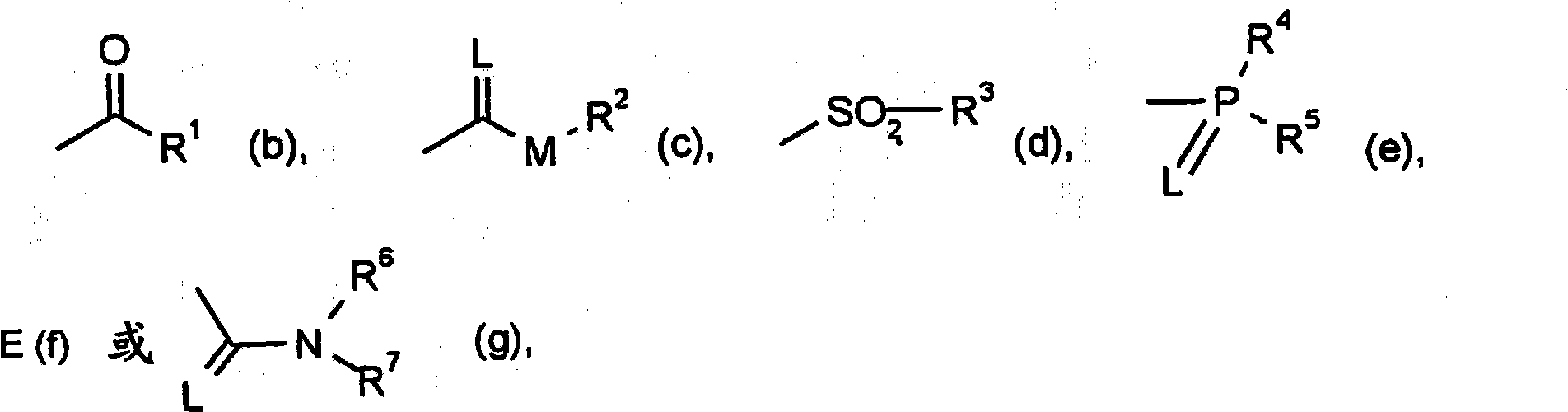
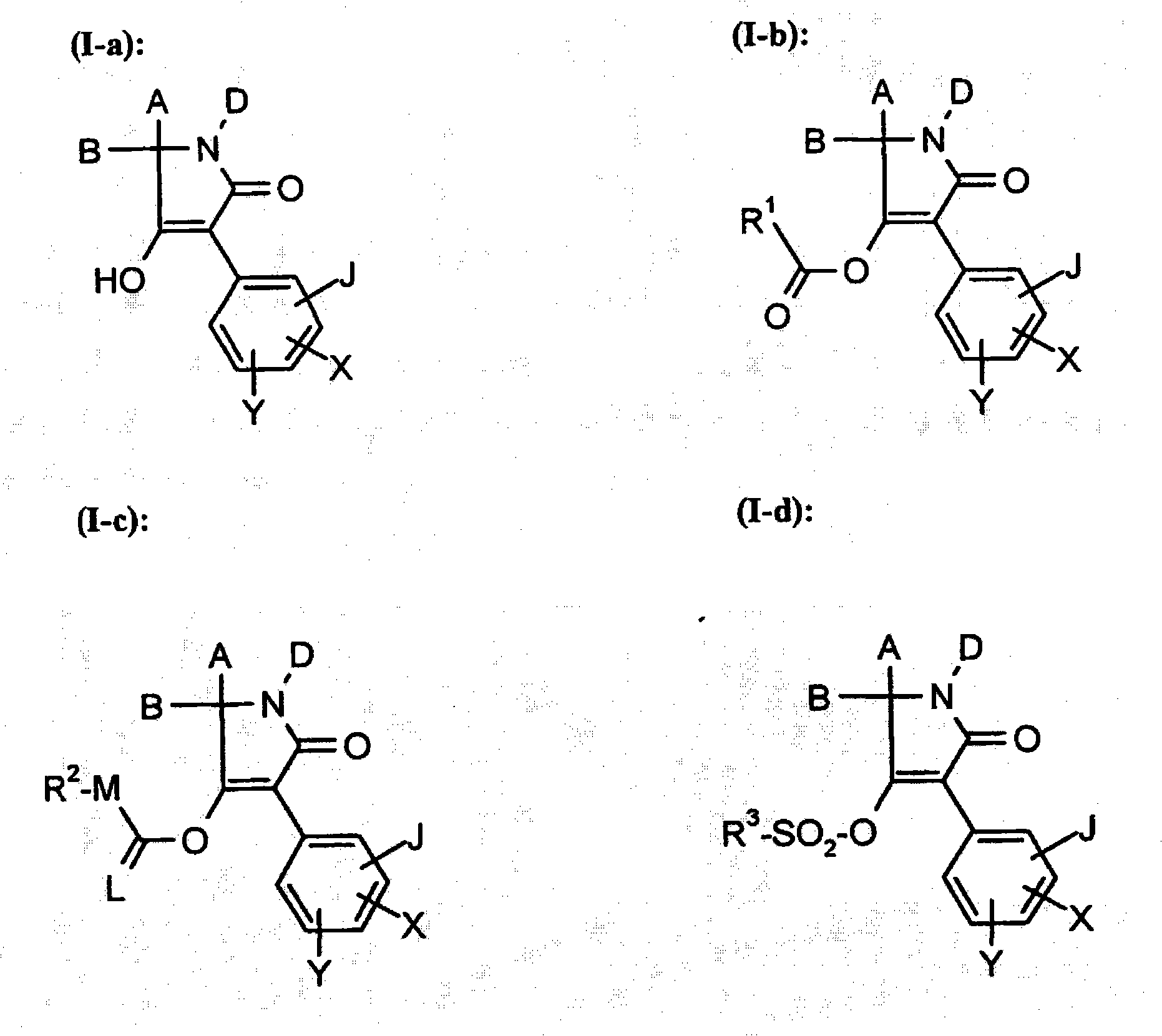
Trifluoromethoxy-phenyl substituted tetramic acid-derivatives (I), are new. Trifluoromethoxy-phenyl substituted tetramic acid-derivatives of formula (I), are new. J1a : trifluoromethoxy; X : H, alkyl, halo, haloalkyl, alkoxy or haloalkoxy; Y1a : H, alkyl or halo, where at least one of J1a, X or Y1a is present at position-2 of the phenyl residue and is at the same time unequally hydrogen; either A : alkyl, alkenyl, alkoxyalkyl, alkylthioalkyl, optionally saturated cycloalkyl (all optionally substituted by halo and at least a ring atom is optionally substituted by a heteroatom), aryl, arylalkyl, hetaryl (all optionally substituted by halo, (halo)alkyl, (halo)alkoxy, cyano or nitro) or H; and B1a : H or alkoxyalkyl; and D : H or a residue optionally substituted by alkyl, alkenyl, alkynyl, alkoxyalkyl, optionally saturated cycloalkyl, in which optionally one or more ring members are substituted by heteroatom, arylalkyl, aryl, hetarylalkyl or hetaryl; or C+A+B1a : optionally saturated, substituted and heteroatom containing cyclic group; or A+D : optionally saturated and at least a heteroatom containing, in A, D-parts optionally substituted cyclic group; G : H, carboxy group of formula (-CO-R 1>) or (-C(=L)-M-R 2>), sulfur dioxide group of formula (-SO 2-R 3>), phosphorus group of formula (-P(R 4>)(R 5>)(=L)), E or (-C(=L)-N(R 6>)(R 7>)); E : an equivalent metal ion or an ammonium ion; L, M : O or S; R 1>optionally halo substituted alkyl, alkenyl, alkoxyalkyl, alkylthioalkyl or polyalkoxyalkyl, optionally halo-, alkyl- or alkoxy substituted cycloalkyl, which is interrupted by at least a heteroatom, optionally substituted phenyl, phenylalkyl, hetaryl, phenoxyalkyl or hetaryloxyalkyl; R 2>optionally halo substituted alkyl, alkenyl, alkoxyalkyl or polyalkoxyalkyl, optionally substituted cycloalkyl, phenyl or benzyl; R 3>-R 5>optionally halo substituted alkyl, alkoxy, alkylamino, dialkylamino, alkylthio, alkenylthio or cycloalkylthio, or optionally substituted phenyl, benzyl, phenoxy or phenylthio; and either R 6>, R 7>optionally halo substituted alkyl, cycloalkyl, alkenyl, alkoxy or alkoxyalkyl, optionally