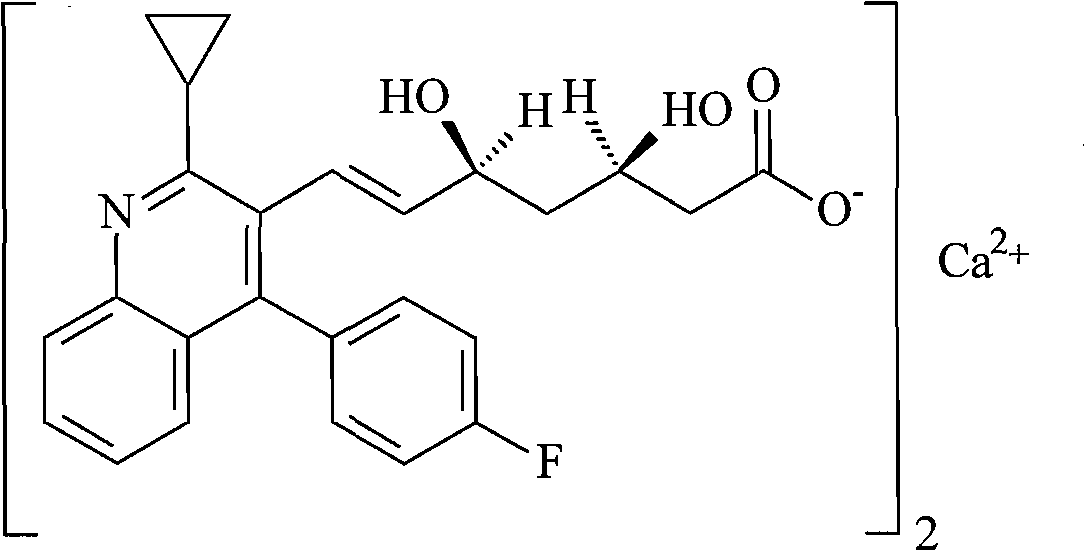
Pitavastatin calcium compound and preparation method thereof
A technology of pitavastatin calcium and compounds, which is applied in the field of medicine, can solve the problems of low purity of pitavastatin calcium, achieve easy control and industrial production, and improve the effect of clinical adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]Get 10g pitavastatin calcium raw material, high-phase liquid chromatography measures content and be 85%, pitavastatin calcium raw material is added in the methyl alcohol-0.1% formic acid aqueous solution that volume ratio is 1: 0.3, is heated to 65-75 ℃, makes Pitavastatin calcium was completely dissolved, filtered, and solid impurities were discarded.
[0037] The above solution was passed through D101 type macroporous adsorption resin, eluted with methanol-0.1% formic acid aqueous solution with a volume ratio of 1:0.3, and the pitavastatin site in the eluate was collected.
[0038] Raise the temperature to 75°C, keep it for 1 hour, adjust the pH to 7 with 0.5M sodium hydroxide, add 10% of the volume of 1M calcium chloride solution while hot, and then add the solution to the solvent volume ratio of 1:2 Tetrahydrofuran. Reduce the temperature to 50-53°C within 1 hour, then reduce the temperature to 22-25°C within 2 hours, and finally reduce the temperature to 5-10°C wit...
Embodiment 2
[0043] Get 10g pitavastatin calcium raw material, high-phase liquid chromatography measures content and be 89%, pitavastatin calcium raw material is added in the methanol-0.1% formic acid aqueous solution that volume ratio is 1: 0.2, is heated to 60-72 ℃, makes Pitavastatin calcium was completely dissolved, filtered, and solid impurities were discarded.
[0044] Pass the above solution through D101 macroporous adsorption resin, elute with methanol-0.1% formic acid aqueous solution with a volume ratio of 1:0.2, and collect pitavastatin in the eluate.
[0045] Raise the temperature to 70°C and keep it for 1 hour, adjust the pH to 6.9 with 0.01M sodium hydroxide, add 10% 0.5M calcium chloride solution while hot, and then add acetone at a volume ratio of solution to solvent of 1:2.5 . Reduce the temperature to 50-52°C within 1 hour, then reduce the temperature to 20-23°C within 3 hours, and finally reduce the temperature to 5-8°C within 10 hours. During this process, crystals con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com