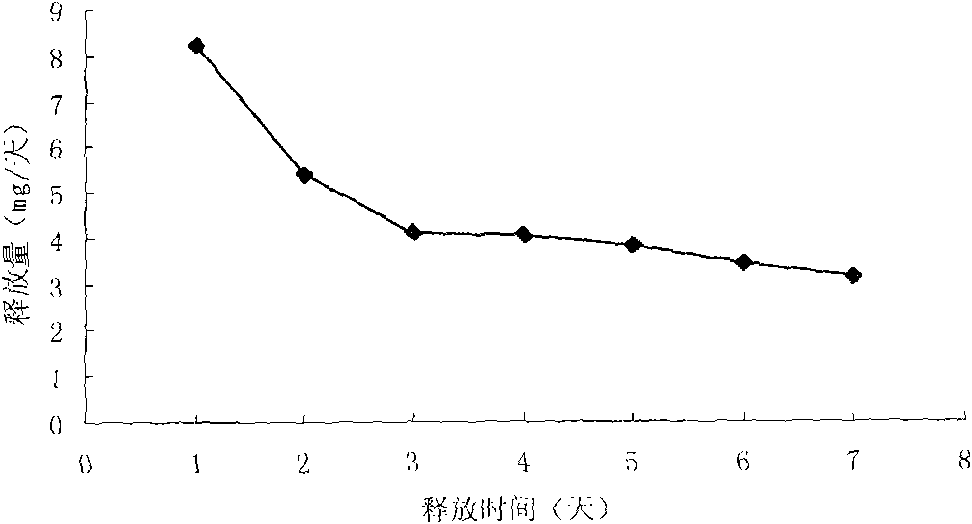
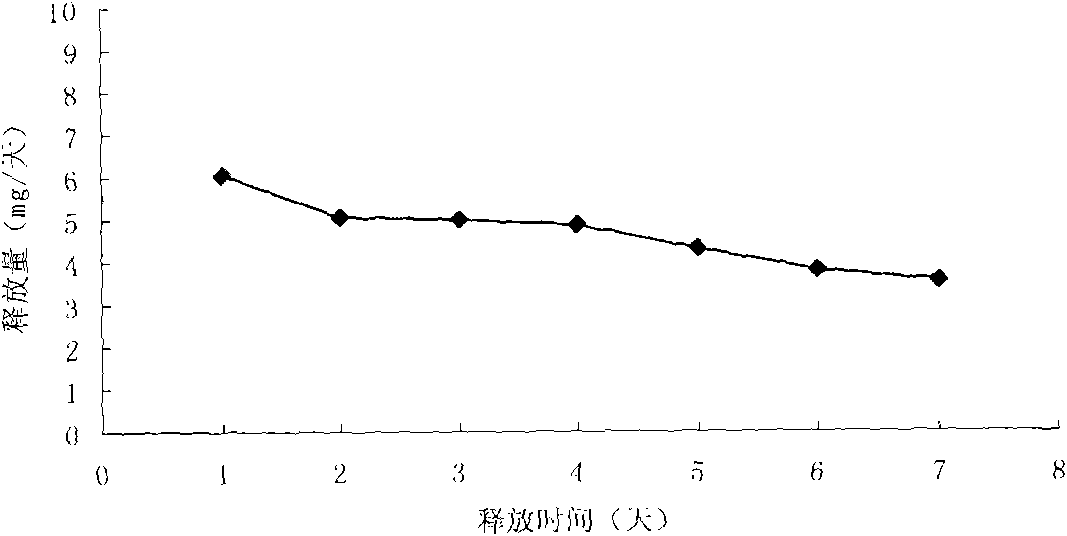
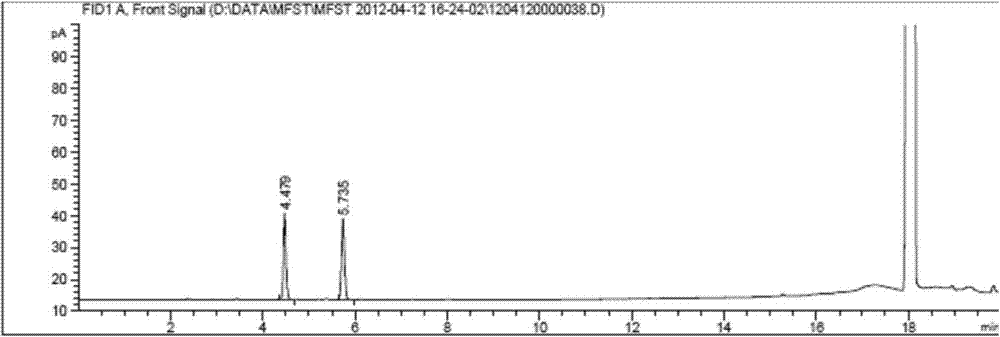
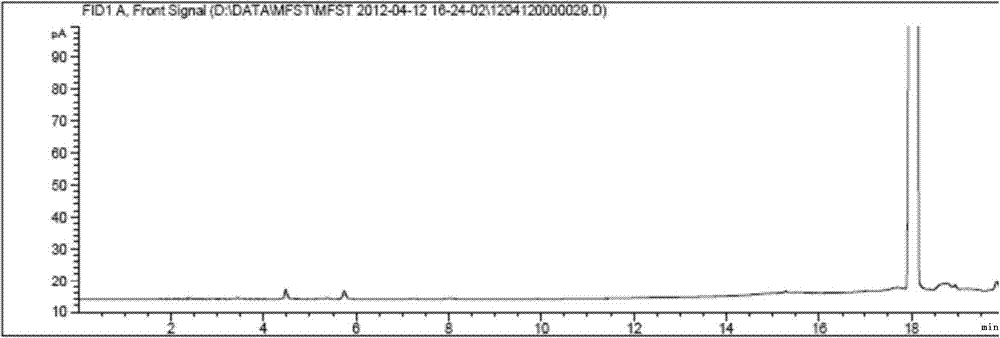
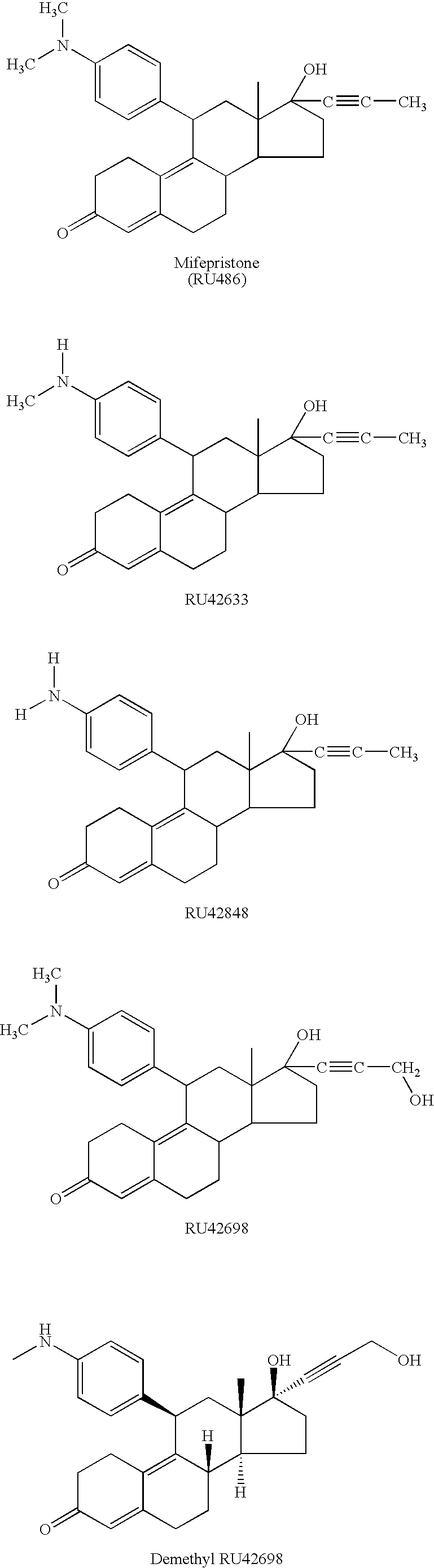
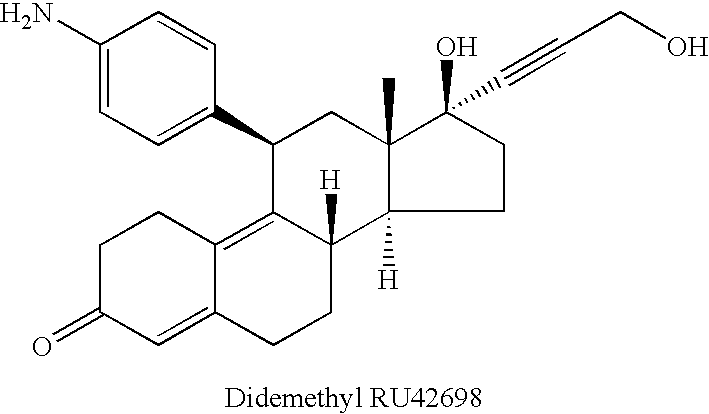
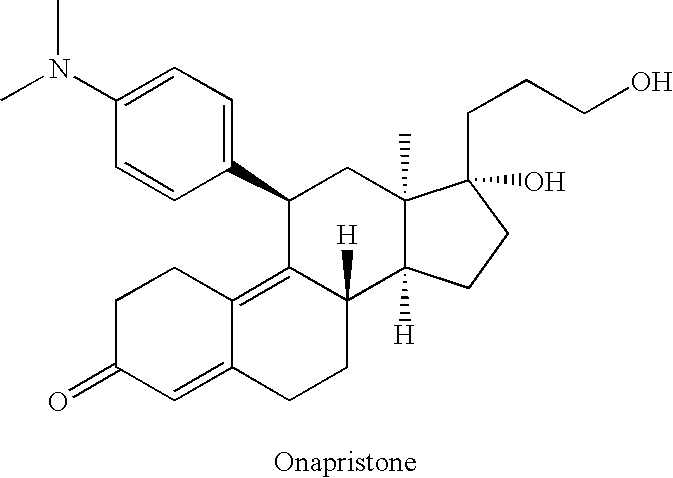
Patents
Literature
74 results about "Mifepristone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
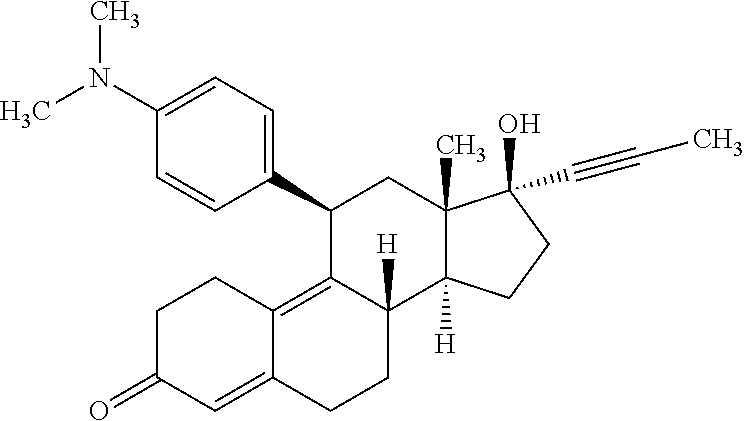
Mifepristone (also known as RU 486) is used to cause an abortion during the early part of a pregnancy. It is used up to week 10 of pregnancy (up to 70 days after the first day of your last menstrual period).