substituted phenyl or benzyl, or H; or NR 6>R 7>cyclic group interrupted optionally by O or S. Independent claims are included for: (1) the preparation of (I); (2) the preparation of an agent to combat parasite and / or undesirable plant growth comprising mixing (I) with a diluent and / or a surface active material; (3) an agent comprising an active agent combination containing (I), at least a compound, which improves the compatibility of cultured plants, e.g. 4-dichloroacetyl-1-oxa-4-aza-spiro[4.5]-decane, 1-dichloroacetyl-hexahydro-3,3,8a-trimethylpyrrolo[1,2-a]-pyrimidin-6 (2H)-one, 4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine, 5-chloro-quinolin-8-oxy-acetic acid-(1-methyl-hexylester), 3-(2-chloro-benzyl)-1-(1-methyl-1-phenyl-ethyl)-urea, alpha -(cyanomethoxyimino)-phenylacetonitrile, diethyl-1-(2,4-dichloro-phen yl)-4,5-dihydro-5-methyl-1H-pyrazol-3,5-dicarboxylate, 2-dichloromethyl-2-methyl-1,3-dioxolane, 2-propenyl-1-oxa-4-azaspiro[ 4.5]decane-4-carbodithioate, 1,8-naphthalic acid anhydride, 1-(2,4-dichloro-phenyl)-5-methyl-1H-pyrazol-3-carboxylic acid-ethylester, 1-(2,4-dichloro-phenyl)-5-isopropyl-1H-pyrazol-3-car boxylic acid-ethylester, 5-chloro-quinolin-8-oxy-acetic acid-1-allyloxy-prop-2-yl-ester, 5-chloro-quinoxalin-8-oxy-acetic acid-methylester, 5-chloro-quinolin-8-oxy-acetic acid-ethylester, 5-chloro-quinoxalin-8-oxy-acetic acid-allylester, 5-chloro-quinolin-8-oxy-acetic acid-2-oxo-prop-1-yl-ester, 5-chloro-quinolin-8-oxy-malonic acid-diethylester, carboxyamide compound of formula (R 1> 6>-CO-N(R 1> 7>)(R 1> 8>)), or a phenyl compound of formula (IId) or (IIe); (4) an N-acylamino acid ester compound of formula (II); (5) a phenyl compound of formula (XV), (XIV) or (XIX); and (6) a phenyl-acetic acid compound of formula (XVII). Either R 1> 7>H, or F, Cl and / or Br substituted 1-6C alkyl, 2-6C alkenyl or 2-6C alkynyl, 1-4C alkoxy-1-4C alkyl, dioxolanyl-1-4C alkyl, furyl, furyl-1-4C alkyl, thienyl, thiazolyl, piperidinyl, or optionally F, Cl and / or Br or 1-4C alkyl substituted phenyl; and R 1> 8>H, optionally F, Cl and / or Br substituted 1-6C alkyl, 2-6C alkenyl or 2-6C alkynyl, 1-4C alkoxy-1-4C alkyl, dioxolanyl-1-4C alkyl, furyl, furyl-1-4C