Patents
Literature
76 results about "Dose reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dose reduction is a challenging process that requires a careful balancing of concerns. In general, higher radiation results in a better image. Too little radiation, therefore, can introduce image noise, leading to an inferior image that may threaten the radiologist's ability to make an accurate diagnosis.
Multiparticulate modified release composition
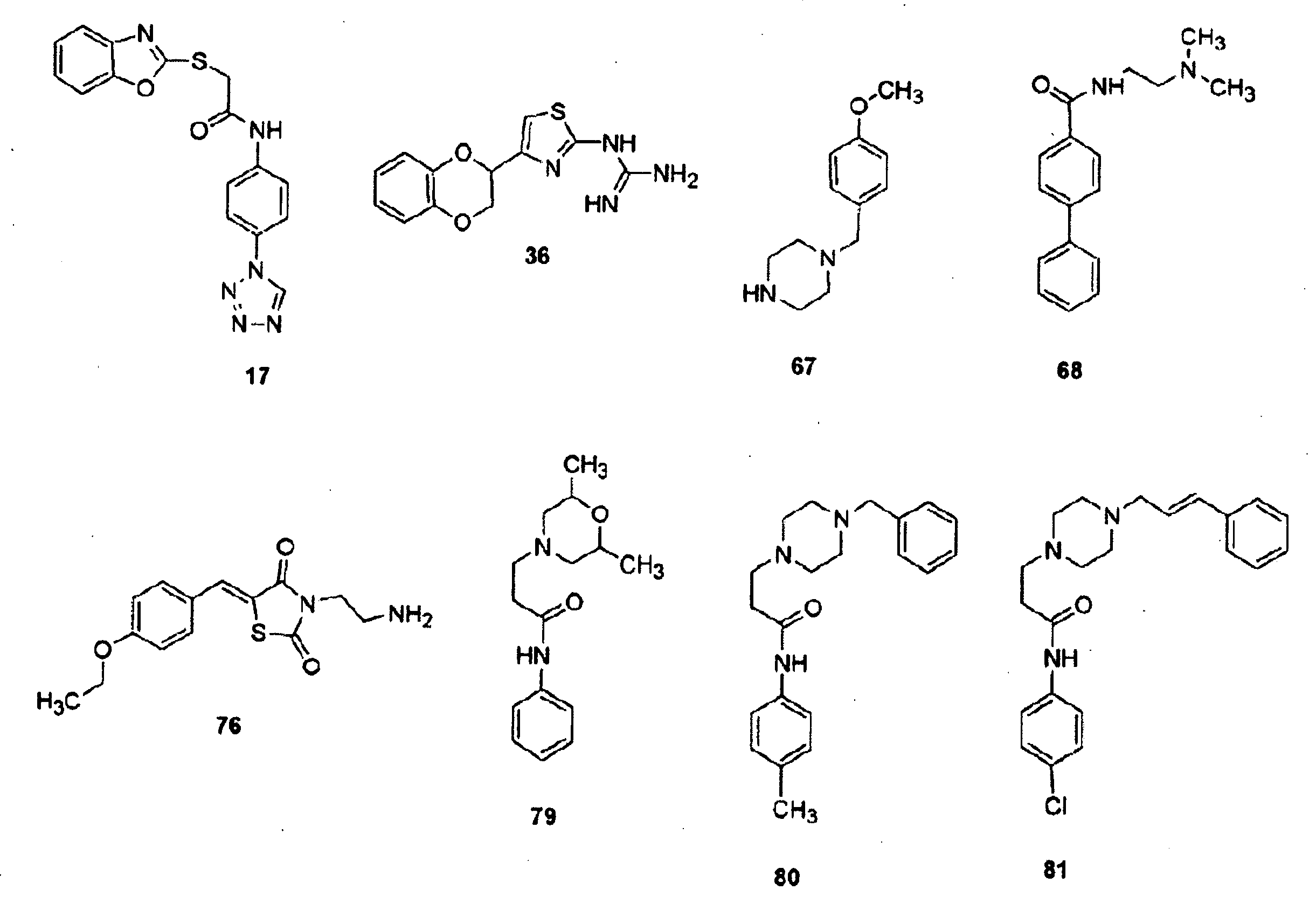
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
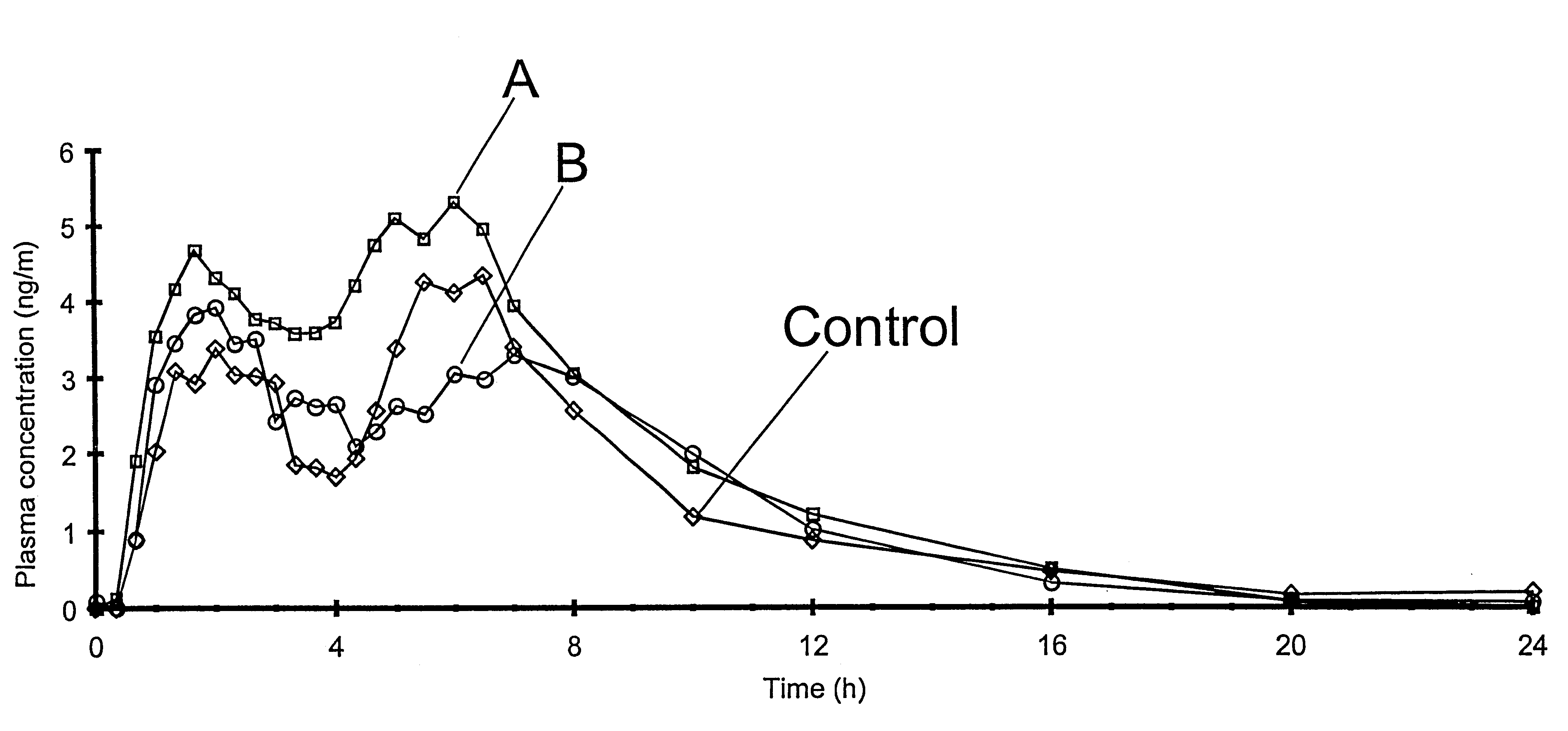
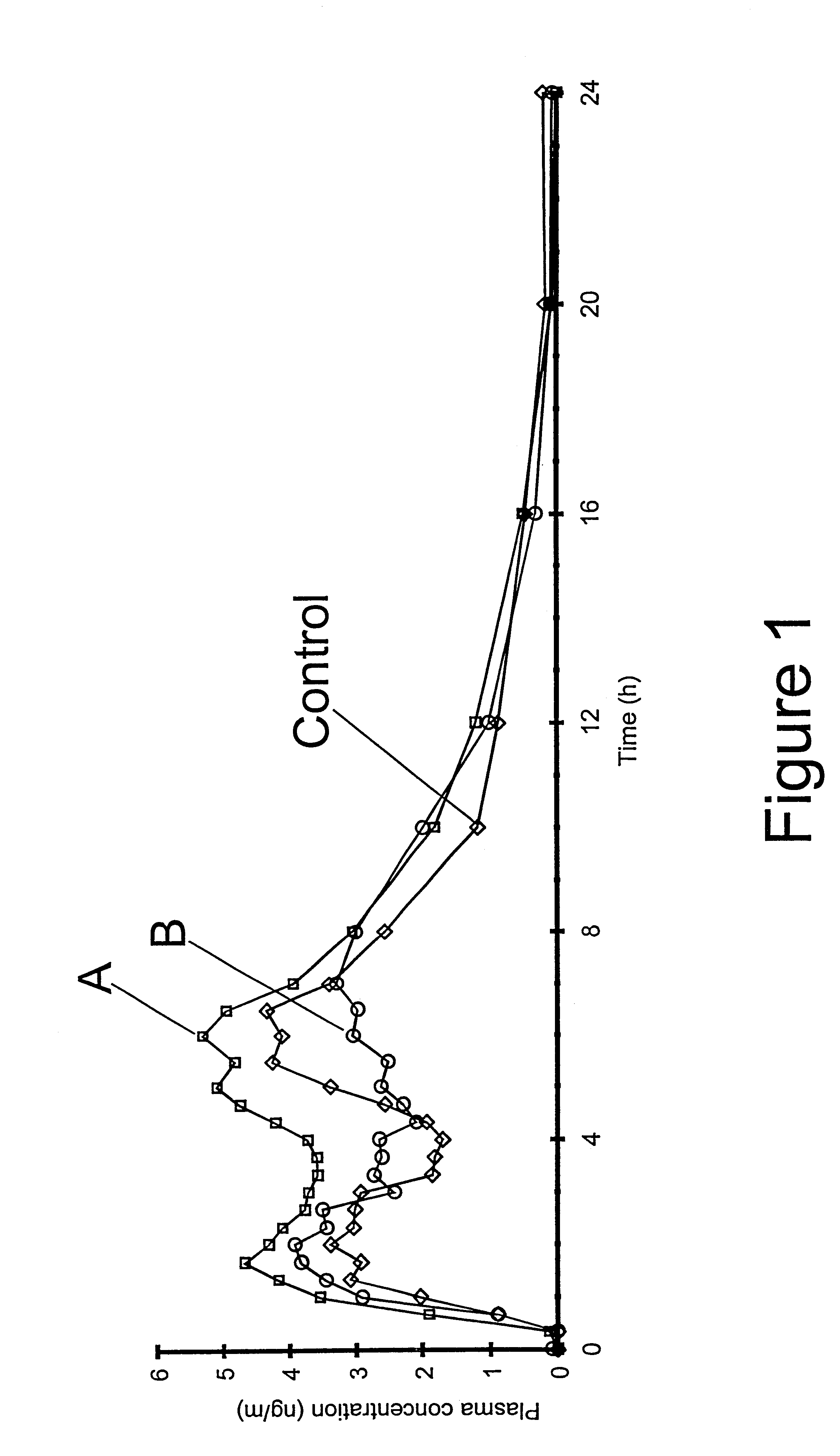
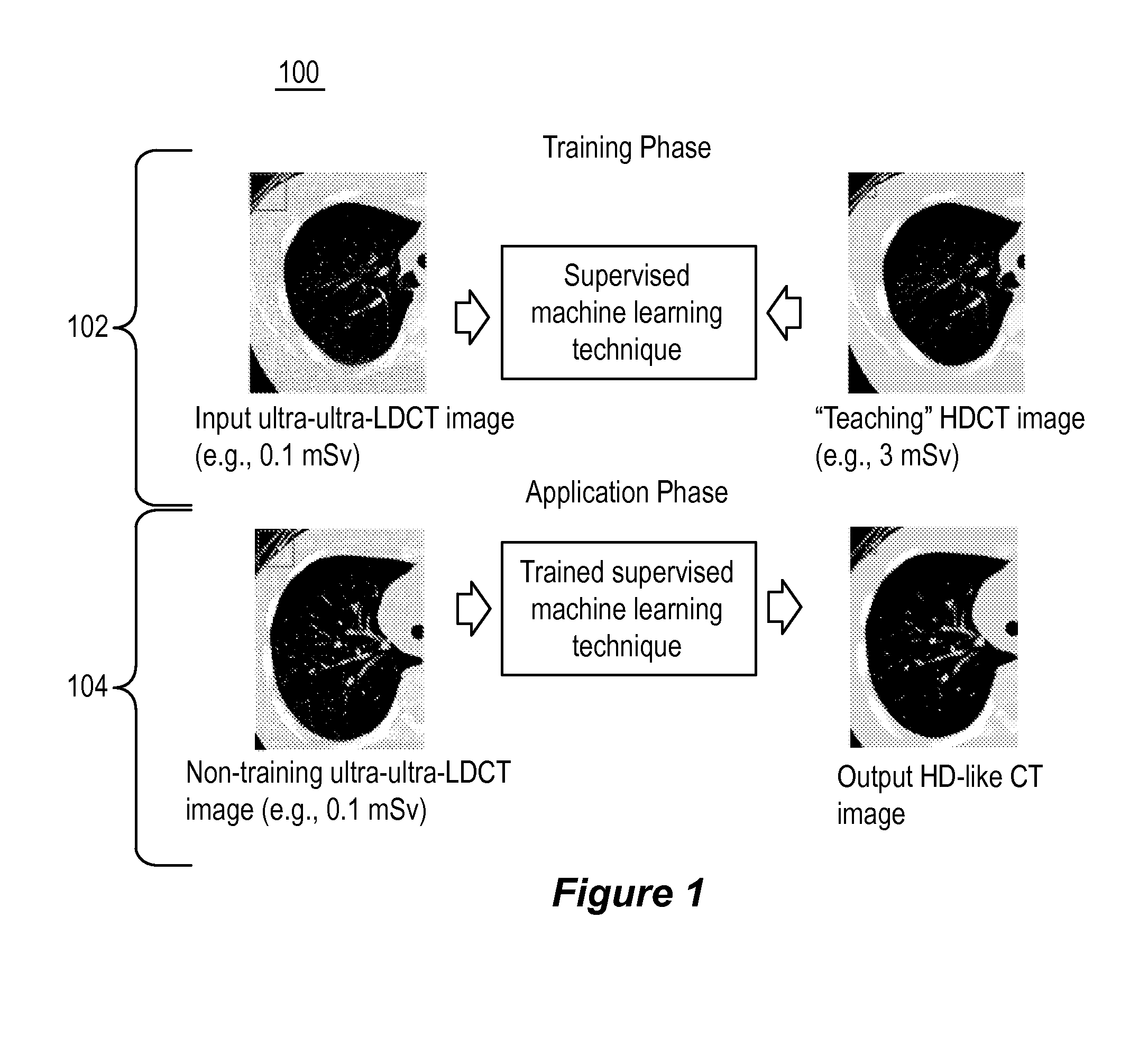
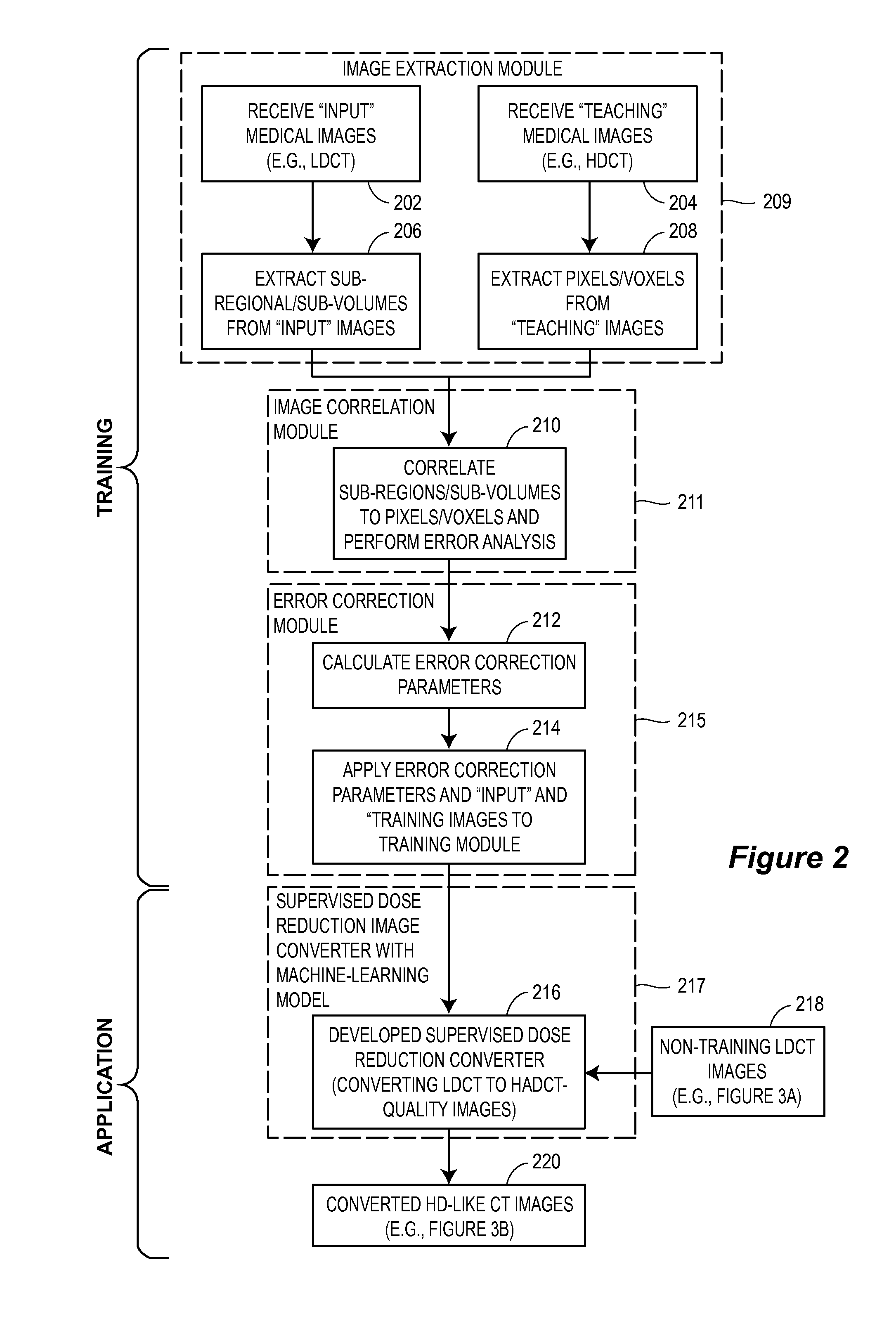
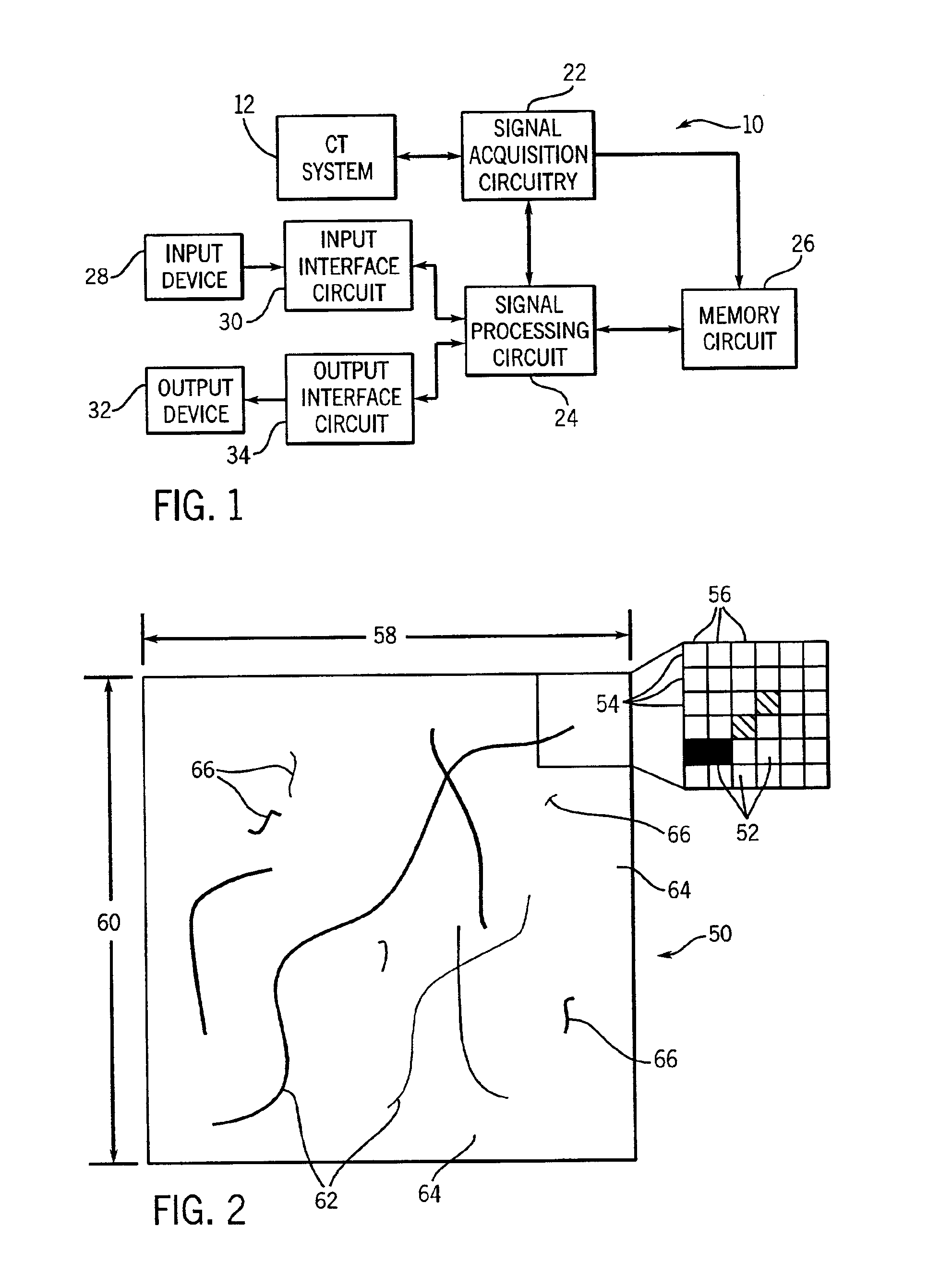
Supervised machine learning technique for reduction of radiation dose in computed tomography imaging