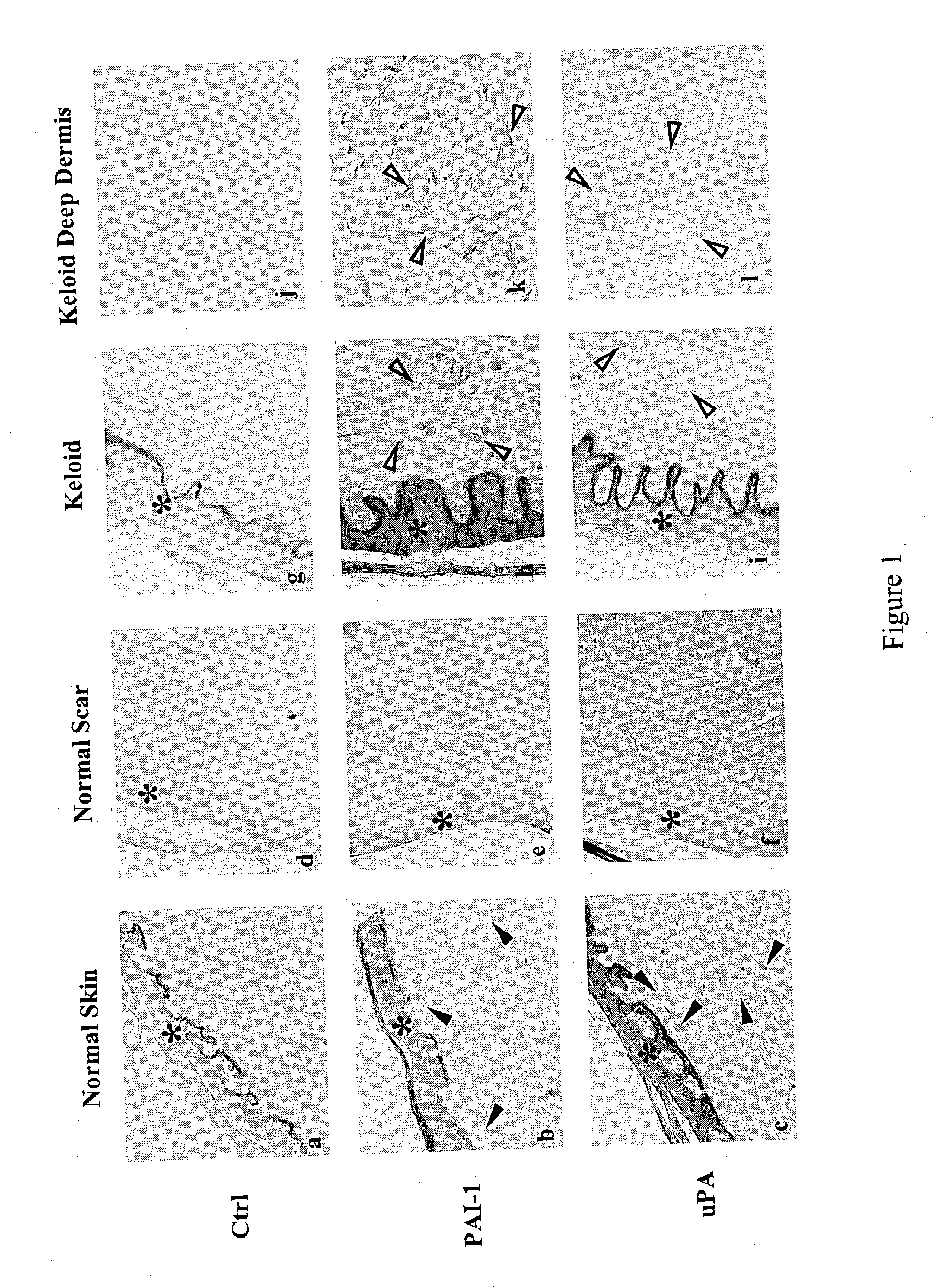
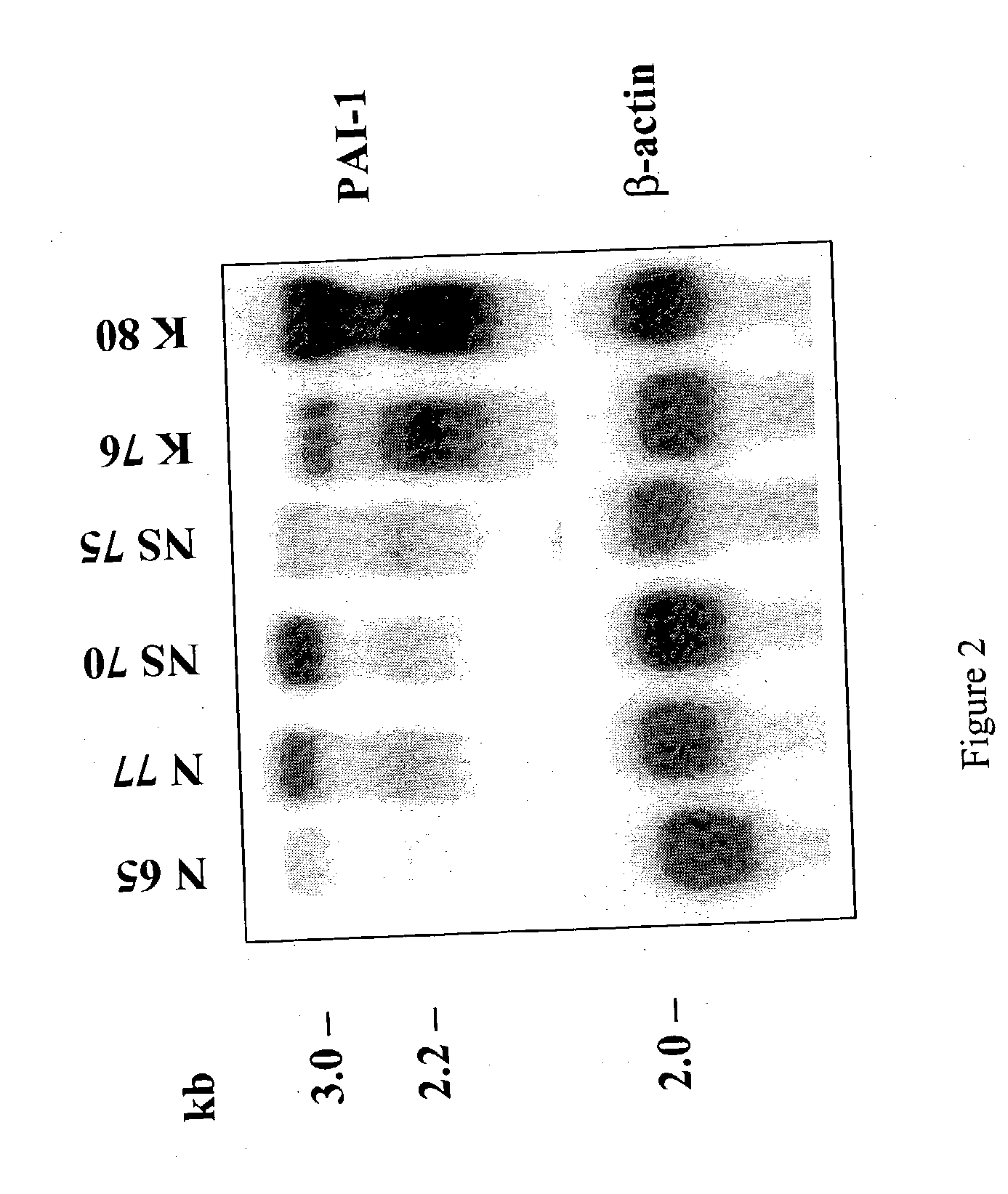
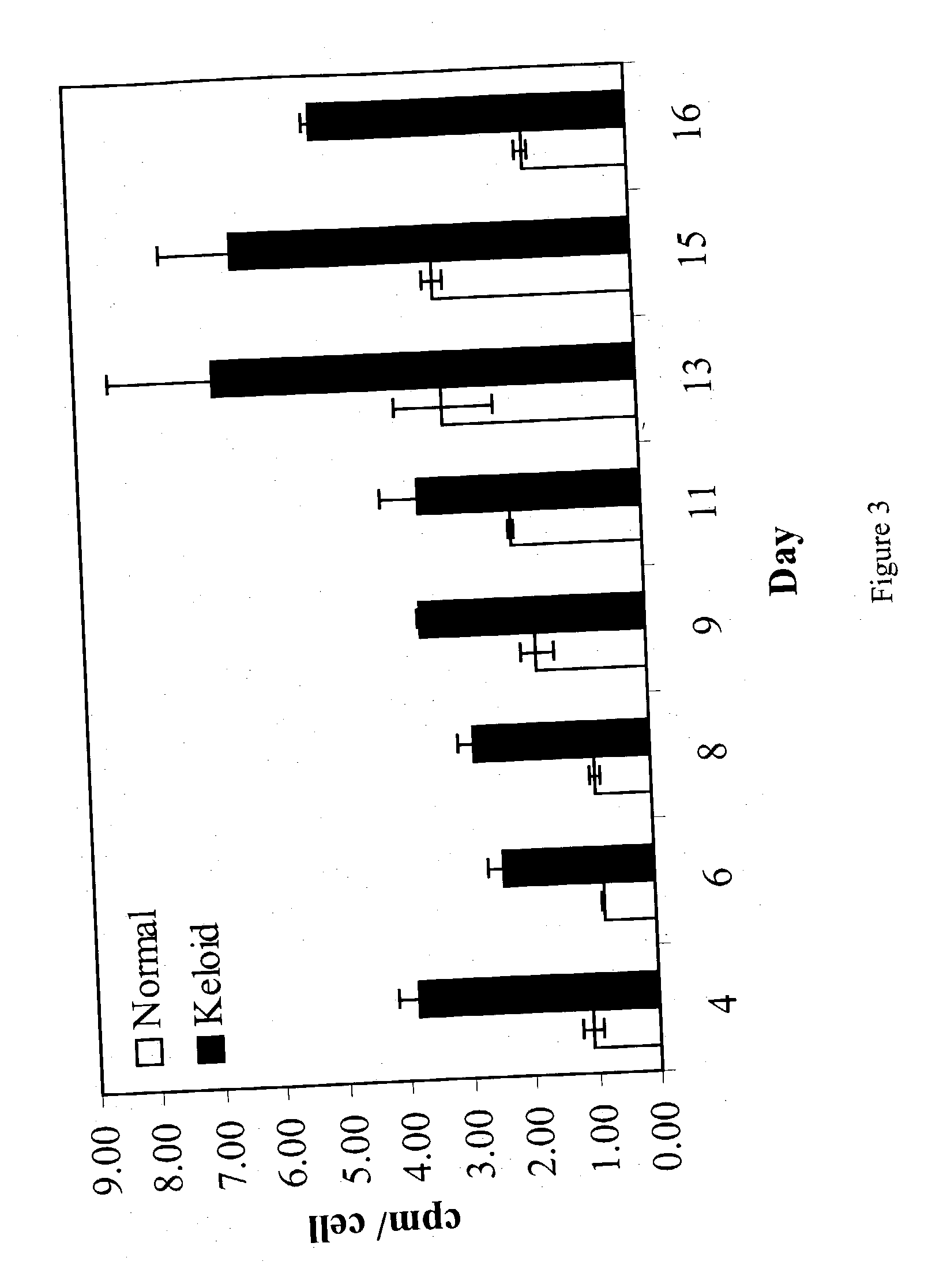
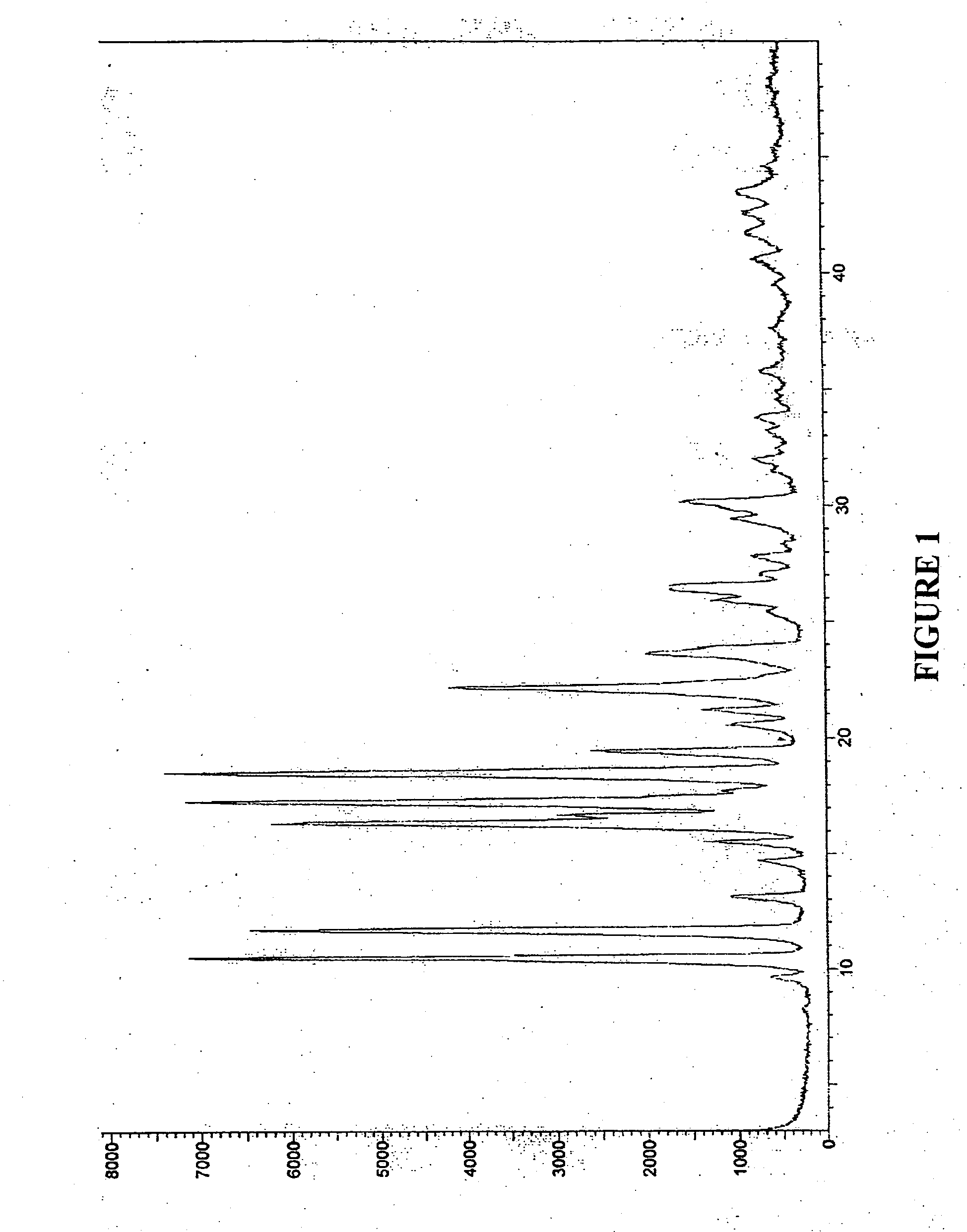
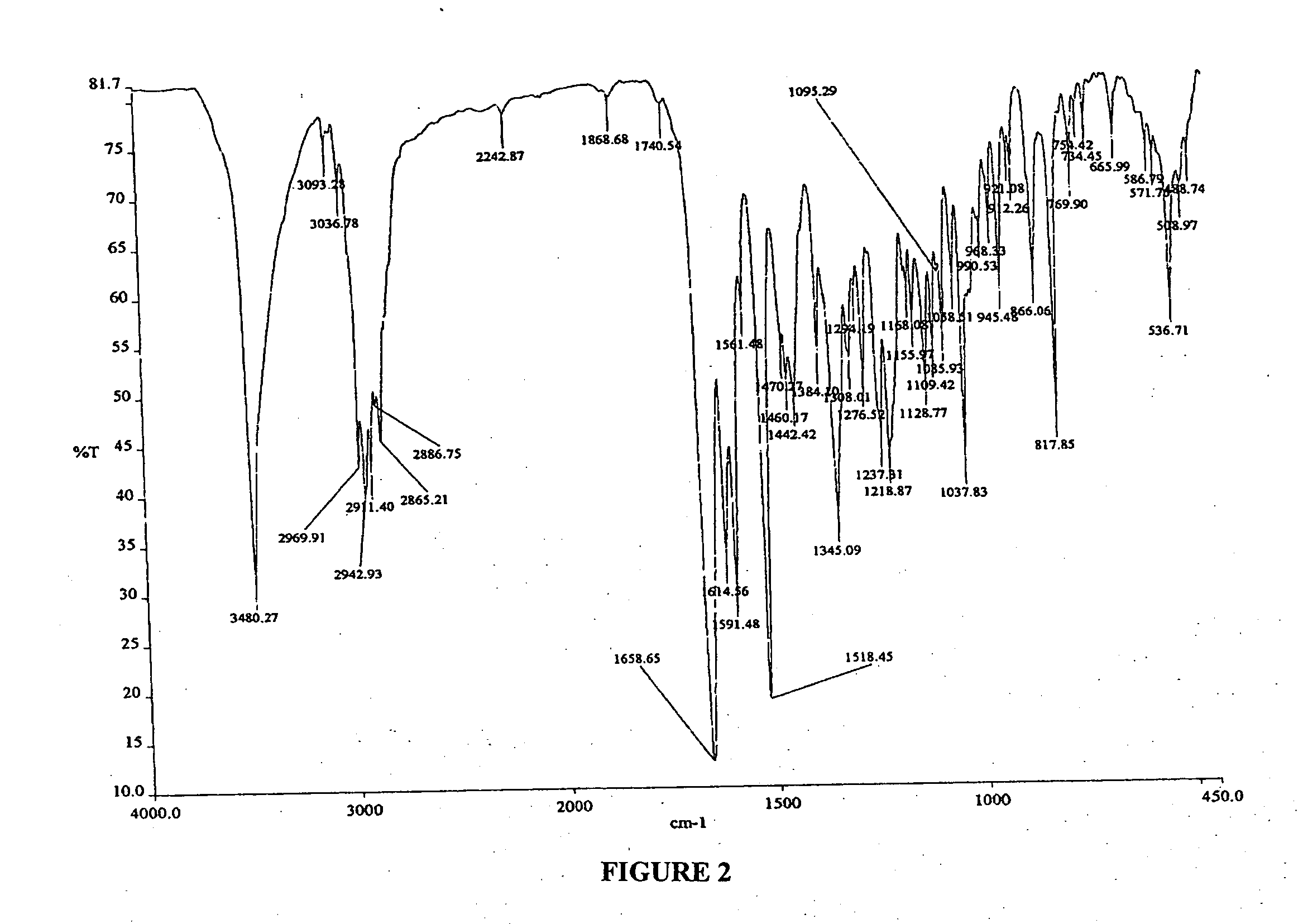
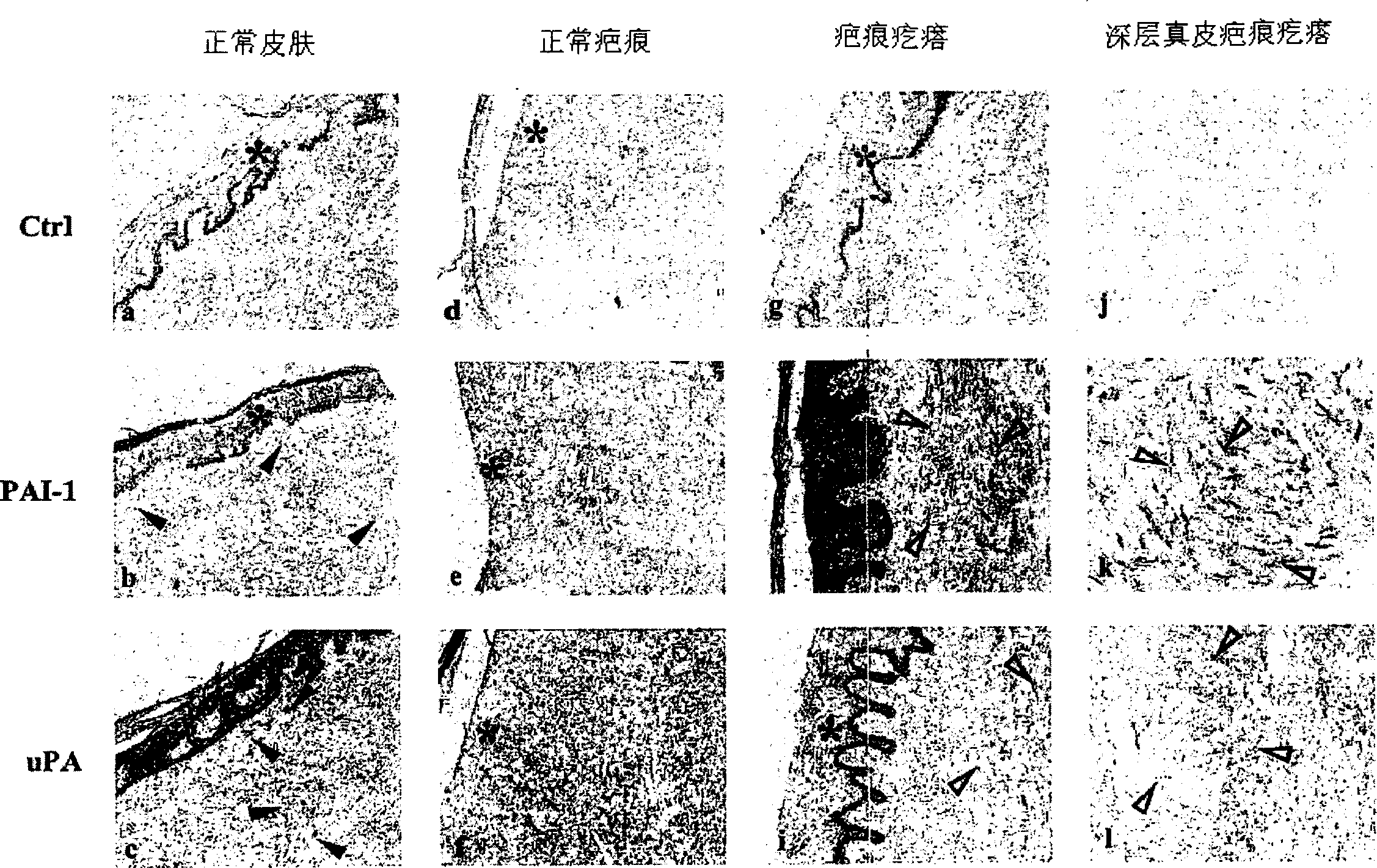
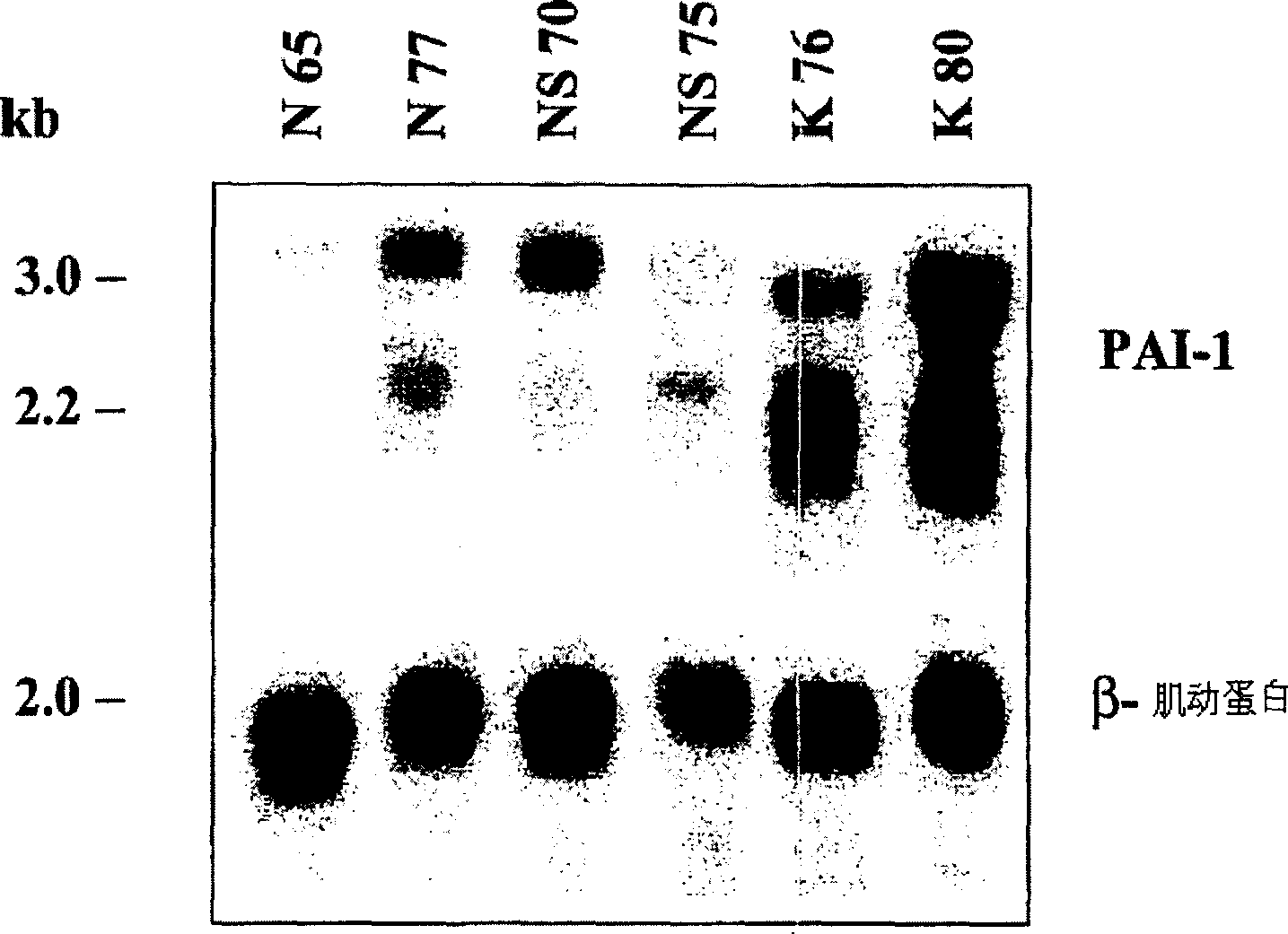
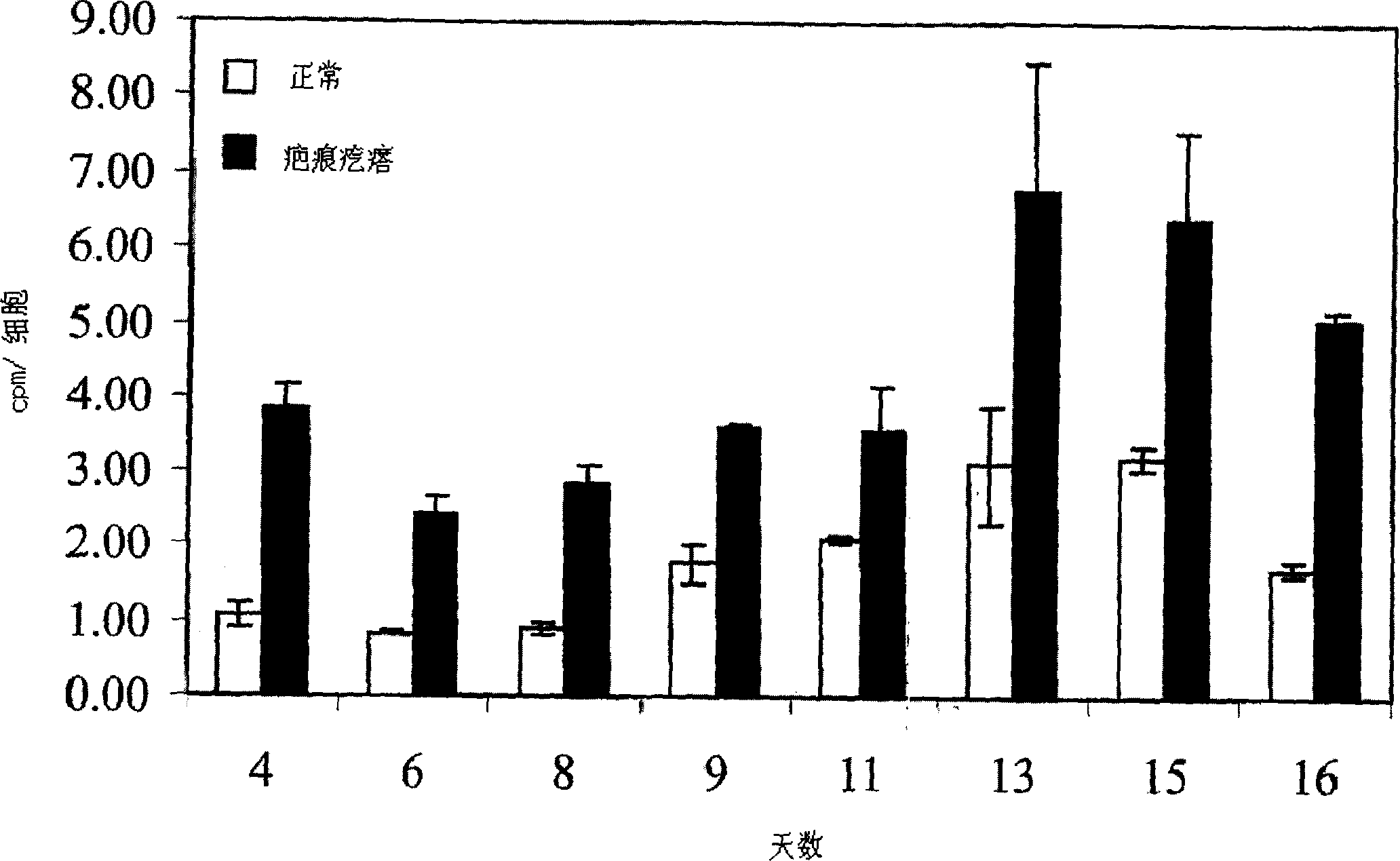
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Vagina administration mifepristone prepn and its composition and prepn process
InactiveCN1846703AHigh affinityImprove adhesionOrganic active ingredientsSuppositories deliverySide effectWhole body
The vagina administrated mifepristone preparation for birth control and health reproduction consists of mifepristone or nanometer mifepristone liposome particle as main component, excipient, osmosis promoter, pH regulator, preservative, surfactant, solvent, water and other pharmaceutically acceptable components, and may be prepared into gel, film or other preparation forms. The vagina administrated mifepristone preparation is absorbed through vagina mucous membrane into blood circulation, and has high bioavailability, lowered dosage, less toxic side effect and less stimulation.
Owner:程定超
Novel polymorph form M of mifepristone and process for its preparation
Mifepristone substantially in polymorph form M is provided. Also provided is a process for the preparation of polymorph form M of mifepristone comprising the steps of (a) dissolving crude mifepristone in a polar solvent at an elevated temperature to obtain a clear solution; (b) cooling the solution to a temperature and for a time period sufficient to form a precipitate of mifepristone crystals; and (c) isolating the precipitate of mifepristone crystals to obtain the polymorph form M of mifepristone.
