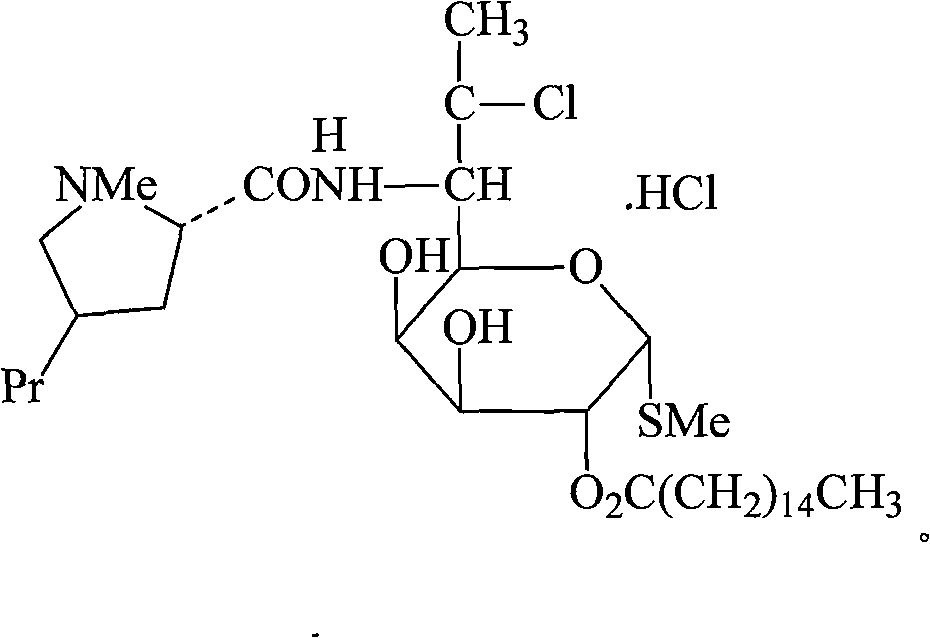
Clindamycin palmitate hydrochloridum compound and preparation method thereof
A technology of clindamycin hydrochloride and palmitate, applied in the field of medicine, can solve the problems of low purity of clindamycin hydrochloride palmitate, difficulty in obtaining high purity and high yield of clindamycin hydrochloride palmitate, etc. The effect of reducing toxic and side effects, improving clinical adverse reactions and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Accurately weigh 10 g of clindamycin palmitate hydrochloride with a purity of 92.34% prepared according to the patent GB 1205083, and dissolve it in 100 ml of water. Heat to 50°C, stir to dissolve completely, cool down after 1 hour and perform suction filtration, discard solid impurities, and collect the filtrate.
[0044] Add the obtained filtrate to the top of the packed column, the filler is fine-pored neutral alumina with a particle size of 20-80 μm and a pore size of 6 nm, and the column pressure is 0.08 MPa, and then pump a mixture of chloroform and methanol with a volume ratio of 1:3. The solvent was subjected to column chromatography, the flow rate was 1.0ml / min, and the column temperature was maintained at 40°C. Timing, sampling, tracking and monitoring were started, and segmental collection was carried out to collect the clindamycin hydrochloride palmitate part in the eluent.
[0045] At a temperature of 65°C, add 0.005M dilute hydrochloric acid aqueous soluti...
Embodiment 2
[0049] Accurately weigh 10 g of clindamycin palmitate hydrochloride bulk drug (Sichuan Creed Pharmaceutical Co., Ltd., H20083170) (purity 94.87%), and dissolve it in 150 ml of methanol. Heat to 50°C, stir to dissolve completely, cool down after 1 hour and carry out suction filtration, discard solid impurities, and collect the filtrate.
[0050] Add the obtained filtrate to the top of the packed column, the filler is fine-pored neutral alumina with a particle size of 100-220 μm and a pore size of 6 nm, and the column pressure is 0.15 MPa, and then pump a mixture of chloroform and methanol with a volume ratio of 1:2. The solution was dissolved for column chromatography, the flow rate was 2.5ml / min, and the column temperature was maintained at 50°C. Timing, sampling, tracking and monitoring were started, and segmental collection was carried out to collect clindamycin hydrochloride palmitate in the eluate.
[0051] At a temperature of 68°C, add 0.01M dilute hydrochloric acid aqueo...
Embodiment 3
[0053] Accurately weigh 10 g of clindamycin palmitate hydrochloride bulk drug (Chongqing Kailin Pharmaceutical Co., Ltd., H20057714) (purity 93.66%), and dissolve it in 120 ml of a mixed solvent of chloroform and methanol with a volume ratio of 1:2. Heat to 45°C, stir to dissolve completely, cool down after 1 hour and filter with suction, discard solid impurities, and collect the filtrate.
[0054] Add it to the top of the packed column. The filler is fine-pored neutral alumina with a particle size of 180-300 μm and a pore size of 6 nm. For analysis, the flow rate was 2ml / min, and the column temperature was maintained at 45°C. Start timing, sampling, follow-up monitoring, segmental collection, and collect the clindamycin hydrochloride palmitate part in the eluent.
[0055] At a temperature of 66°C, add 0.05M dilute hydrochloric acid aqueous solution to the obtained clindamycin palmitate hydrochloride solution, adjust the pH value between 5-6, and lower the temperature to 45-50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com