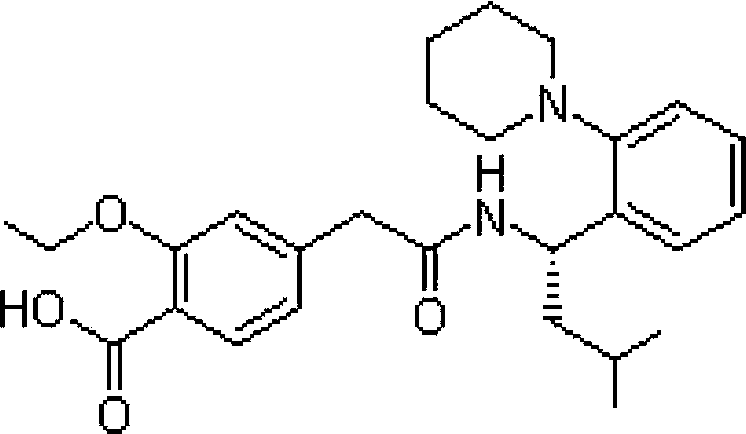
Repaglinide compound and novel production method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Take 10 g of repaglinide raw material with a long production date (Wante Pharmaceutical (Hainan) Co., Ltd., batch number: 20091101), and the content of repaglinide was determined to be 95% by high performance liquid chromatography. The crude repaglinide was dissolved in 80ml of chloroform, and after stirring thoroughly, the repaglinide was dissolved, and the insoluble impurities were removed by filtration, and the filtrate was collected once.
[0048] Add 0.6 g of activated carbon to the primary filtrate, keep warm at 35° C., stir for 20 min, filter the deactivated carbon, and collect the secondary filtrate.
[0049] Add 20ml of 1M sodium ethoxide aqueous solution to the above-mentioned secondary filtrate for treatment, the treatment temperature is 60°C, and the treatment time is 3 hours, then cool down to room temperature, stand to separate layers, and then separate the aqueous phase to obtain repaglinide-containing the organic phase.
[0050] Heat the obtained organi...
Embodiment 2
[0053] Take 10 g of expired repaglinide bulk drug, and the content of repaglinide as measured by high performance liquid chromatography is 92%. Dissolve the crude repaglinide in 100ml of toluene, stir well, dissolve the repaglinide, filter to remove insoluble impurities, and collect the filtrate once.
[0054] Add 0.5 g of alumina to the primary filtrate, keep warm at 40° C., stir for 30 min, filter the de-alumina, and collect the secondary filtrate.
[0055] Add 30ml of 0.5M sodium methoxide aqueous solution to the above-mentioned secondary filtrate for treatment, the treatment temperature is 65°C, the treatment time is 2 hours, then the temperature is lowered to room temperature, the layers are left to stand, and then the aqueous phase is separated to obtain the Nai's organic phase.
[0056] Heat the obtained organic phase to 70-75°C for concentration, then add anhydrous methanol accounting for 70% of the volume of the solution, slowly lower the temperature while stirring, ...
Embodiment 3
[0059] Take 10 g of repaglinide raw material (Wante Pharmaceutical (Hainan) Co., Ltd., batch number: 20111101), and the content of repaglinide as measured by high performance liquid chromatography is 96.5%. The crude repaglinide was dissolved in 90 ml of ethyl acetate, and after stirring well, the repaglinide was dissolved, filtered to remove insoluble impurities, and the filtrate was collected once.
[0060] Add 0.5 g of A-type molecular sieves to the primary filtrate, keep warm at 40° C., stir for 25 minutes, filter and remove the molecular sieves, and collect the secondary filtrate.
[0061] Add 25ml of 0.8M potassium ethoxide aqueous solution to the above-mentioned secondary filtrate for treatment, the treatment temperature is 50°C, and the treatment time is 4 hours, then cool down to room temperature, let stand to separate layers, and then separate the water phase to obtain repaglide-containing Nai's organic phase.
[0062] Heat the obtained organic phase to 65-75°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com