alkyl, thienyl, thiazolyl, piperidinyl, or optionally F, Cl and / or Br or 1-4C alkyl substituted phenyl; or R 1> 7>R 1> 8>optionally 1-4C alkyl substituted phenyl, furyl or annealed benzene ring; or CR 1> 7>R 1> 8>5- or 6-membered carbocyclic (substituted by 3-6C alkandiyl or 2-5C oxaalkandiyl); either R 2> 5>H, optionally cyano, OH, halogen or 1-4C alkoxy substituted 1-6C alkyl, optionally cyano or halo substituted 3-6C alkenyl or 3-6C alkynyl, optionally cyano, halogen or 1-4C alkyl substituted 3-6C cycloalkyl; and R 2> 6>R 2> 5>, optionally nitro, cyano, halo, 1-4C alkyl, 1-4C haloalkyl, 1-4C alkoxy or 1-4C haloalkoxy substituted phenyl; or R 2> 6>R 2> 5>2-6C alkandiyl (substituted optionally with 1-4C alkyl or 2-5C oxaalkandiyl); and R 1> 6>F, Cl and / or Br substituted 1-4C alkyl. [Image] [Image] [Image] [Image] - ACTIVITY : Antiparasitic; Herbicide; Insecticide; Arachnicide; Anthelmintic; Nematocide. - MECHANISM OF ACTION : None given.
Owner:BAYER IP GMBH
Method for purification of a biphenol tetraacid composition and a biphenol tetraacid composition
PendingCN113365970APreparation from carboxylic acid saltsCarboxylic compound separation/purificationTetramic acidAlcohol
A method for purification of a biphenol tetraacid composition includes contacting the biphenol tetraacid composition with a solvent including a C1-6 alcohol to form a slurry and isolating the purified biphenol tetraacid from the slurry. The biphenol tetraacid composition includes a biphenol tetraacid and a biphenol. A purified biphenol tetraacid composition is also described.
Owner:SABIC GLOBAL TECHNOLOGIEIS BV
4-oxo-alkylated tetramic acid compound, and preparation method thereof
The present invention relates to a new compound and a preparation method thereof. The general structural formula of the compound is shown in Formula I. Animal experiments show that the compound has the effect of saving the memory of animal models. It is of high safety, has no mutagenicity, can remain in blood for several hours after oral or intravenous injection, and can enter the brain. The compound can be used for preparing a medicament for treating Alzheimer's disease.
Owner:BEIJING JOEKAI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com