Improved production process for repaglinide
A process, the technology of reggae acid, applied in the field of medicine, can solve the problems of low yield and product purity, and achieve the effects of simple operation, low risk and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the improved process of producing repaglinide
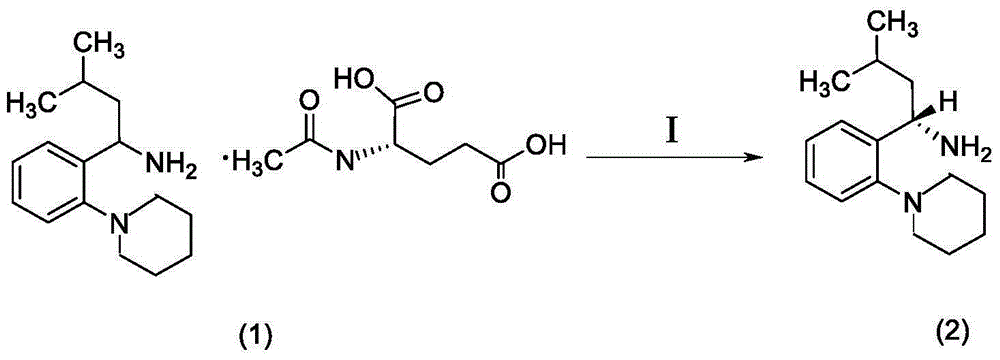
[0025] (1) Preparation of compound (S)-(+)-3-methyl-1-(2-piperidinyl-phenyl) butylamine (2), the reaction formula is as (I):
[0026] Get 1.0kg of compound (S)-(+)-3-methyl-1-(2-piperidinyl-phenyl)butylamine-N-acetyl-L-glutamic acid (1) and add 1.5kg of water and 5.0 kg of toluene mixed solution, then add 3.5kg of ammonia and stir until fully dissolved, extract the organic layer after the reaction, dry, filter, and concentrate under reduced pressure, the obtained compound (S)-(+)-3-methyl-1-( 2-Piperidinyl-phenyl)butylamine (2).
[0027]
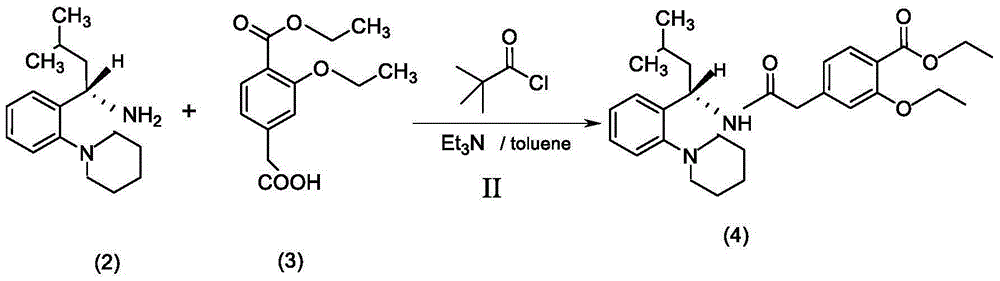
[0028] (2) Preparation of compound (S)-2-ethoxy-4-[2-[3-methyl-1-[2-(1-piperidinyl)-phenyl]-butanyl]-amino] -2-carbonylethylbenzoic acid ethyl ester (4), reaction formula such as (II):
[0029] Pump 3.0kg of toluene into the reaction tank and put in 680g of Regal acid (3), put in 310g of triethylamine, add the mixture of pivaloyl chloride and toluene dropwise at -5~0°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com