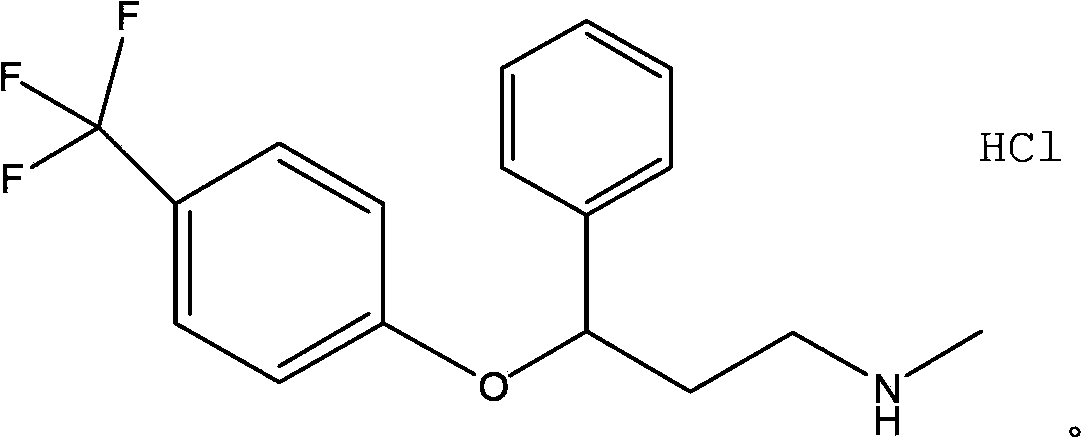
Fluoxertine hydrochloride compound and new preparation method thereof
A technology of fluoxetine hydrochloride and compounds, which is applied in the field of fluoxetine hydrochloride compounds, can solve the problems of low purity of fluoxetine hydrochloride, achieve the effects of reducing toxic and side effects, reducing residues, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Get 10g of fluoxetine hydrochloride crude drug with a long production date (manufacturer Shanxi Qianyuan Pharmaceutical Co., Ltd., batch number 20090501), and the purity measured by high performance liquid chromatography is 94.48%. Dissolve this fluoxetine hydrochloride raw material in 40ml acetone, fully stir, make it dissolve completely, filter to remove insoluble impurity, collect filtrate once.
[0054] Add 0.2 g of activated carbon to the primary filtrate, stir and adsorb at 40°C for 15 minutes, filter the deactivated carbon, collect the secondary filtrate, and concentrate under reduced pressure at 60°C.
[0055] Add 10g of alumina to the above-mentioned concentrated filtrate, stir evenly, evaporate the solvent and add to the upper end of the preparative chromatographic column, and then use the preparative chromatographic column to separate and purify, wherein the mobile phase used in the chromatographic column has a volume ratio of 2: 5:2 mixed solution of acetoni...
Embodiment 2
[0061] Take 10 g of expired fluoxetine hydrochloride raw material (manufacturer Shanxi Qianyuan Pharmaceutical Co., Ltd., batch number 20081201), and the purity measured by high performance liquid chromatography is 92.24%. Dissolve this fluoxetine hydrochloride raw material in 50ml ethanol, fully stir, make it dissolve completely, filter to remove insoluble impurity, collect filtrate once.
[0062] Add 0.3 g of alumina to the primary filtrate, stir and adsorb at 35°C for 10 minutes, filter the de-alumina, collect the secondary filtrate, and concentrate under reduced pressure at 55°C.
[0063] Add the above-mentioned concentrated filtrate to the upper end of the preparative chromatographic column, and then use the preparative chromatographic column to separate and purify the filtrate, wherein the mobile phase used in the chromatographic column is a mixed solution of acetonitrile:methanol:water with a volume ratio of 2:5:2 , the flow rate is 2ml / min, the stationary phase filler ...
Embodiment 3
[0069] Take 10 g of fluoxetine hydrochloride crude product prepared according to US2004102561A1, and the purity is 93.28% as measured by high performance liquid chromatography. Dissolve this fluoxetine hydrochloride raw material in the mixed solvent of 60ml ethanol and methanol, fully stir, make it dissolve completely, filter to remove insoluble impurity, collect filtrate once.
[0070] Add 0.2 g of A-type molecular sieve to the primary filtrate, stir and adsorb at 35°C for 15 minutes, filter and remove the molecular sieve, collect the secondary filtrate, and concentrate under reduced pressure at 60°C.
[0071] Add the above-mentioned concentrated filtrate to the upper end of the preparative chromatographic column, and then use the preparative chromatographic column to separate and purify the filtrate, wherein the mobile phase used in the chromatographic column is a mixed solution of acetonitrile:methanol:water with a volume ratio of 2:5:2 , the flow rate is 1ml / min, the stati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com