Owner:GLENMARK GENERRICS LTD
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
InactiveCN1668312AReduced uPA activityImmunoglobulins against animals/humansMuscular disorderVitamin CFibrosis
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Mifepristone medicinal preparation and preparation method thereof
InactiveCN101455671APromote dissolutionIncrease miscarriage rateOrganic active ingredientsSexual disorderMedicineAdhesive
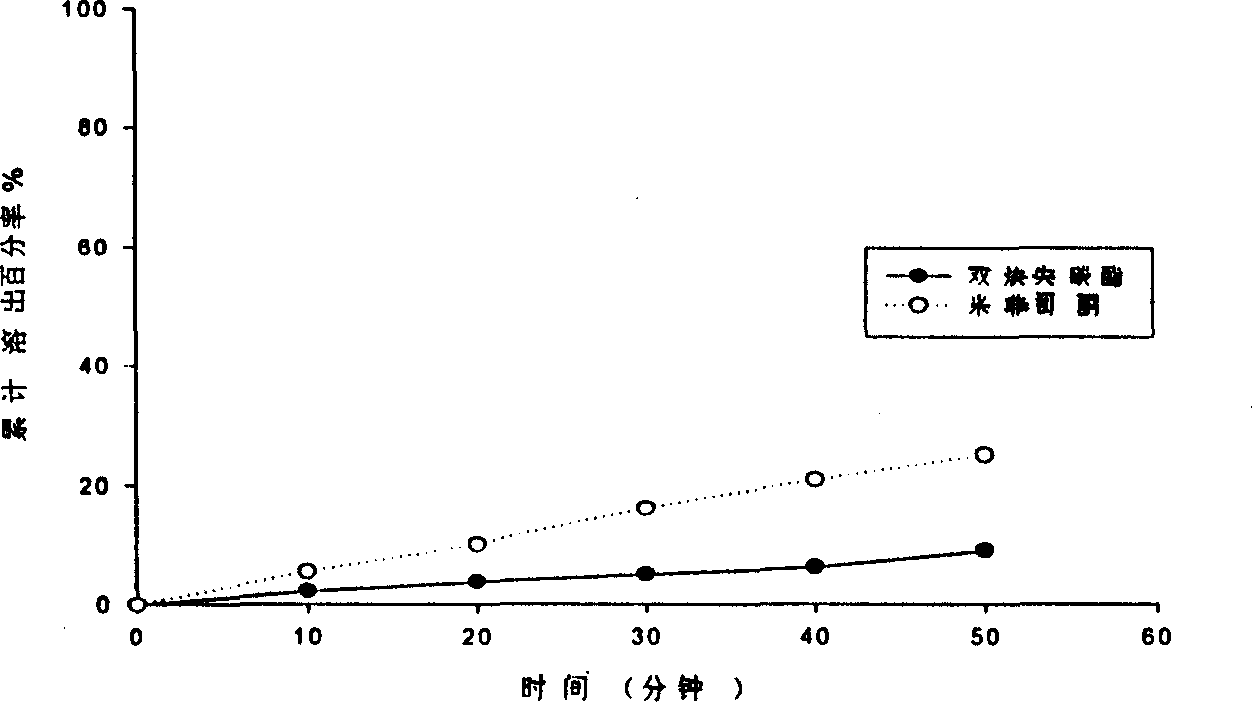
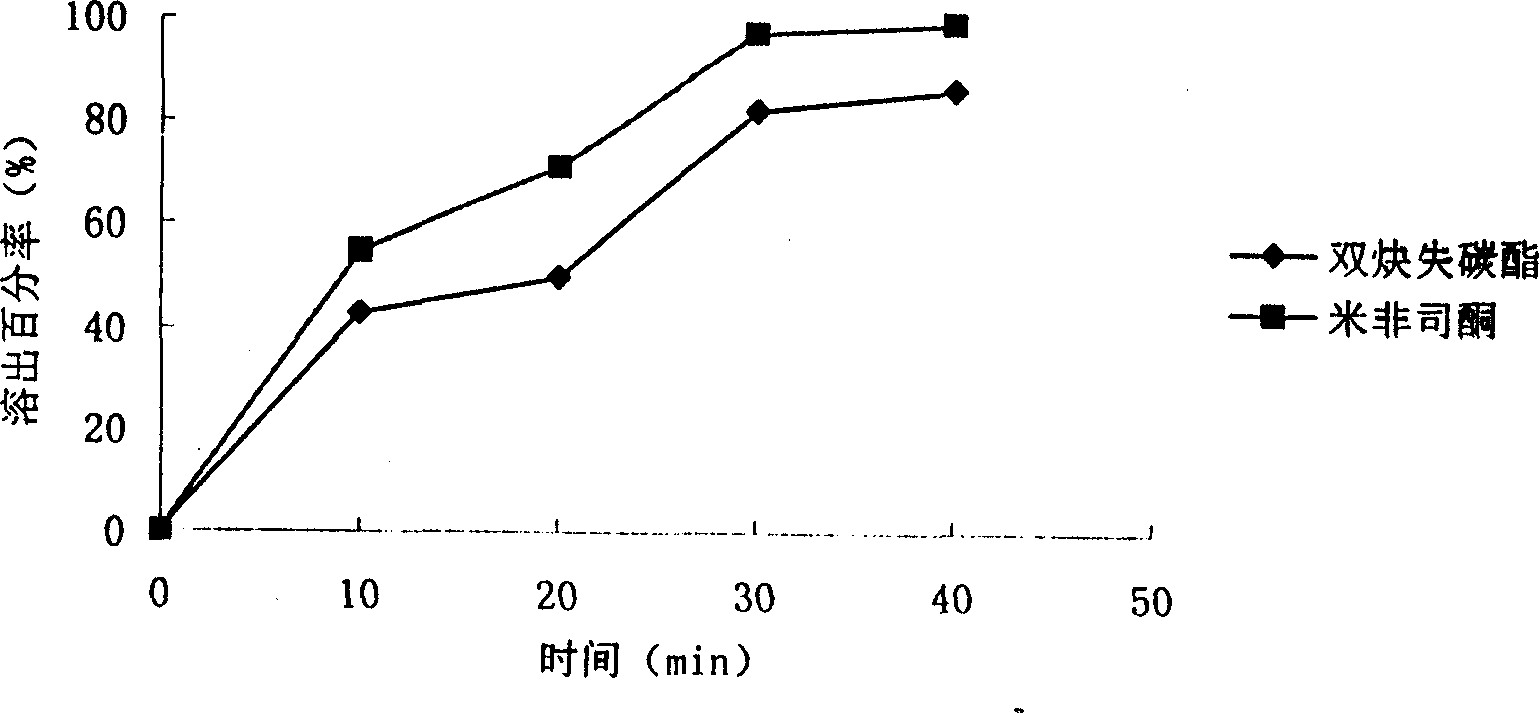
The invention relates to a pharmaceutical preparation of mifepristone and preparation method thereof. The pharmaceutical preparation of mifepristone is prepared by the step of mixing the raw materials as follows according to mass ratio: 5-30 % of mifepristone particles, 10-40% of microcrystalline cellulose, 2-10% of adhesive, 20-40% of starch, 20-40% of dextrin, 05.-2% of lubricant, and 0.5-1% of glidant. The invention can improve dissolution and bioavailability of the mifepristone preparation having obviously increased action against early pregnancy of animals, comparatively simple preparation method, and low manufacturing cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Compositions and means for treating uterine leiomyomata, leiomyoma, myoma, uterine fibroids, endometriosis, adenomyosis and related disorders by mifepristone
The present invention provides a vaginally administrable tablet useful for treating leiomyomata, leiomyoma, myoma, uterine fibroids, endometriosis, adenomyosis and other related disorders. The tablet comprises mifepristone, at least one non-effervescent excipient or diluent, and at least one effervescent excipient.
