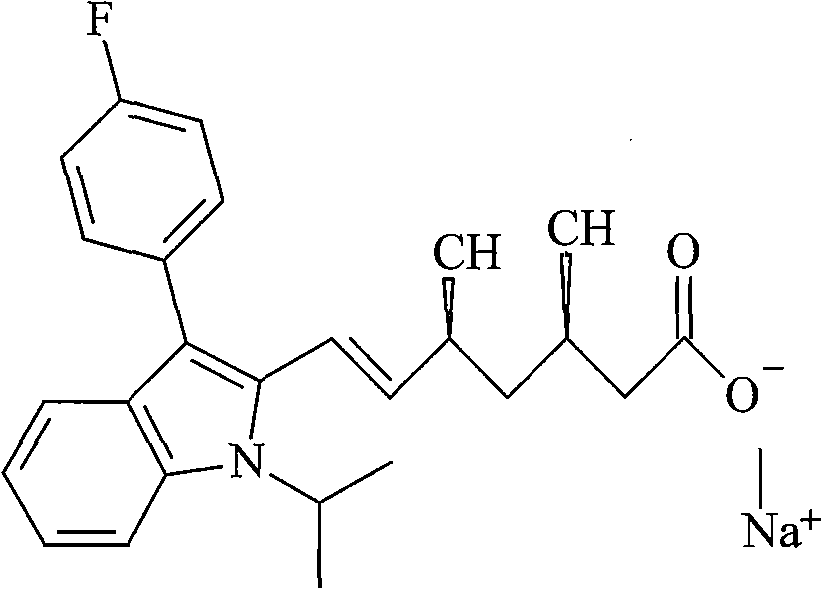
Fluvastatin sodium compound and preparation method thereof
A technology of fluvastatin sodium and its compound, which is applied in the field of fluvastatin sodium compound and its preparation method, can solve the problems of low purity of fluvastatin sodium, achieve easy control and industrial production, improve clinical adverse reactions, and improve product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Get 10g of fluvastatin sodium crude product with a purity of 86.3% prepared according to route 1, dissolve it in 200ml of water, add 0.2g of gac, stir, absorb for 10 minutes, filter with suction, and collect the filtrate.
[0060] 1 g of sodium methoxide was added to the above filtrate and treated at room temperature for 1 hour, the precipitated precipitate was filtered off, and the filtrate was collected.
[0061] Add 100ml of acetone to the above-mentioned filtrate, during this process crystals are slowly precipitated, placed at room temperature for 5 hours, the crystallization is complete, washed with 10ml of purified water, and dried to obtain 8.4g of refined fluvastatin sodium with a purity of 98.6% and a yield of 95.6% .
[0062] 1 H-NMR (CD 3 OD): δ1.52~1.48(1H, m), 1.60~1.71(7H, m), 2.35~2.20(2H, m), 3.99~3.94(1H, m), 4.38~4.34(1H, m), 4.92(1H, hept, J=6.8Hz), 5.73(1H, dd, J=16.0, 6.0Hz), 6.67(1H, dd, J=16.2, 1.2Hz), 6.98(1H, t, J=7.2Hz ), 7.12~7.08 (3H, m),...
Embodiment 2
[0068] Get 10g of fluvastatin sodium crude product with a purity of 88.9% prepared according to route 2, dissolve it in 1000ml of water, add 3g of gac, stir, absorb for 30 minutes, filter with suction, and collect the filtrate.
[0069] Add 2 g of sodium ethylate to the above filtrate and treat at room temperature for 1 hour, filter the precipitated precipitate, and collect the filtrate.
[0070] Add 800ml of acetone to the above-mentioned filtrate, during this process crystals are slowly precipitated, placed at room temperature for 12 hours, the crystallization is complete, washed with 20ml of purified water, and dried at 50°C to obtain 8.5g of refined fluvastatin sodium with a purity of 98.8%. 94.5%. mp196°C.
Embodiment 3
[0076] Get 10g of fluvastatin sodium crude product with a purity of 84.1% prepared according to route 3, dissolve it in 500ml of water, add 1g of activated carbon, stir, absorb for 20 minutes, filter with suction, and collect the filtrate.
[0077] Add 1 g of sodium ethylate to the above filtrate and treat at room temperature for 1 hour, filter out the precipitated precipitate, and collect the filtrate.
[0078] Add 500ml of acetone to the above filtrate, during this process crystals slowly precipitate out, place at room temperature for 12 hours, the crystallization is complete, wash with purified water, and dry at 50°C to obtain 8.2g of refined fluvastatin sodium with a purity of 98.8% and a yield of 96.3 %. mp196℃
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com