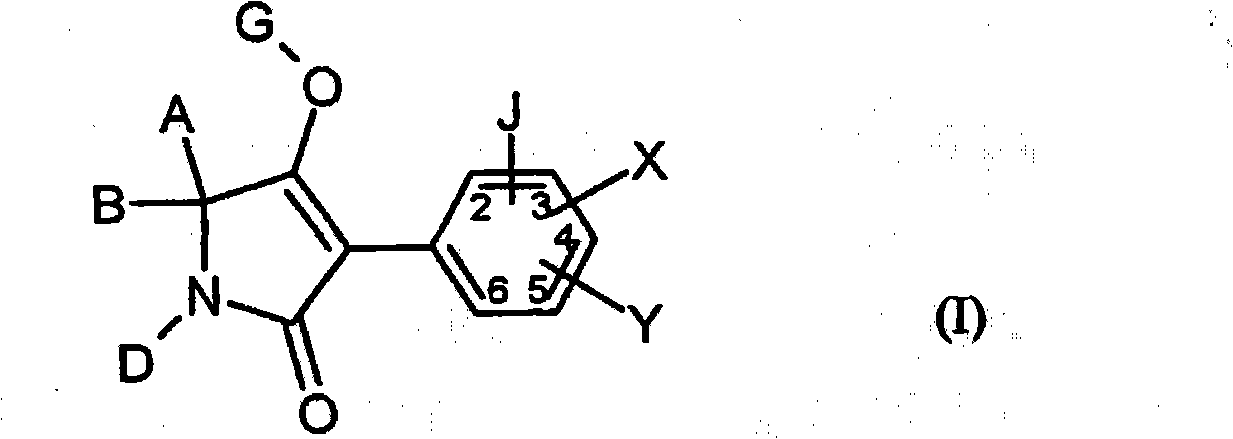
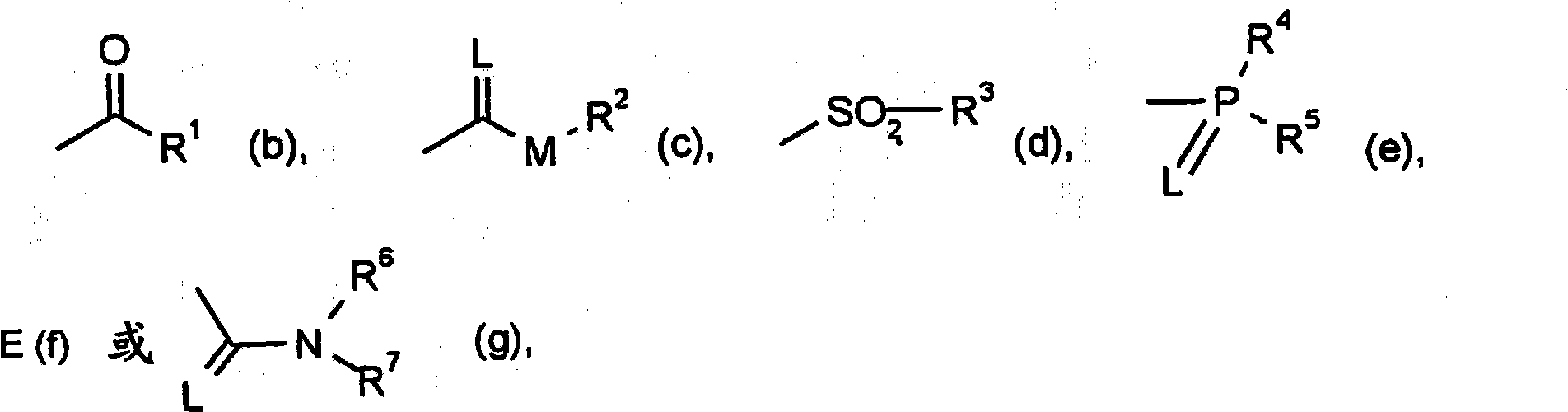
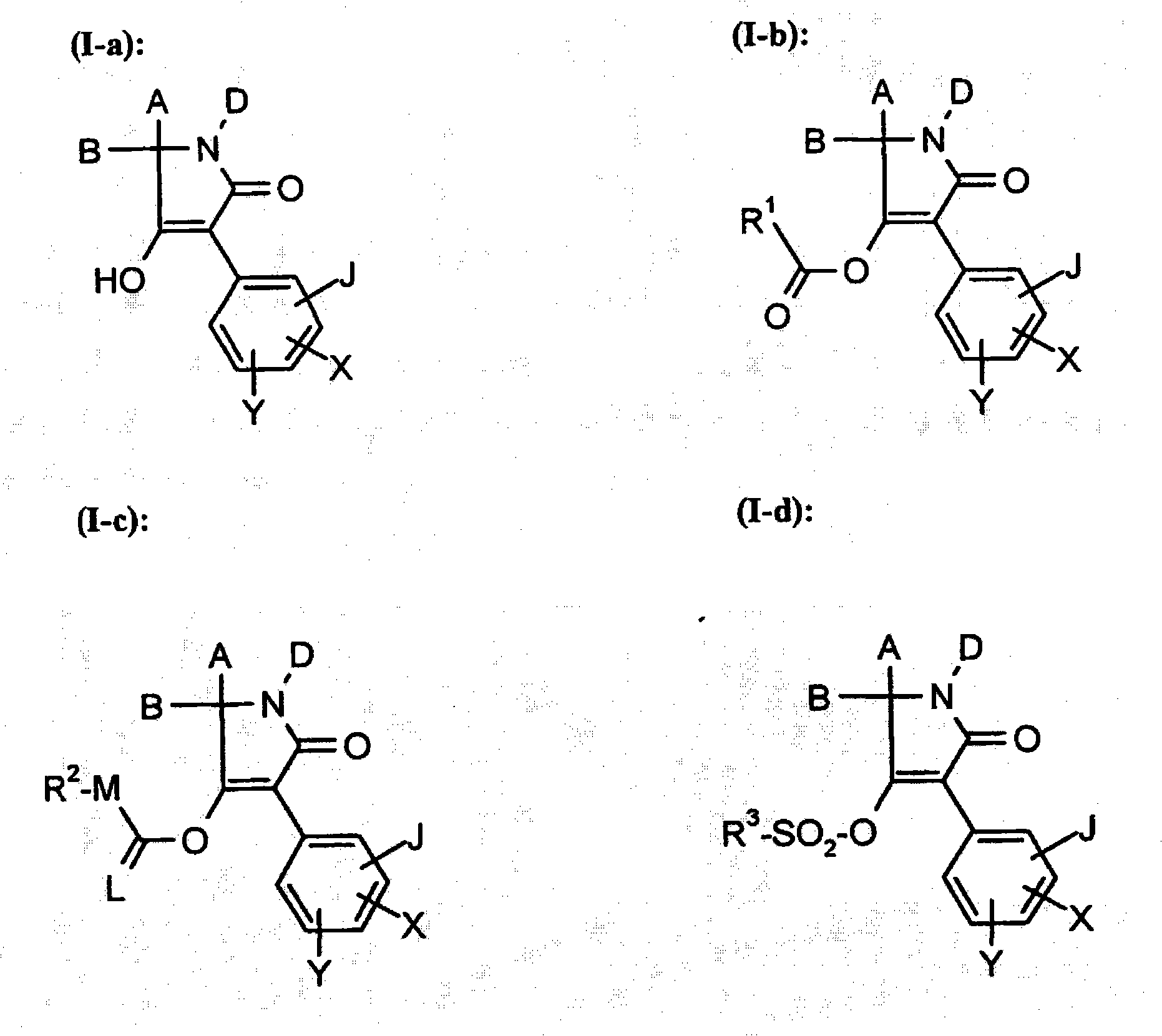
New trifluoromethoxy-phenyl substituted tetramic acid-derivatives useful to combat parasites including insects, arachnid, helminth, nematode and mollusk and/or undesirable plant growth and in hygienic sectors
A compound, meaning technology, applied in the field of Tetramic acid derivatives, can solve the problems that the activity and activity spectrum of the compound are not always completely satisfactory, and the plant compatibility is not always sufficient, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-a-1
[0969]
[0970] Under argon, 4.26 g of potassium tert-butoxide (36 mmol) were first added to 10 ml of dimethylacetamide. 8.5 g (16.4 mmol) of the compound of Example II-1 in 15 ml of dimethylacetamide were added dropwise at -20 to -30°C. The mixture was stirred at 20° C. for 1 h (the reaction was monitored by thin layer chromatography). After the reaction, the reaction solution was stirred into 100 ml of ice water, the pH was adjusted to 2 with concentrated hydrochloric acid, and the precipitate was filtered off with suction. The precipitate was redissolved