Owner:LAPIDOT MEDICAL IMPORT & MARKETING
Methods for treating stress disorders using glucocorticoid receptor-specific antagonists
InactiveUS6964953B2Preventing and delaying and lessening emergenceOrganic active ingredientsNervous disorderHydrocortisonePsychiatry
This invention generally pertains to the field of psychiatry. In particular, this invention pertains to the discovery that agents which inhibit the binding of cortisol to its receptors can be used in methods for treating stress disorders. Mifepristone, a potent specific glucocorticoid receptor antagonist, can be used in these methods. The invention also provides a kit for treating stress disorders in a human including a glucocorticoid receptor antagonist and instructional material teaching the indications, dosage and schedule of administration of the glucocorticoid receptor antagonist.
Owner:CORCEPT THERAPEUTICS INC
Use of mifepristone for the treatment of amyotrophic lateral sclerosis
InactiveUS20110166115A1Effective and improved treatmentNervous disorderHydroxy compound active ingredientsAmyotrophic lateral sclerosisHydrocortisone
The invention generally pertains to the discovery that agents capable of inhibiting the binding of cortisol to its receptor can be used in methods for treating patients diagnosed with Amyotrophic Lateral Sclerosis (ALS).
Owner:CORCEPT THERAPEUTICS INC
Optimizing mifepristone levels in plasma serum of patients suffering from mental disorders treatable with glucocorticoid receptor antagonists
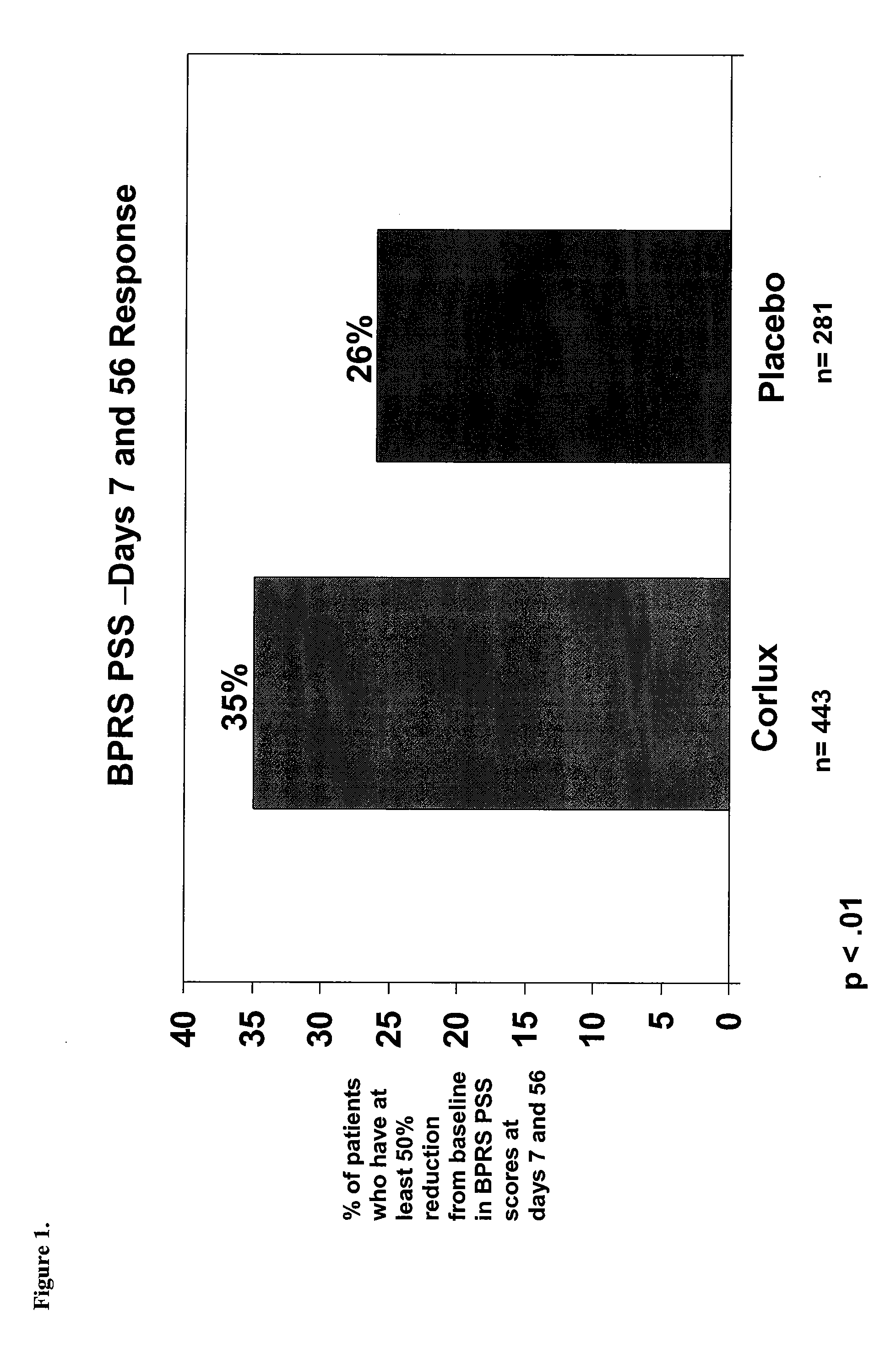
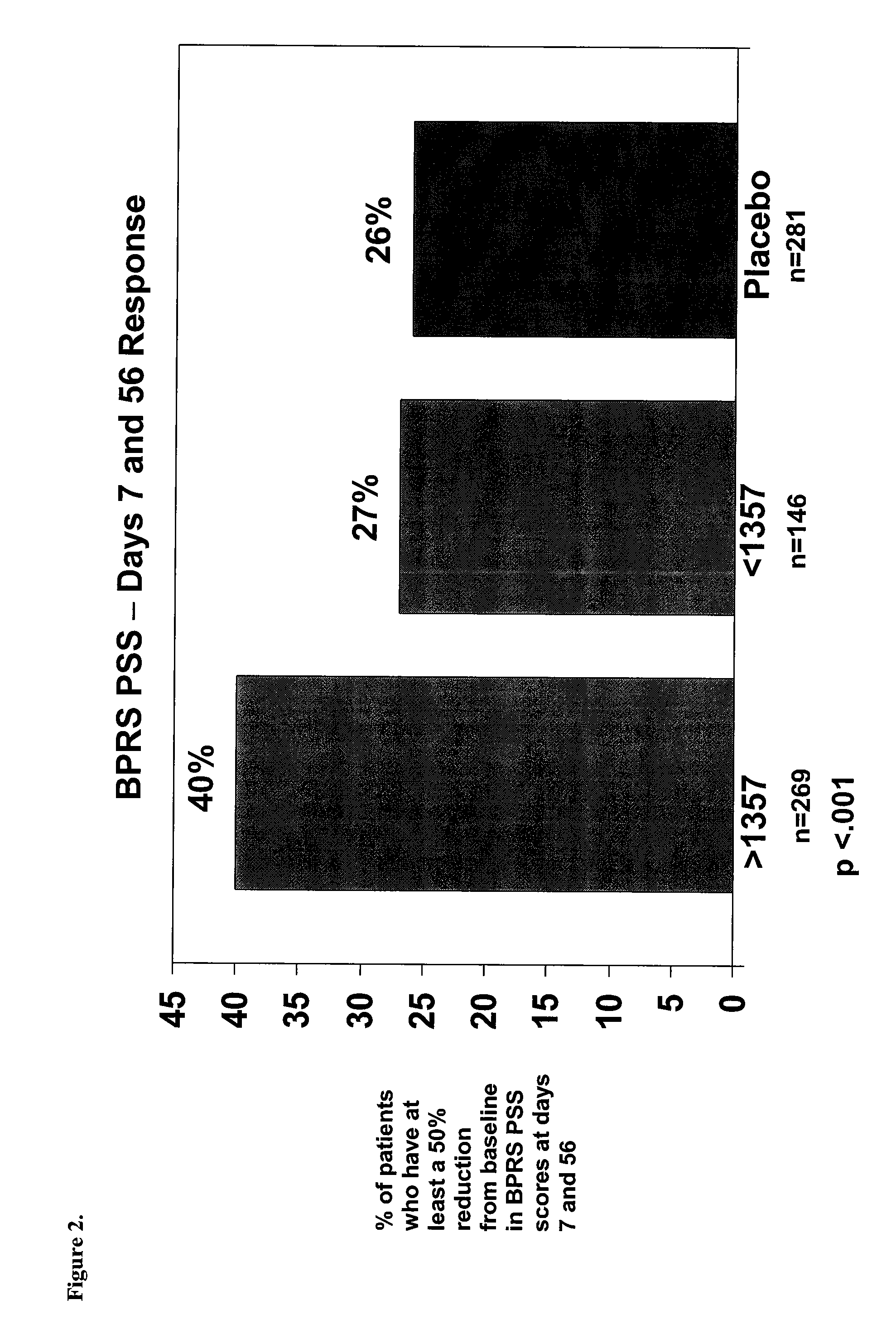
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from a mental disorder amenable to treatment by mifepristone. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1300 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1300 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
Optimizing mifepristone levels for cushing's patents
ActiveUS20160310507A1Good curative effectOrganic active ingredientsDisease diagnosisBlood levelMifepristone
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from Cushing's syndrome. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1631 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1631 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
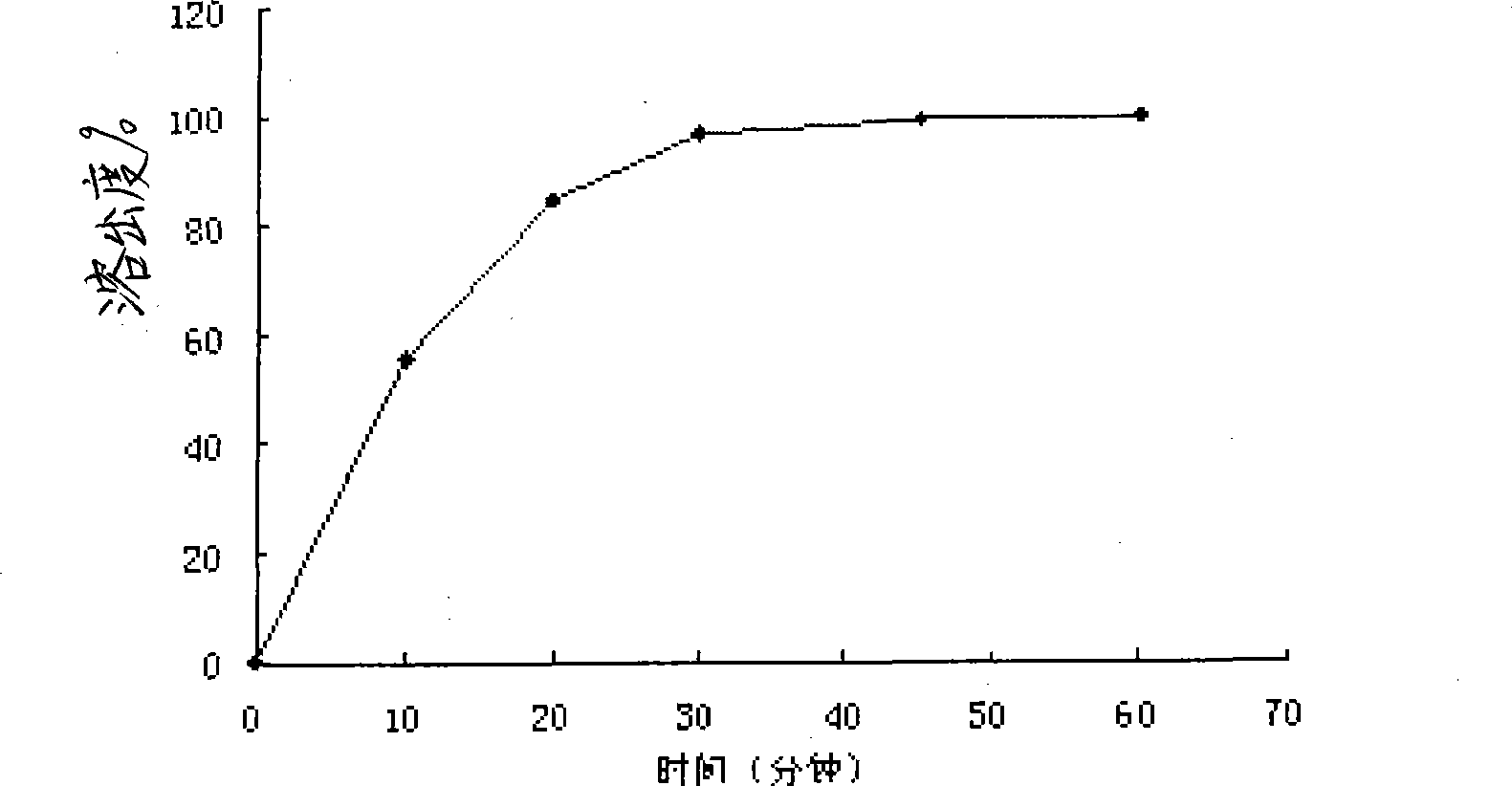
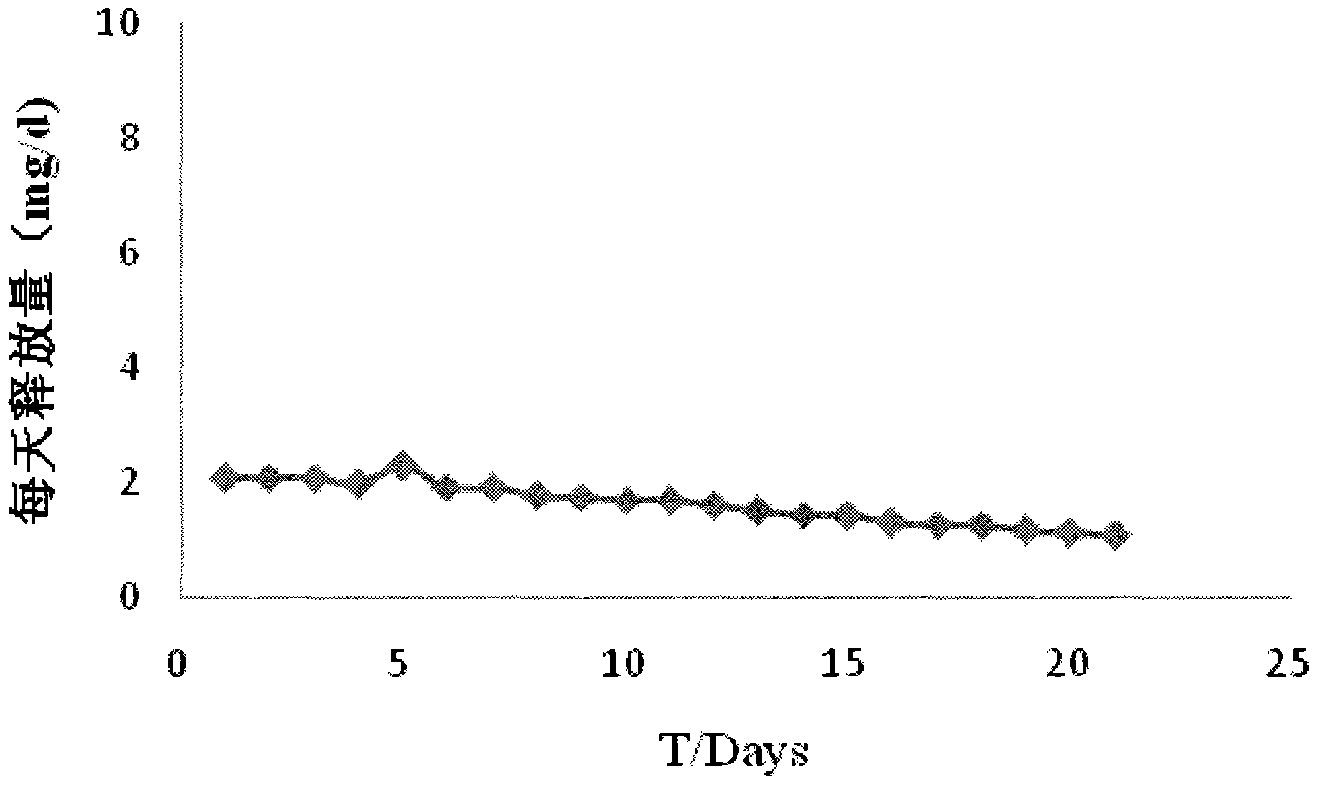
Slow-release mifepristone vaginal ring preparation and application thereof
InactiveCN102600001APromote dissolutionImprove bioavailabilityOrganic active ingredientsFemale contraceptivesPharmacyHepatic first pass effect
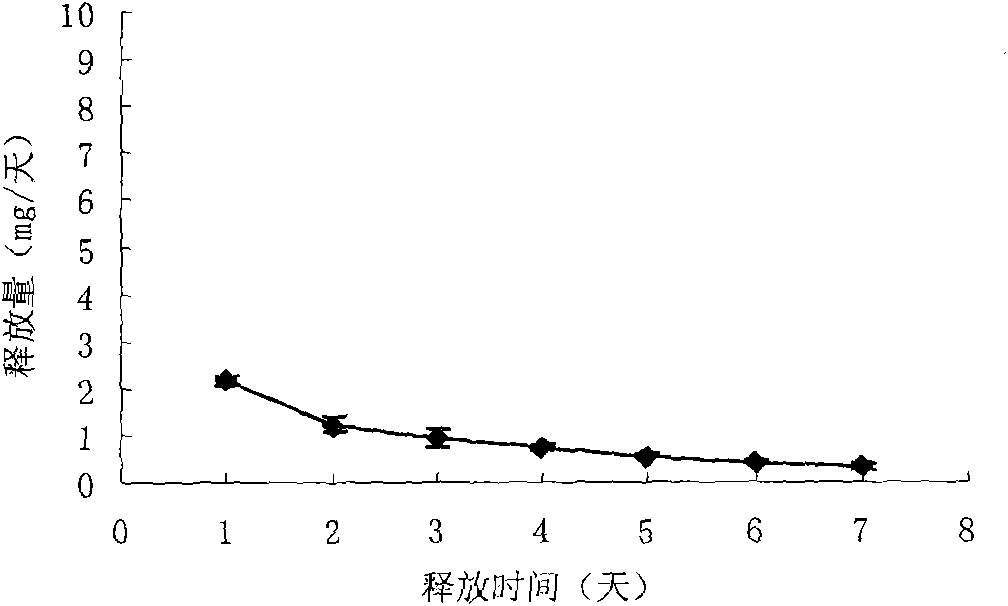
The invention relates to a preparation method for two types of slow-release mifepristone vaginal ring preparations. The first type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant and from 62 to 97.45wt% of medical high polymer release control materials. The second type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant, from 62 to 97.45wt% of medical high polymer release control materials, and a thin release control membrane covered on the outside of each slow-release mifepristone vaginal ring preparation. Due to the preparation method, a slow-release mifepristone vaginal ring can slowly release the mifepristone within seven days, a first pass effect and gastrointestinal reaction of mifepristone orally taken drug are avoided, bioavailability of the drug and pharmacy compliance of a patient are improved.
Owner:NAT RES INST FOR FAMILY PLANNING
Method for measuring residual solvent in bulk drug mifepristone
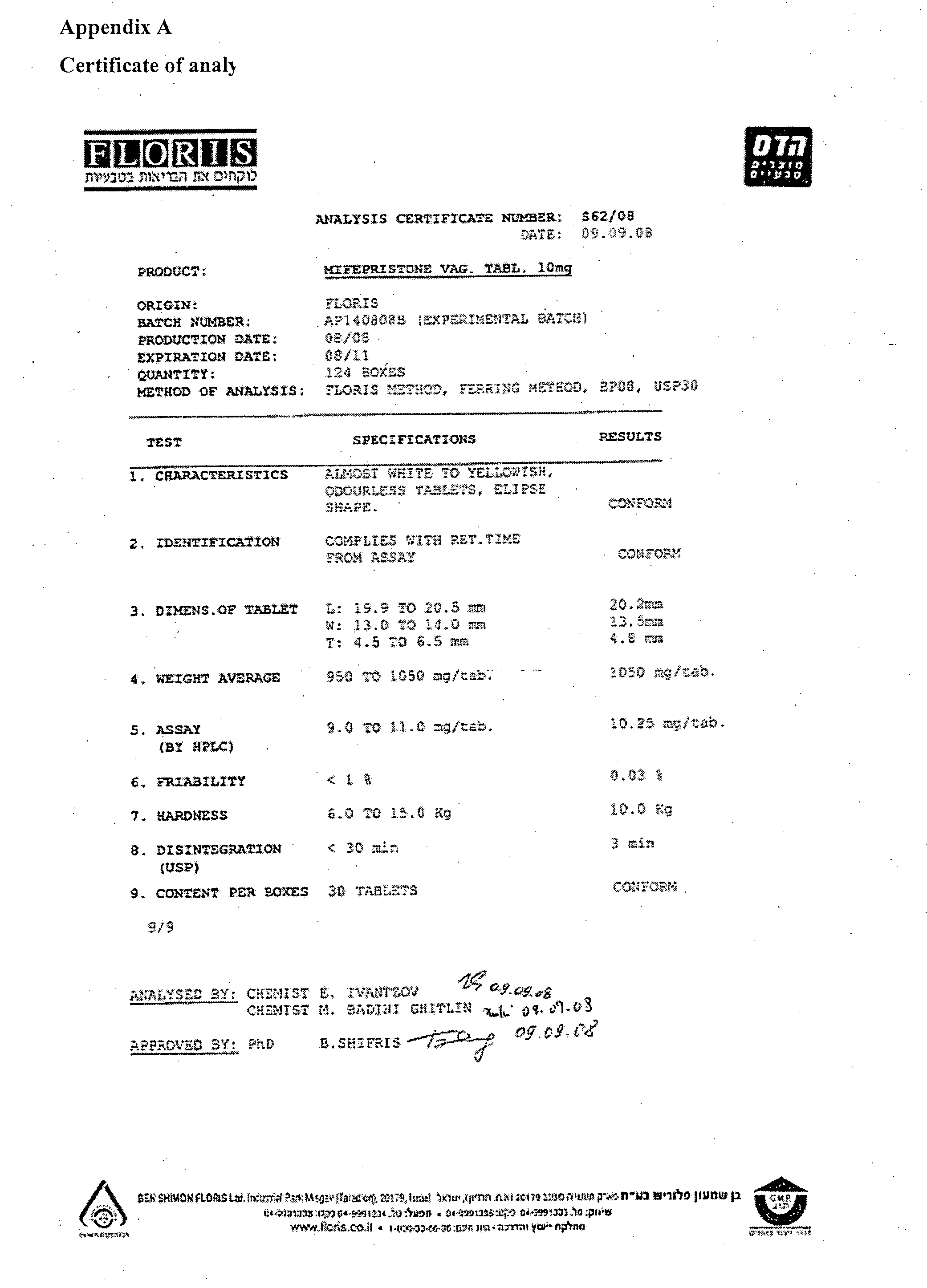
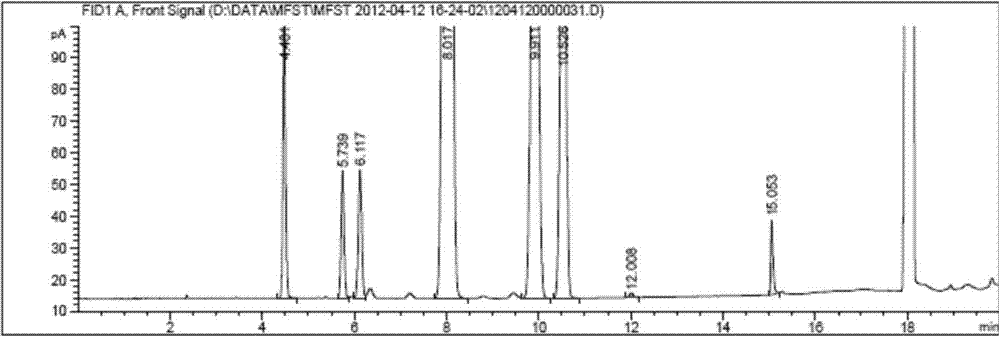
ActiveCN103926359ASimple and fast operationHigh sensitivityComponent separationEthyl acetateTest object
