Novel method for synthesizing bisoprolol importance intermediate
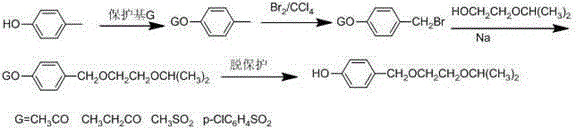
A technology for bisoprolol and intermediates, which is applied in the field of synthesis of important intermediates of bisoprolol, can solve the problems of consumption of 2-isopropoxyethanol, inability to process and reuse, and high production costs, and achieve easy industrial production, Low-cost, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
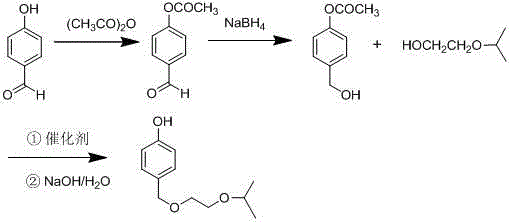
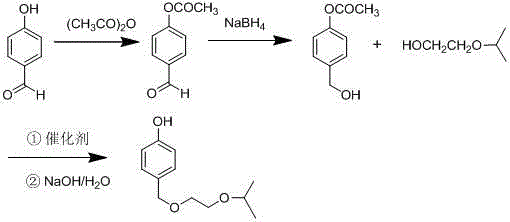
[0026] Add 12.2g (0.1mol) of p-hydroxybenzaldehyde and 0.3g of concentrated sulfuric acid into a 100ml three-necked flask, slowly add 12.12g (0.12mol) of acetic anhydride dropwise, and heat to reflux. After TLC detects that the reaction is complete, distill off the solvent and add After stirring with 20ml of water, solids were precipitated, cooled to crystallize, separated by suction filtration, and dried to obtain 16.1 g of 4-acetoxybenzaldehyde, with a yield of 98% and a purity of 99.3% by HPLC.
Embodiment 2
[0028] In a 100ml three-neck flask, add 16.1g (0.098mol) 4-acetoxybenzaldehyde and 20ml ethanol, stir to dissolve, add 1.48g sodium borohydride in batches in an ice-water bath, continue stirring, and heat up to 25°C after 1h Reaction, TLC detects that after the reaction is complete, evaporate the solvent, add 30ml of ethyl acetate and 10ml of water to the residue, keep the organic phase, continue to add 10ml of water to the organic phase for washing, remove the water layer, and concentrate the organic phase to obtain 4- Acetoxybenzyl alcohol was 15.8 g, the yield was 97.4%, and the HPLC purity was 99.5%.
Embodiment 3
[0030] In a 100ml three-neck flask, add 16.1g (0.098mol) 4-acetoxybenzaldehyde and 20mlTHF, stir to dissolve, add 2.23g sodium borohydride in ice water bath, continue stirring, after 1h, heat up to 25°C for reaction , after the TLC detection reaction was complete, evaporate the solvent, add 30ml ethyl acetate and 10ml water to the residue, keep the organic phase, continue to add 10ml water to the organic phase for washing, remove the water layer, and evaporate the organic phase to dryness to obtain 4- Acetoxybenzyl alcohol 15.5g, yield rate 95%, HPLC purity 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com