in 40 ml of dichloromethane, and 20 ml of 0.5N NaOH solution was added dropwise. The mixture was stirred at 20 °C for 1 h. The aqueous phase was adjusted to pH 2 and the precipitate was filtered off with suction.
[0971] The product was purified by silica gel column chromatography (dichloromethane:ethyl acetate=5:3).
[0972] Yield: 4.2 g (51% of theory), melting point 259°C.
[0973] In a similar manner to Example...
Embodiment I-b-1
[0982]
[0983] Under argon, first add 0.5 g (1 mmol) of the compound of Example I-a-1 into ethyl acetate, add 0.14 ml of triethylamine and 10 mg of Steglich base, and add the compound dissolved in 5 ml of ethyl acetate dropwise under reflux. 0.1 ml of 2-methylpropanoyl chloride in , and the mixture was stirred under reflux.
[0984] After completion of the reaction (reaction monitored by thin layer chromatography), the mixture was separated by RP column chromatography (water / acetonitrile: 50 / 50→10 / 90).
[0985] Yield: 0.23 g (40% of theory), melting point 219°C.
[0986] In a similar manner to Example (I-b-1) and according to the general description of the preparation, the following compound of formula (I-b) is obtained:
[0987]
[0988]
[0989] 1) 2.61 (m, 1H, C H (C5)); 2.70(m, 1H(C H (CH 3 ) 2 ); 7.31, 7.44 (respectively dd, 1H, Ar H )
[0990] 2) 2.70(m, 1H, CH(C H 3 ) 2 ); 4.79 (m, 1H, CH(C5); 7.47 (dd, 2-H, Ar H )
[0991] 3) 2.62(m, 1H CH(C5); 2.70...
Embodiment I-c-1
[0999]
[1000] Under argon, first add 0.5 g (1 mmol) of the compound of Example I-a-1 into dichloromethane, add 0.14 ml of triethylamine, and add dropwise methyl chloride dissolved in 5 ml of dichloromethane at about 20° C. acid ester (0.1 ml), and the mixture was stirred at 20-30°C.
[1001] After completion of the reaction (reaction monitored by thin layer chromatography), the mixture was separated by RP column chromatography (water / acetonitrile: 50 / 50→10 / 90).
[1002] Yield: 0.415 g (74% of theory), melting point 194°C.
[1003] In a similar manner to Example (I-c-1) and according to the general description of the preparation, the following compound of formula (I-c) is obtained:
[1004]
[1005]
[1006]
[1007] 1) 4.29 (m, 2H, O CH 2 -CH 3 ); 4.81 (m, 1H, C H (C5)); 7.33, 7.47 (dd, 1H, Ar respectively H )
[1008] 2) 2.62(m, 1H, CH (C5)); 4.26 (m, 2H, O CH 2 CH 3 ); 7.32, 7, 45 (respectively dd, 1H, Ar H )
[1009] 3) 4.27(q, 2H, OCH 2 ); 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com