The invention discloses a method for measuring residual solvent in bulk drug mifepristone, which is characterized in that a gas chromatograph method is established, the chromatogram of measured standard substance solutions of tetrahydrofuran, ethanol, isopropyl ether, ethyl acetate, dichloromethane, pyridine, acetonitrile and benzene can be compared with chromatogram of a bulk drug mifepristone tested object solution, and then calculated to complete the detection method of the residual solvent in the bulk drug mifepristone. The measuring method has the advantages of rapid and simple operation, high sensitivity, good repeatability and accurate result, solvents of residual tetrahydrofuran, ethanol, isopropyl ether, ethyl acetate, dichloromethane, pyridine, acetonitrile and benzene in the bulk drug mifepristone can be detected in a quantification mode, the method provides a good reference for controlling the residue of an organic solvent in a bulk drug mifepristone production technology, ensures the quality of the mifepristone bulk drug, increases the security of clinical medication, and provides a method basis for increasing the quality standard of the mifepristone bulk drug.
Owner:SHANGHAI NEW HUALIAN PHARMA
Composition of mifepristone semi-solid skeleton preparation
The composition consists of: mifepristone 0.5-5 wt%, surfactant with HLB greater than 12 1-50 wt%, and semi-solid sleleton carrier 45-98.5 wt%. The composition has good leaching performance, powerful early pregnancy resisting effect, rat Ed50 as high as 17 times that of the marketed tablet and dog's biological utilization as high as 5 times that of the marketed tablet. Central double-blind clinical research shows that the present invention has threefold clinical effect as the marketed tablet. The mifepristone capsule of the present invention has the advantages of small dosage, less negative effect and high biological utilization.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Methods for treating delirium glucocorticoid receptor-specific antagonists
This invention generally pertains to the field of psychiatry. In particular, this invention pertains to the discovery that agents which inhibit the binding of cortisol to its receptors can be used in methods for treating delirium. Mifepristone, a potent specific glucocorticoid receptor antagonist, can be used in these methods. The invention also provides a kit for treating delirium in a human including a glucocorticoid receptor antagonist and instructional material teaching the indications, dosage and schedule of administration of the glucocorticoid receptor antagonist.
Owner:CORCEPT THERAPEUTICS INC
Composition and tablet of mifepristone and anorethidrane dipropionate
The invention relates to a composition and tablet of mifepristone and anorethidrane dipropionate, wherein the compound comprises (by weight ratio) Mifepristone 6 parts, anorethidrane dipropionate 1 part. The tablet provided by the invention comprises the compound of Mifepristone and anorethidrane dipropionate and auxiliary solvent, wherein the Mifepristone and anorethidrane dipropionate have rather high dissolving degree.
Owner:武汉九珑人福药业有限责任公司
Optimizing mifepristone levels for cushing's patients
ActiveUS9943526B2Good curative effectOrganic active ingredientsDisease diagnosisBlood levelMifepristone
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from Cushing's syndrome. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1631 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1631 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
Treatment of Macular Degeneration
InactiveUS20100143346A1Reducing angiogenesisInhibits agonist activityAntibacterial agentsBiocideRetinal neovascularisationOcular disease
An agent having progesterone antagonist properties may be used to treat eye conditions associated with pathological blood vessel formation, for example age-related macular degeneration, choroidal neovascularisation, retinal neovascularisation or corneal neovascularisation. The agent may be mifepristone.
Owner:SUMMIT
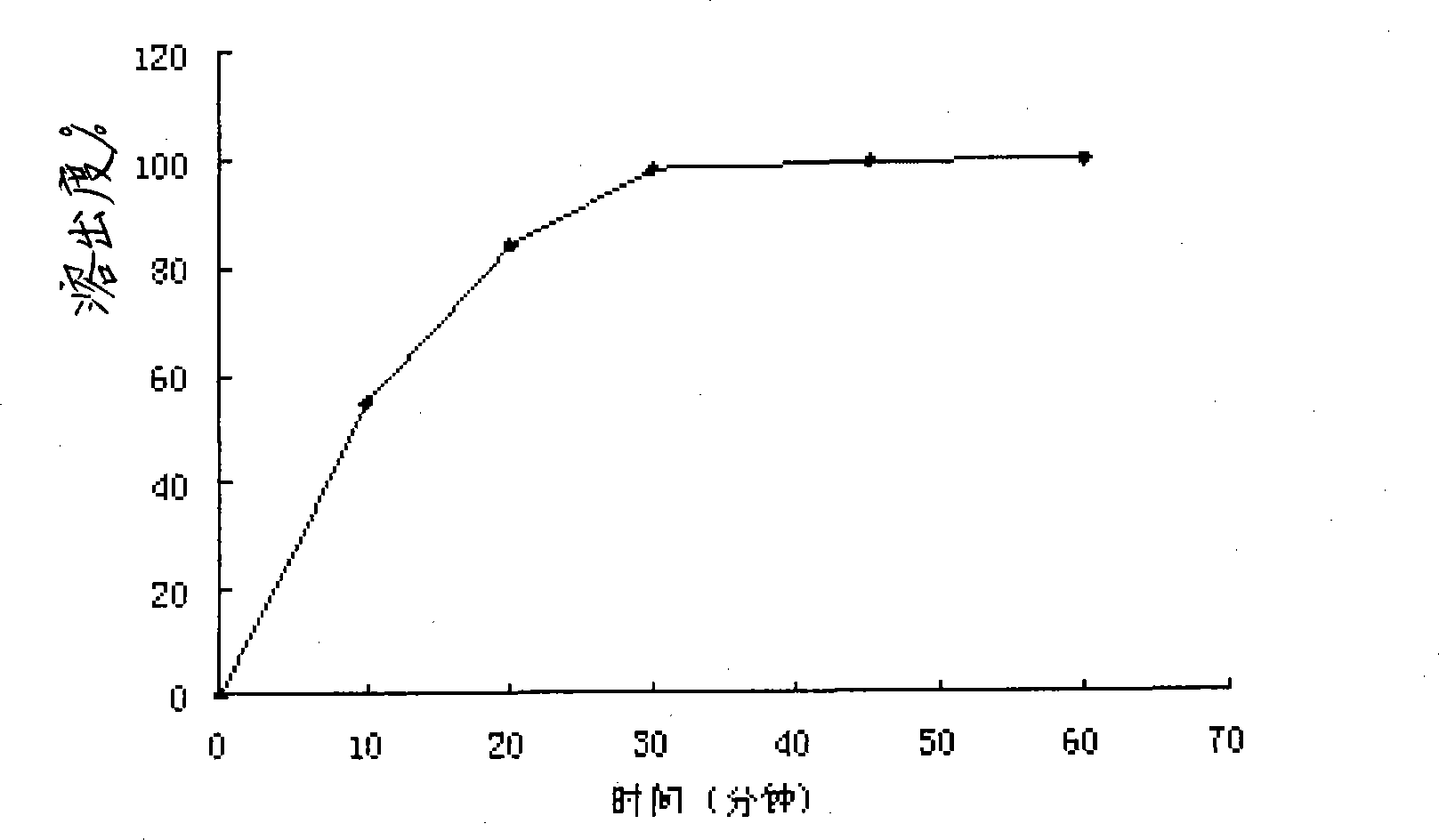
Mifepristone shell-type vaginal ring preparation and application
InactiveCN103505802AImprove solubilityReduce solubilityOrganic active ingredientsSurgeryPhysiologyGYNECOLOGIC DISORDERS
The invention relates to mifepristone shell-type vaginal ring preparation and application. A miferprisone shell-type vaginal ring comprises, by weight, 1.0-6.0% of mifepristone, 0.5-3.0% of medicine dispersion carriers, 0.3-2.0% of additives and 89.0-98.2% of medical polymer controlled release materials. Firstly, a solid dispersion technology is adopted for improving the solubility in water and release degree in vitro of the mifepristone, then a method of mold pressing vulcanization forming is adopted for preparing the mifepristone shell-type vaginal ring, the inner layer is a vacancy silicon rubber skeleton layer, the middle is a medicated layer, and the outmost layer is a controlled release film. By the implementation of the mifepristone shell-type vaginal ring preparation and application, the vaginal ring can release the mifepristone within 21 days at almost constant speed slowly and continuously for a long time, so that the medicine can be released more stably, the first-pass effect and gastrointestinal reaction caused by oral medication of the mifepriston are avoided, and the biological availability of the medicine and medication compliance of a patient are improved. The vaginal ring can be used for routine contraception and treating gynecological diseases such as the endometriosis and the uterine fibroid.
Owner:NAT RES INST FOR FAMILY PLANNING
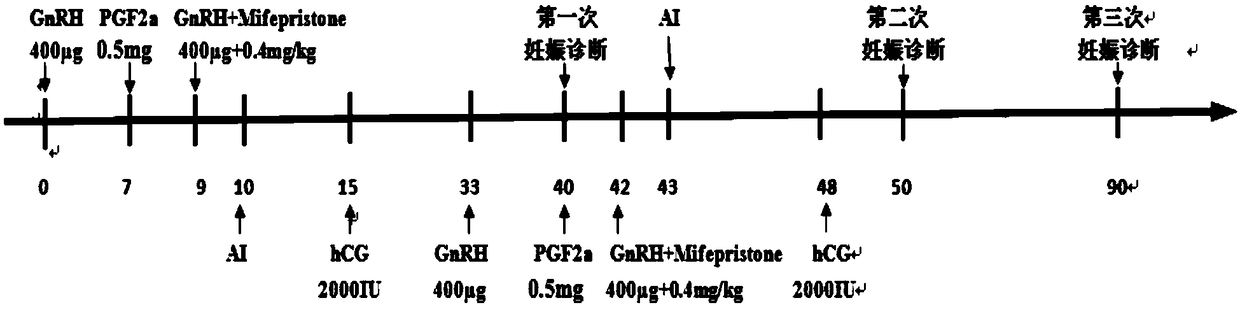
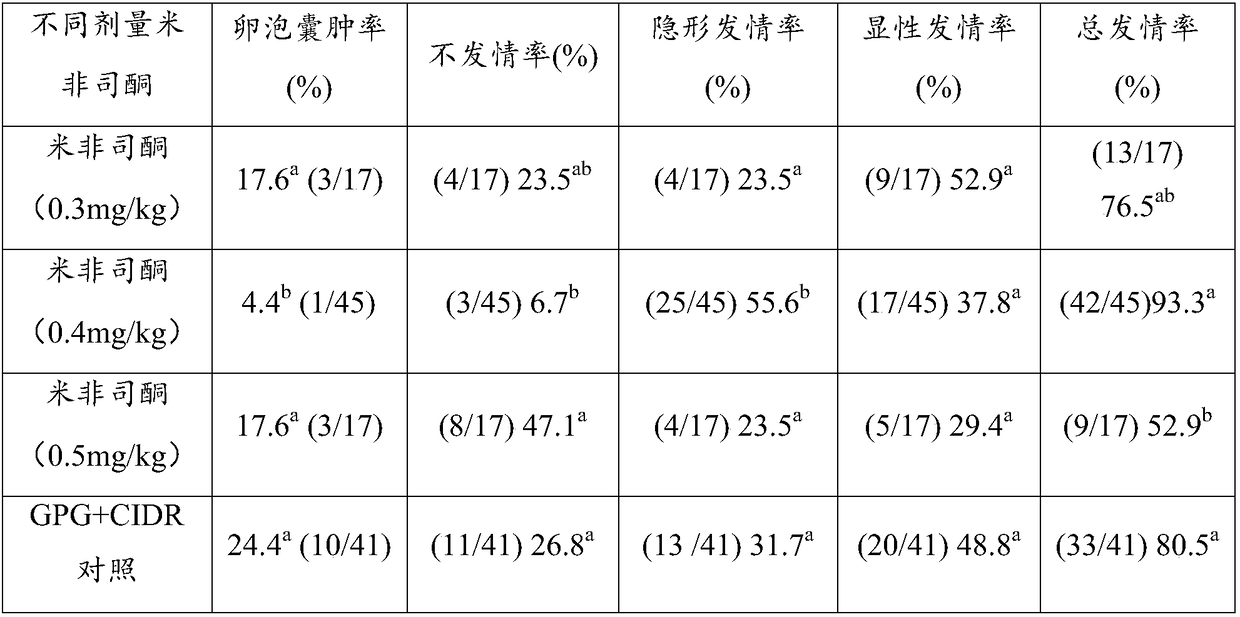
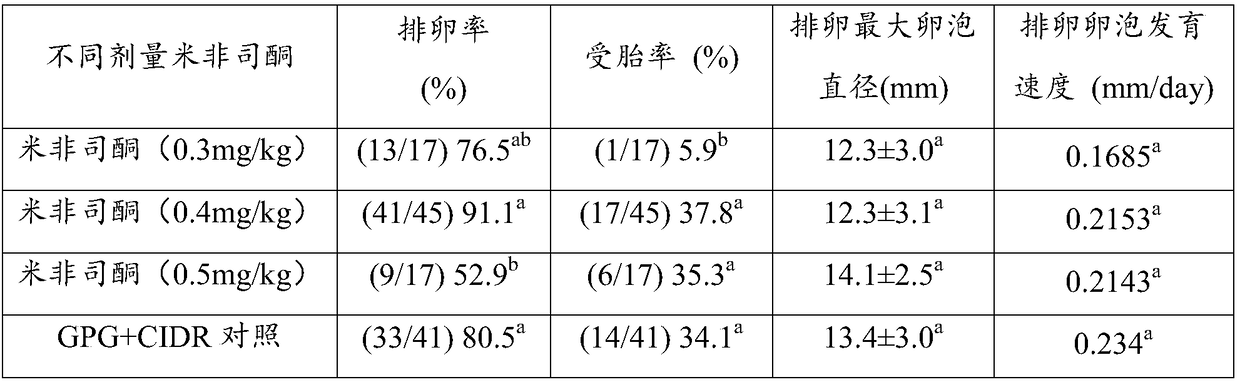
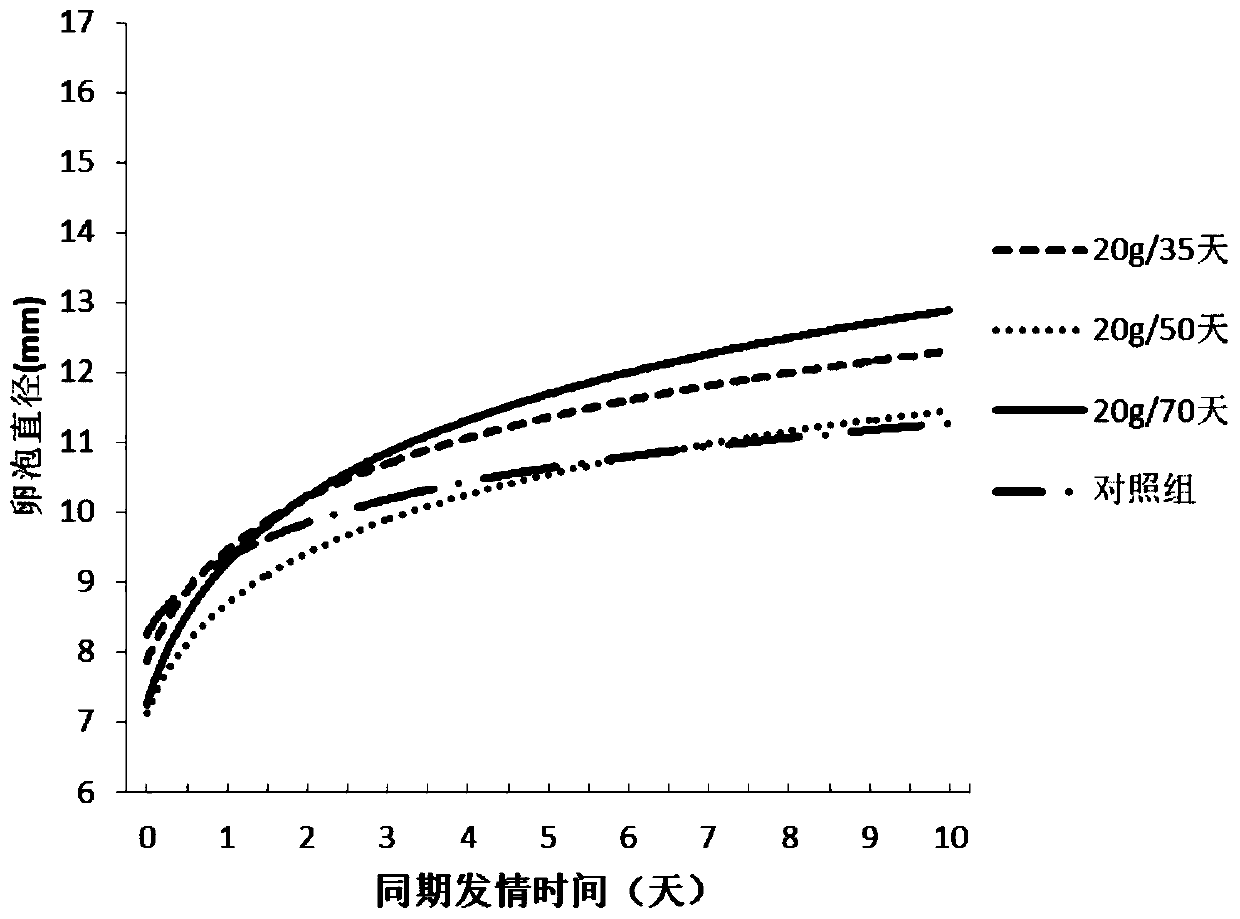
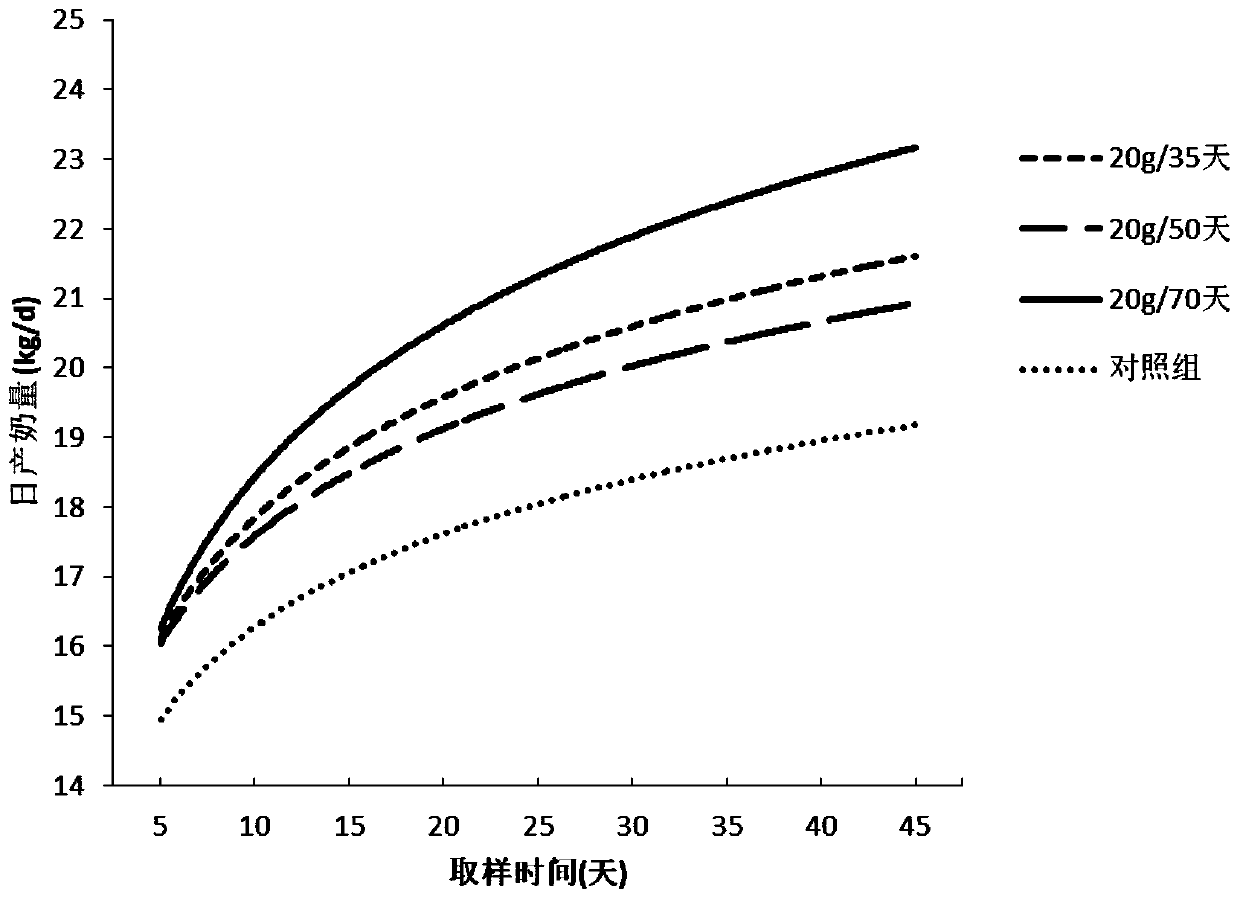
Simultaneous estrus-timed insemination treatment method for hybrid buffaloes
ActiveCN109431647AIncrease estrus rateImprove the breeding rateAnimal reproductionAnimal husbandryEstrus DetectionEmbryo
The invention provides a simultaneous estrus-timed insemination treatment method for hybrid buffaloes. The method comprises the following steps that S1, intramuscular injection of gonadotropin releasing hormones is conducted on one or more healthy cows in the estrous cycle; S2, intramuscular injection of sodium cloprostenol is conducted; S3, gonadotropin releasing hormones are injected for the second time, and mifepristone is simultaneously injected to promote ovulation; S4, 24 hours after the injection of mifepristone, artificial insemination is conducted; S5, 5 days after the artificial insemination, intramuscular injection of human chorionic gonadotropin is conducted. By means of the method, the estrous cycle of the buffaloes is effectively controlled, and the simultaneous estrus rate,breeding rate and conception rate of the hybrid buffaloes are increased; the survival rate of early embryos is effectively increased; estrus detection is not needed, timed insemination is achieved, and the operation is convenient.
Owner:HUAZHONG AGRI UNIV
Cyclodextrin mifepristone clathrate compound, preparation method thereof, and pharmaceutical composition containing clathrate compound
InactiveCN103505740ASolve the problem of water solubilityGood water solubilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityDrug metabolism
The invention discloses a cyclodextrin mifepristone clathrate compound. The clathrate compound takes beta-cyclodextrin as a skeleton to include mifepristone; the ratio of the beta-cyclodextrin to the mifepristone is (1-5):1. Meanwhile, the invention discloses a preparation method of the clathrate compound, and a pharmaceutical composition containing the clathrate compound. The pharmaceutical composition is a solid preparation and comprises the cyclodextrin mifepristone clathrate compound and at least two auxiliary materials. The cyclodextrin mifepristone clathrate compound focuses on solving the problem of water-solubility of the mifepristone; the solubility of the cyclodextrin mifepristone clathrate compound in water is about 30 times of that of pure mifepristone; an oral preparation is prepared from the cyclodextrin mifepristone clathrate compound instead of the traditional mifepristone crude drug. Therefore, the absorption rate of the drug is greatly improved; the curative effect of the drug is greatly improved; the effect of drug metabolism is reduced; the toxic and side effects are reduced; the adaptability of a sufferer on the drug is increased.
Owner:SHANGHAI NEW HUALIAN PHARMA
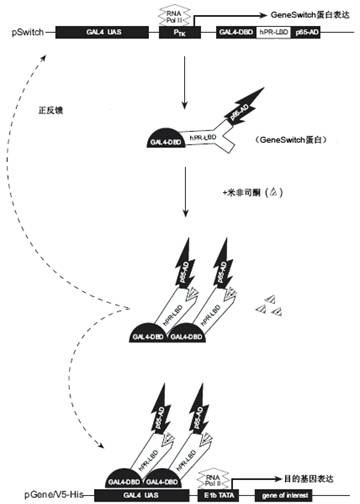
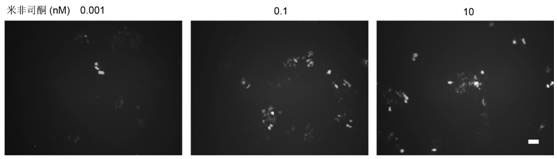
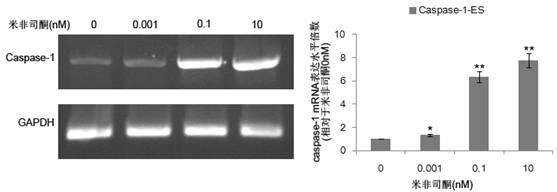
Caspase-1-ES cell line induced by mifepristone
InactiveCN102424818ADoes not affect differentiation potentialReduce the risk of transplantation tumorNervous disorderAntipyreticIntracellularCaspase
The invention relates to a caspase-1-ES cell line induced by mifepristone. The collection number of the caspase-1-ES cell line is CCTCC No.C201193. The invention also provides a preparation method and application of the cell line. The invention has the advantages that: caspase-1 is transferred into an embryonic stem (ES) cell for the first time, and the differential potential of the cell is not influenced; the caspase-1 is highly expressed in the ES cell for the first time, it is proved that activated caspase-1 initiates DNA damage to cause death of undifferentiated caspase-1-ES cells in vitro, and a risk of nodule formation caused by ES cell transplantation is reduced; it is discovered that the highly expressed caspase-1 in the differentiated ES cell does not obviously influence the cell for the first time, and the treatment effect of the ES cell is maintained; and the safety of embryonic stem cell transplantation is improved, the effect of treating parkinson disease and other neurologic diseases is remained, and the caspase-1-ES cell line can be widely applied to stem cell transplantation.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
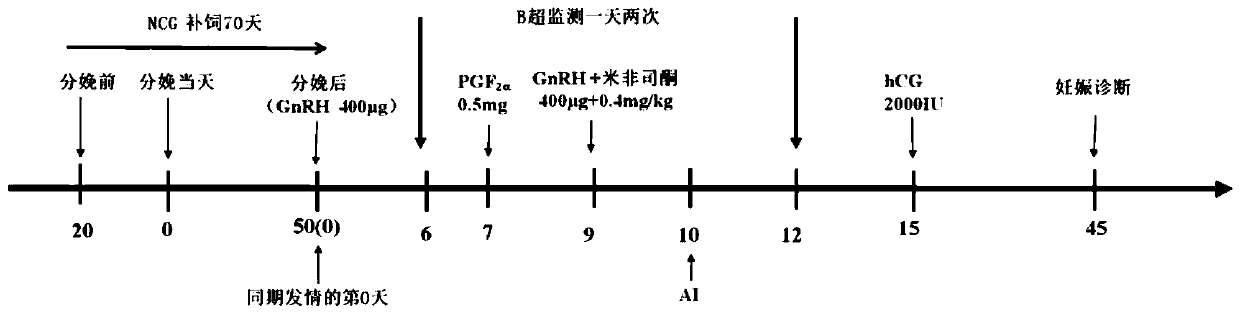
Treatment method for improving effect of synchronous estrus and timing insemination in dairy cows in summer
ActiveCN110368132AIncrease estrus rateImprove the breeding rateAnimal reproductionAccessory food factorsEmbryoB mode ultrasound
The invention provides a treatment method for improving the effect of synchronous estrus and timing insemination in dairy cows in summer. The treatment method includes the steps of S1, adding NCG (N-carbamyl glutamate) to daily ration of the dairy cows for continuous feeding of 70 days from the day 260 of the cows' pregnancy or from 20 days before the expected date of delivery to the post natal period of 50 days; S2, on the 0th day of synchronous estrus treatment, intramuscularly injecting gonadotropin-releasing hormone to the cows of estrous cycle; S3, on the 7th day, intramuscularly injecting PGF2a; S4, on the 9th day, injecting the gonadotropin-releasing homore and meanwhile injecting mifepristone; S5, on the 10th day, carrying out artificial indoctrination; S6, on the 15th day, intramuscularly injecting human chorionic ganodotropin; S7, on the 55th day, determining pregnancy of the cows by B-mode ultrasound. With the treatment method, the estrus rate, the distribution rate and thepregnancy rate of the postpartum dairy cows can be effectively increased, the milk yield and the early embryo survival rate of the dairy cows can be increased, detection of estrus is not needed, timing insemination is achieved, and operation is facilitated.
Owner:HUAZHONG AGRI UNIV
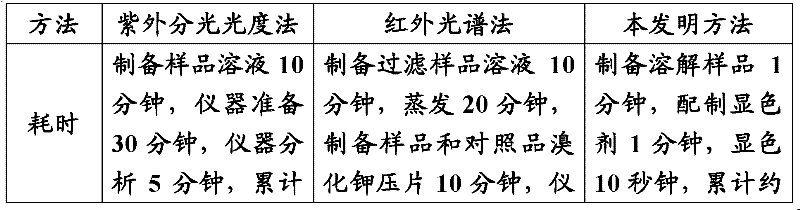
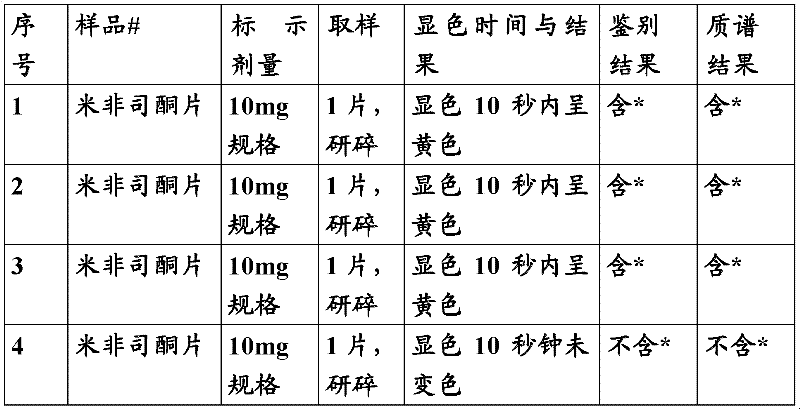
Method for rapidly detecting mifepristone
ActiveCN102565046AHigh speedSimple and fast operationMaterial analysis by observing effect on chemical indicatorAcetic anhydrideColor changes
The invention relates to a method for rapidly detecting mifepristone, in particular to a method for detecting mifepristone in medicines. The method comprises the steps that (1) proper amount of medicine to be detected is mixed with an extraction solvent, the mixture is shaken, and extract is obtained; (2) the extract obtained in the step 1 is mixed with sulfuric acid-acetic anhydride reagent, and mixed liquid is obtained; (3) the color change of the mixed liquid obtained in step 2 is observed, if the color of the mixed liquid suddenly changes to obvious yellow, orange yellow and / or orange red, a judgment is made that the medicine possibly contains mifepristone; and if the color of the mixed liquid has no change, a judgment is made that the medicine does not contain mifepristone. The method has the characteristics of accuracy, simplicity, rapidness, low specialty requirement and the like.
Owner:BEIJING INST FOR DRUG CONTROL
Methods for treating delirium using glucocorticoid receptor-specific antagonists
ActiveUS20060194713A1Effective for symptomEffective treatmentBiocideNervous disorderHydrocortisoneAcute confusional states
This invention generally pertains to the field of psychiatry. In particular, this invention pertains to the discovery that agents which inhibit the binding of cortisol to its receptors can be used in methods for treating delirium. Mifepristone, a potent specific glucocorticoid receptor antagonist, can be used in these methods. The invention also provides a kit for treating delirium in a human including a glucocorticoid receptor antagonist and instructional material teaching the indications, dosage and schedule of administration of the glucocorticoid receptor antagonist
Owner:INFINEON TECH AG
Traditional Chinese medicine for clearing embryonic tissues and preparation method and application thereof
InactiveCN104288697AEasy to shrinkEasy dischargeOrganic active ingredientsAnthropod material medical ingredientsBleeding timePollen
The invention relates to a traditional Chinese medicine for clearing embryonic tissues and a preparation method and an application thereof. The traditional Chinese medicine is made from the following raw material medicines in parts by weight: 9-22 parts of ground beeltle, 12-27 parts of leonurus, 4-14 parts of rhizoma sparganii, 4.2-14.5 parts of curcuma zedoary, 6-13.7 parts of peach kernel, 5-16.4 parts of safflower, 1.3-5.8 parts of rheum officinale, 1.2-8 parts of pollen typhae, 6-16 parts of red peony root, 8-24.5 parts of radices trichosanthis, 5-13 parts of corydalis tuber, 5.1-17 parts of radix clematidis, 5-13 parts of talc, 5-15.8 parts of rhizoma alismatis, 5-18.6 parts of angelica and 5-12 parts of radix cyathulae. The traditional Chinese medicine provided by the invention has the function of removing blood stasis, and thus is capable of clearing placenta tissues left in a uterus and promoting the uterus to contract, and the traditional Chinese medicine is singly used or cooperatively used with mifepristone to obviously accelerate the discharge of the fertilized egg, obviously reduce colporrhagia, shorten the bleeding time and obviously improve the clearing rate of embryonic residues in the uterine cavity.
Owner:SHANDONG PROVINCIAL HOSPITAL
Use of mifepristone for the treatment of amyotrophic lateral sclerosis
InactiveUS20140005158A1Nervous disorderHydroxy compound active ingredientsAmyotrophic lateral sclerosisHydrocortisone
The invention generally pertains to the discovery that agents capable of inhibiting the binding of cortisol to its receptor can be used in methods for treating patients diagnosed with Amyotrophic Lateral Sclerosis (ALS).
Owner:CORCEPT THERAPEUTICS INC
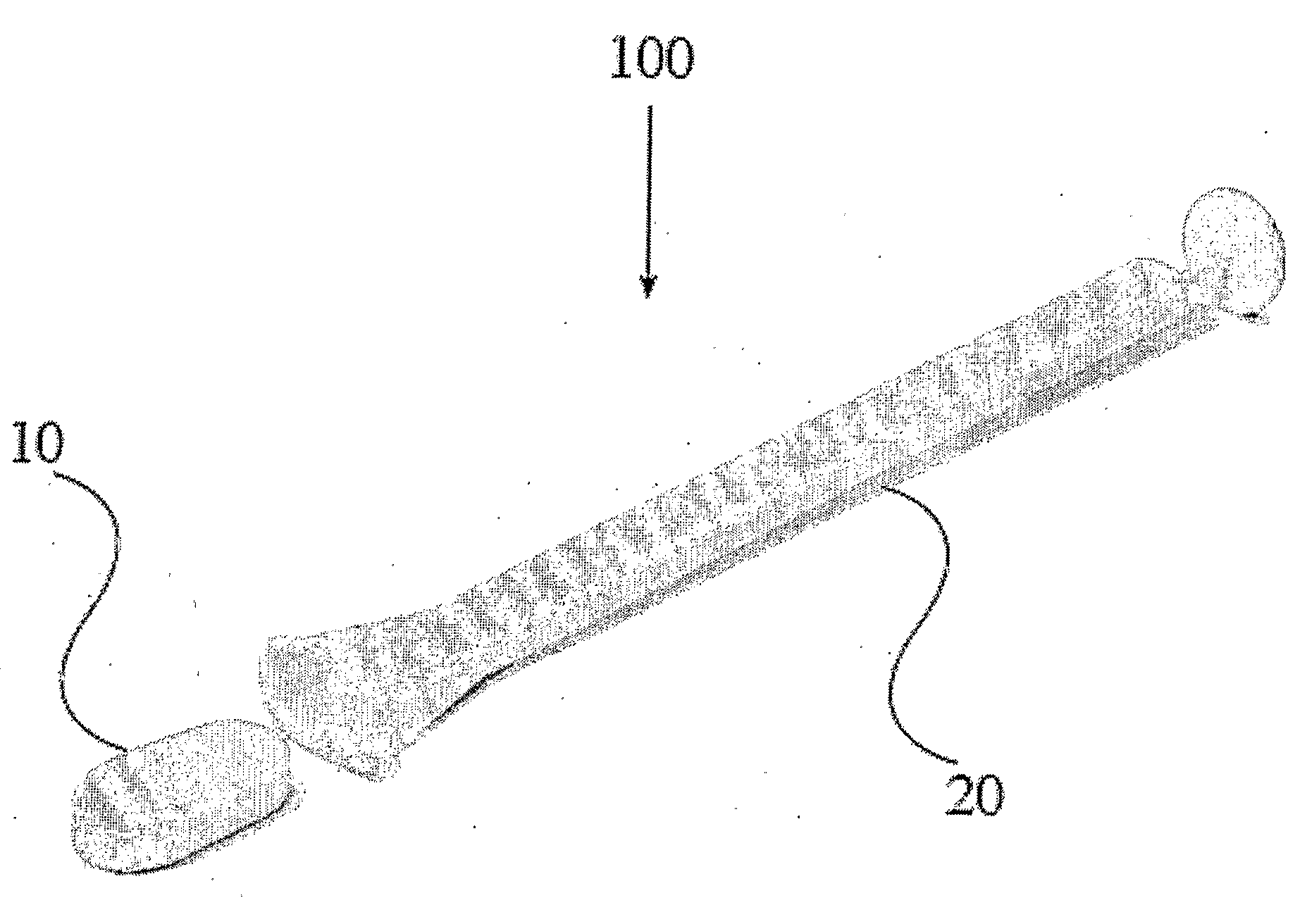
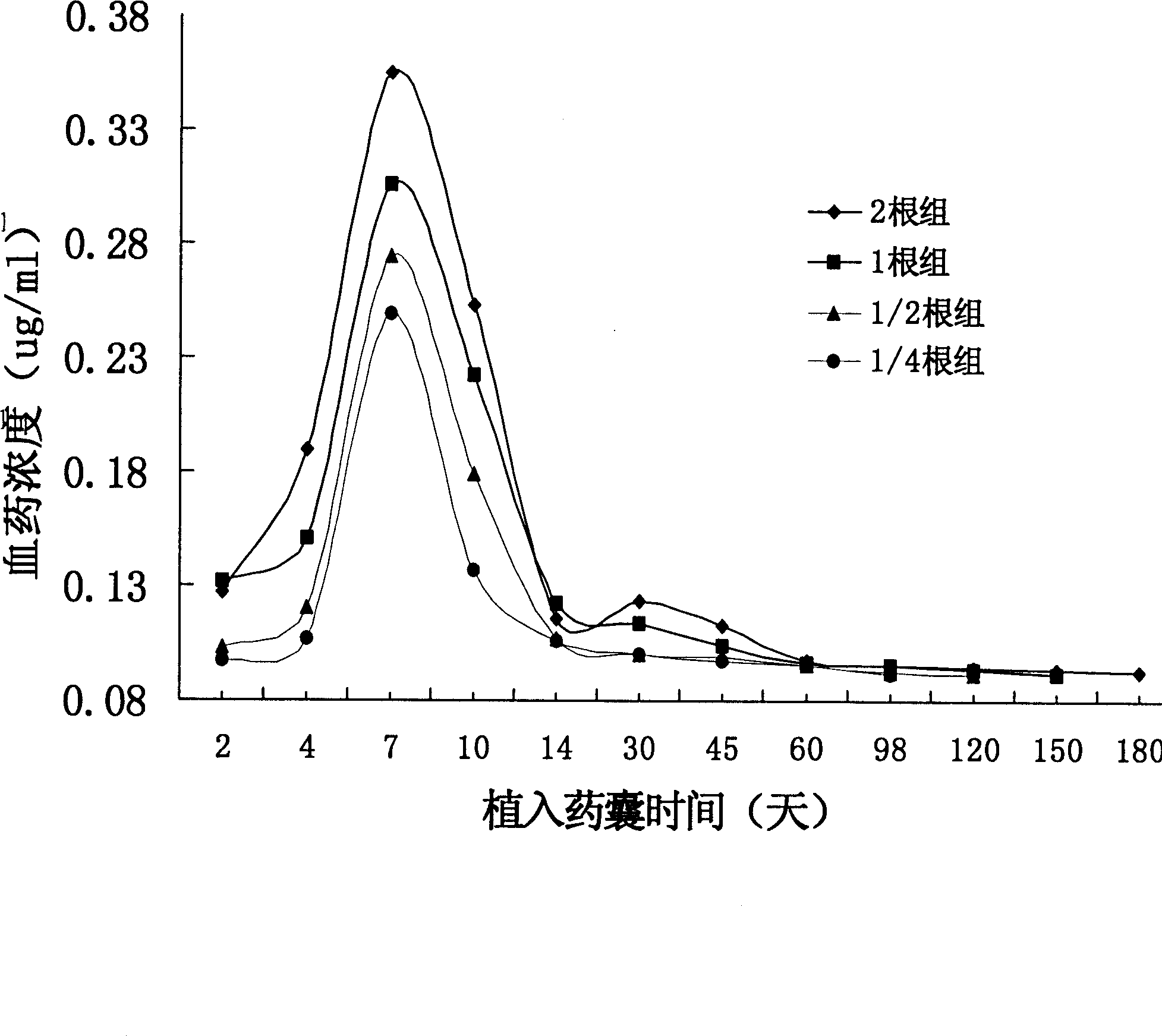
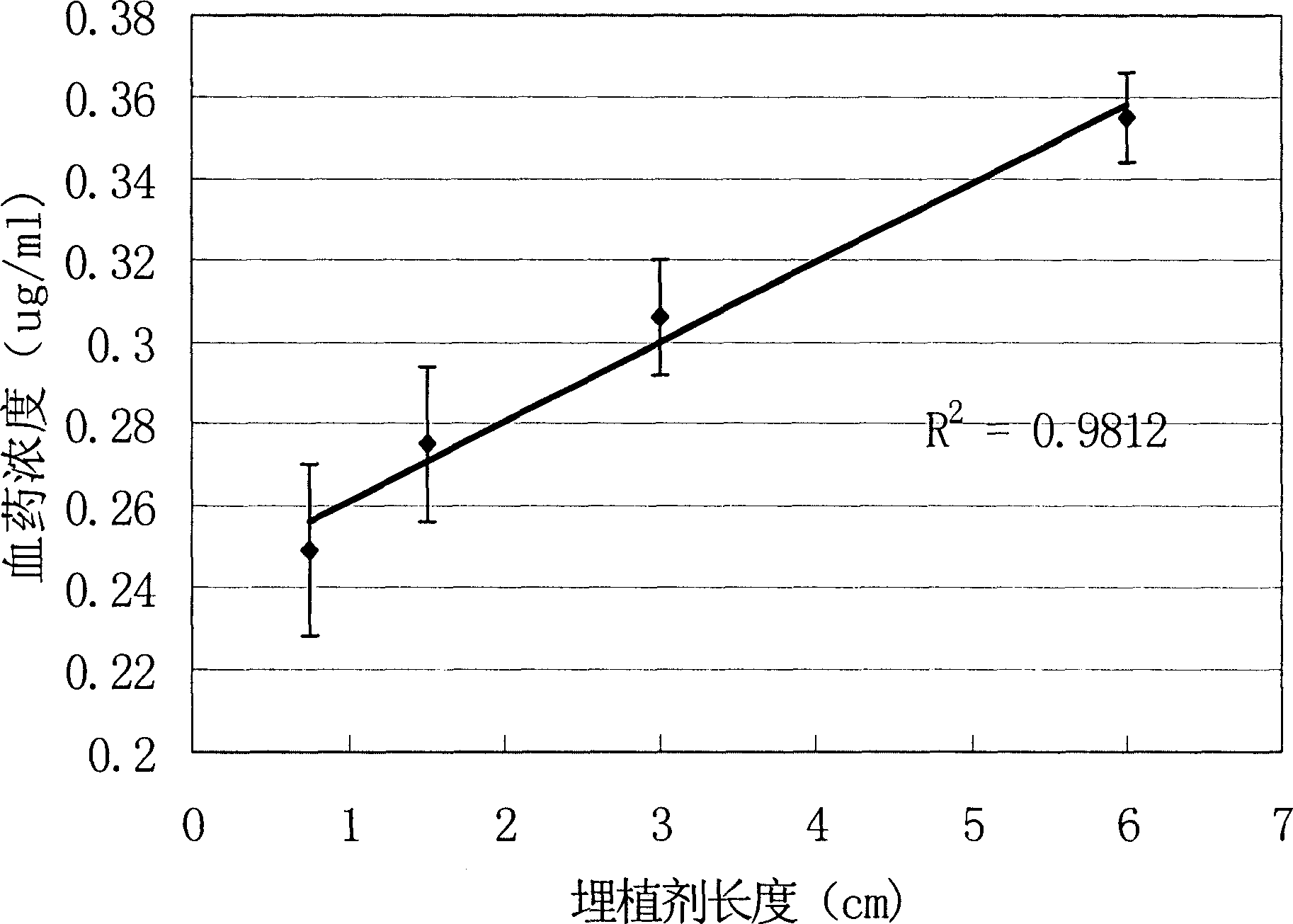
Subcutaneous implant of mifespristone and use thereof in treatment of endometriosis
InactiveCN1864689ALPain reliefOrganic active ingredientsPharmaceutical delivery mechanismEndometriosisSubcutaneous implant
The present invention provides one kind of subdermal embedded mifepristone preparation containing mifepristone in effective treating amount and mifepristone holding sac. The sac has pharmaceutically acceptable polymer as basic material and dispersed pore creating agent, and the polymer may be polylactic acid, polylactide, polylactic acid-hydroxyacetic acid, polycaprolactone, etc. and is preferably polycaprolactone. The present invention also provides the use of the subdermal embedded mifepristone preparation in preparing medicine for treating endometriosis.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
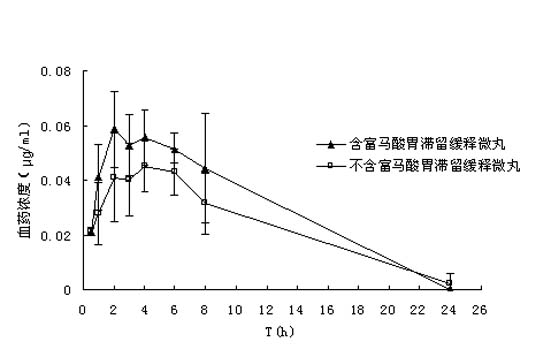
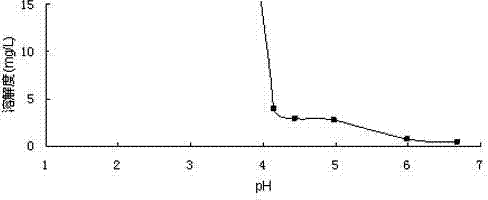
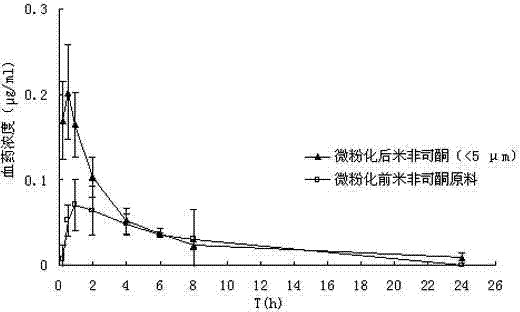
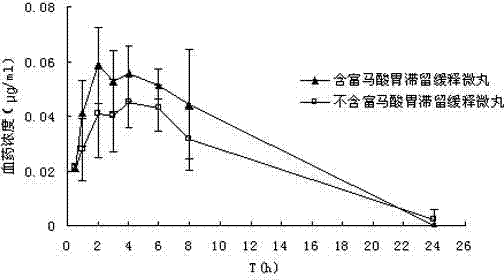
Mifepristone slow release preparation characterized by gastric stasis and preparation method thereof
ActiveCN102048681AImprove bioavailabilityControl for individual differencesOrganic active ingredientsNervous disorderEnhanced bioavailabilitySustained-Release Preparations
The invention relates to a mifepristone slow release preparation characterized by gastric stasis and a preparation method thereof. The preparation comprises a quick release mifepristone system and a slow release mifepristone cellular system. The quick release mifepristone system accounts for 10-80 wt% of the total weight and the balance is the slow release mifepristone celluar system. The slow release preparation consists of micronized mifepristone, solubilizer, pH regulator, diluent, bond, slow release material and adhesion material. The experimental results show that the bioavailability of the mifepristone slow release preparation characterized by gastric stasis is increased by more than 50% in comparison with the bioavailability of common mifepristone tablets, and the individual difference is obviously reduced. Therefore, the mifepristone slow release preparation characterized by gastric stasis can enhance the bioavailability and improve the individual difference so that the dosage reduction is possible.
Owner:REGENEX PHARMA LTD
Preparation method of radix arnebiae seu lithospermi phenolic acids, adjuvant resistant to early pregnancy
InactiveCN102973631AIncrease contractilityIncreased complete miscarriage rateSexual disorderPlant ingredientsComplete abortionAdjuvant
The invention discloses a preparation method of lithospermum phenolic acids, adjuvant resistant to early pregnancy. The method comprises the steps of smashing natural lithospermum, extracting for two times (1.5 hours for each time) through water, concentrating extracted solution in decompression mode, enabling the extracted solution to go through macroporous resin, washing the extracted solution with ethanol to be colorless, eluting with the ethanol, collecting, continuously concentrating, washing concentrate in MCI gel CHP20P column stilled water until occurrence of chemical compound dipotassium salt, eluting with the ethanol, collecting the chemical compound dipotassium salt and 30% ethanol eluting part, and obtaining eluant of lithospermum phenolic acids effective parts. The lithospermum phenolic acids made in the preparation method can directly kill chorionic villus cells, obviously enhances contractility of ingravidation uterus muscles, improves complete abortion rates of mifepristone, and is effective adjuvant resistant to early pregnancy.
Owner:武汉康鸿达科技股份有限公司
Mifepristone slow release preparation characterized by gastric stasis and preparation method thereof
ActiveCN102048681BImprove bioavailabilityControl for individual differencesOrganic active ingredientsNervous disorderDiluentDose reduction
Owner:REGENEX PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com