ActiveUS20150201895A1Quality improvementReduce noiseImage enhancementReconstruction from projectionComputed tomographyDose reduction
Substantial reduction of the radiation dose in computed tomography (CT) imaging is shown using a machine-learning dose-reduction technique. Techniques are provided that (1) enhance low-radiation dosage images, beyond just reducing noise, and (2) may be combined with other approaches, such as adaptive exposure techniques and iterative reconstruction, for radiation dose reduction.
Owner:UNIVERSITY OF CHICAGO
Multiparticulate modified release composition
InactiveUS20020054907A1Organic active ingredientsNervous disorderControlled releasePatient compliance
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component comprising a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD
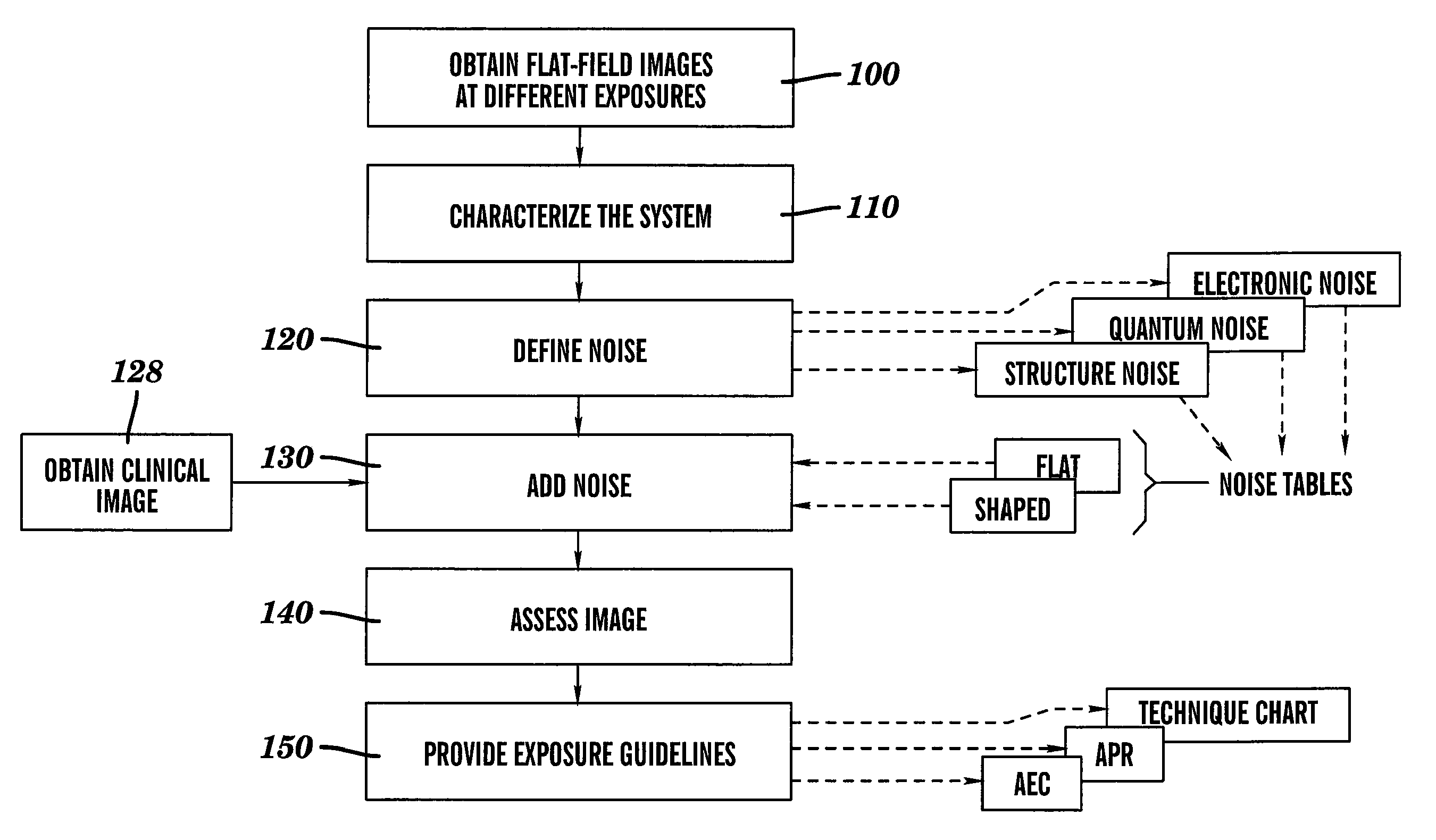
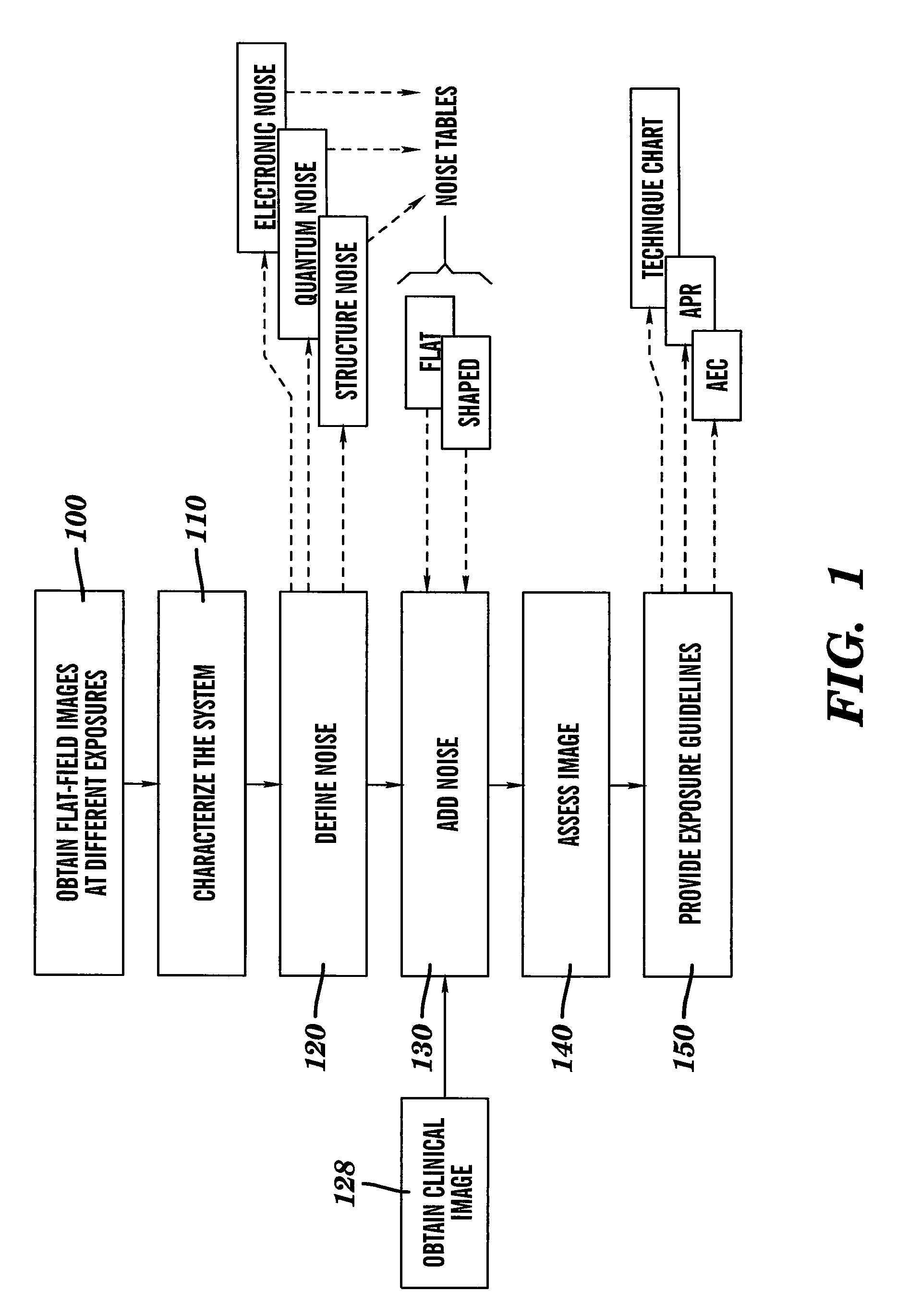
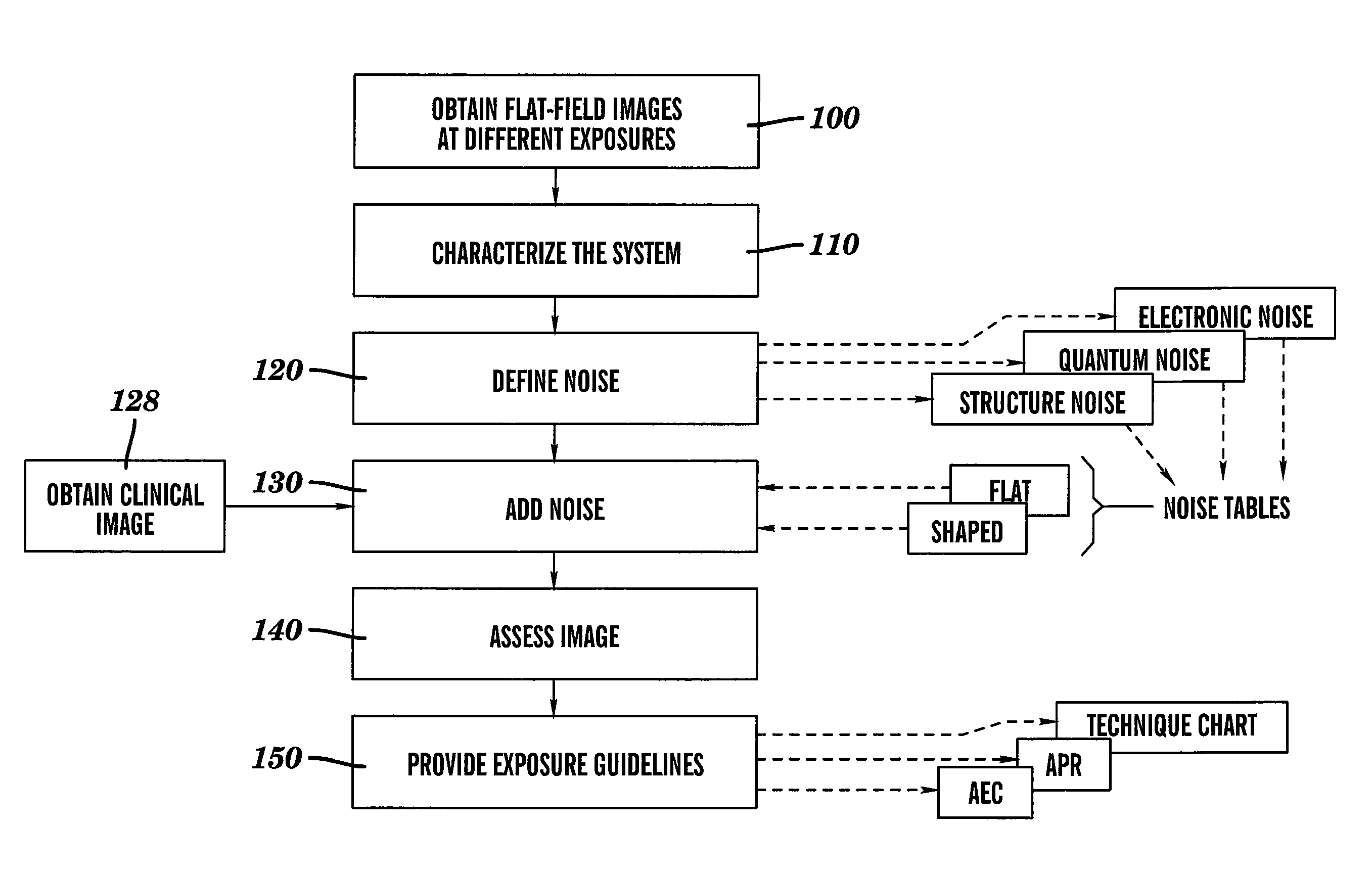
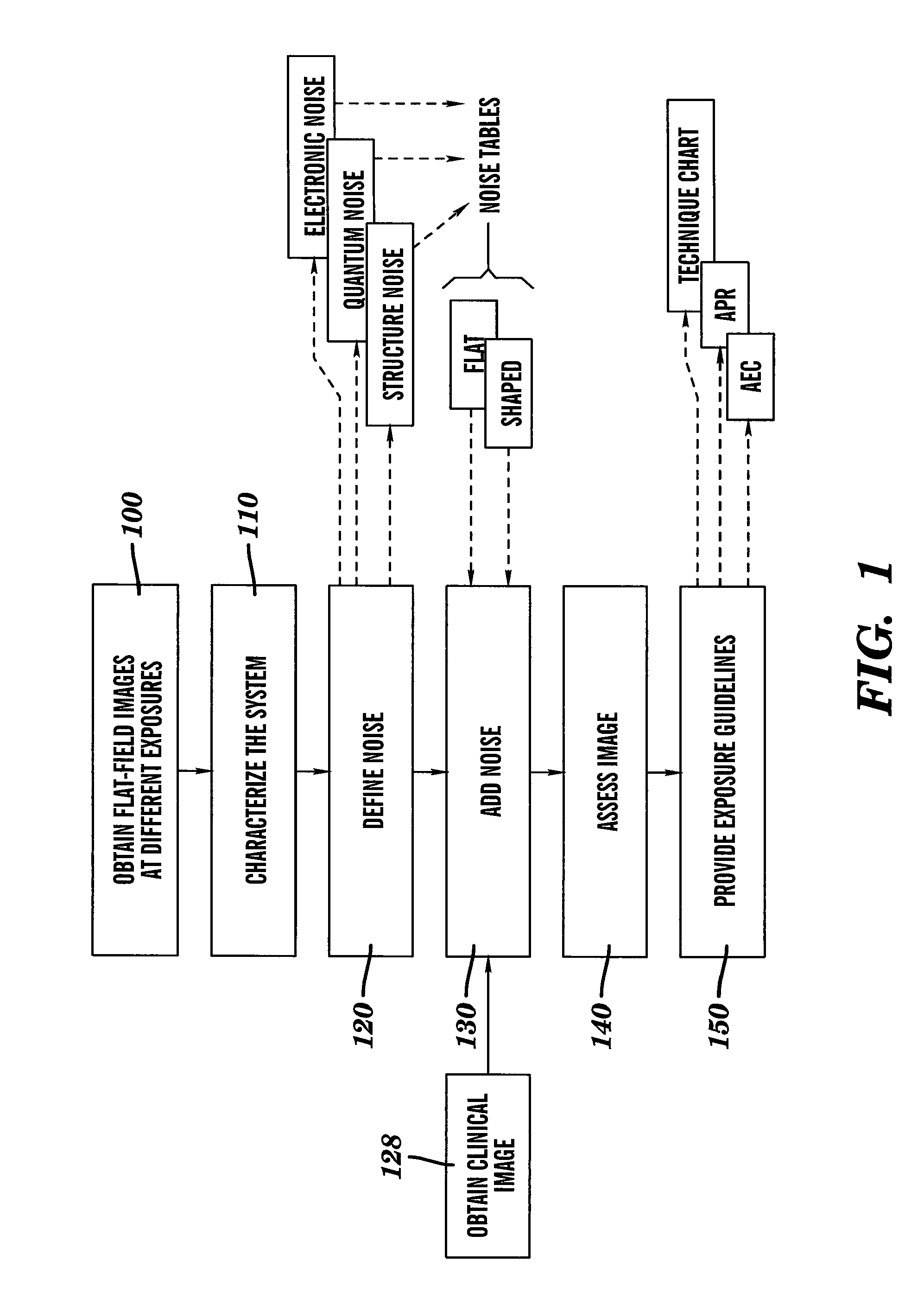
Dose reduced digital medical image simulations
ActiveUS7480365B1Reducing x-ray exposure levelReduce exposureTomosynthesisTomographyNoise power spectrumExposure value
A method for providing a reduced exposure value for radiographic imaging obtains a set of flat-field images at two or more exposure values and measures the noise power spectra using the flat field images. At least one noise table is generated according to interpolated noise power spectra for a set of predetermined exposure values. Values from the at least one noise table are applied to a clinical image to form a reduced exposure simulation image. A noise mask is generated according to at least one noise table and the exposure values of the reduced exposure simulation image and added to the reduced exposure simulation image. The reduced exposure simulation image is assessed to generate a desired dose reduction factor.
Owner:CARESTREAM HEALTH INC
Apparatus for spatial modulation of an x-ray beam
InactiveUS7209547B2Accurate measurementAdditive manufacturing apparatusMaterial analysis using wave/particle radiationUltrasound attenuationElectricity
Owner:SIEMENS HEALTHCARE GMBH
Iterative methods for dose reduction and image enhancement in tomography
InactiveUS20090232377A1Reduce doseReduce in quantityReconstruction from projectionCharacter and pattern recognitionImage resolutionImaging quality
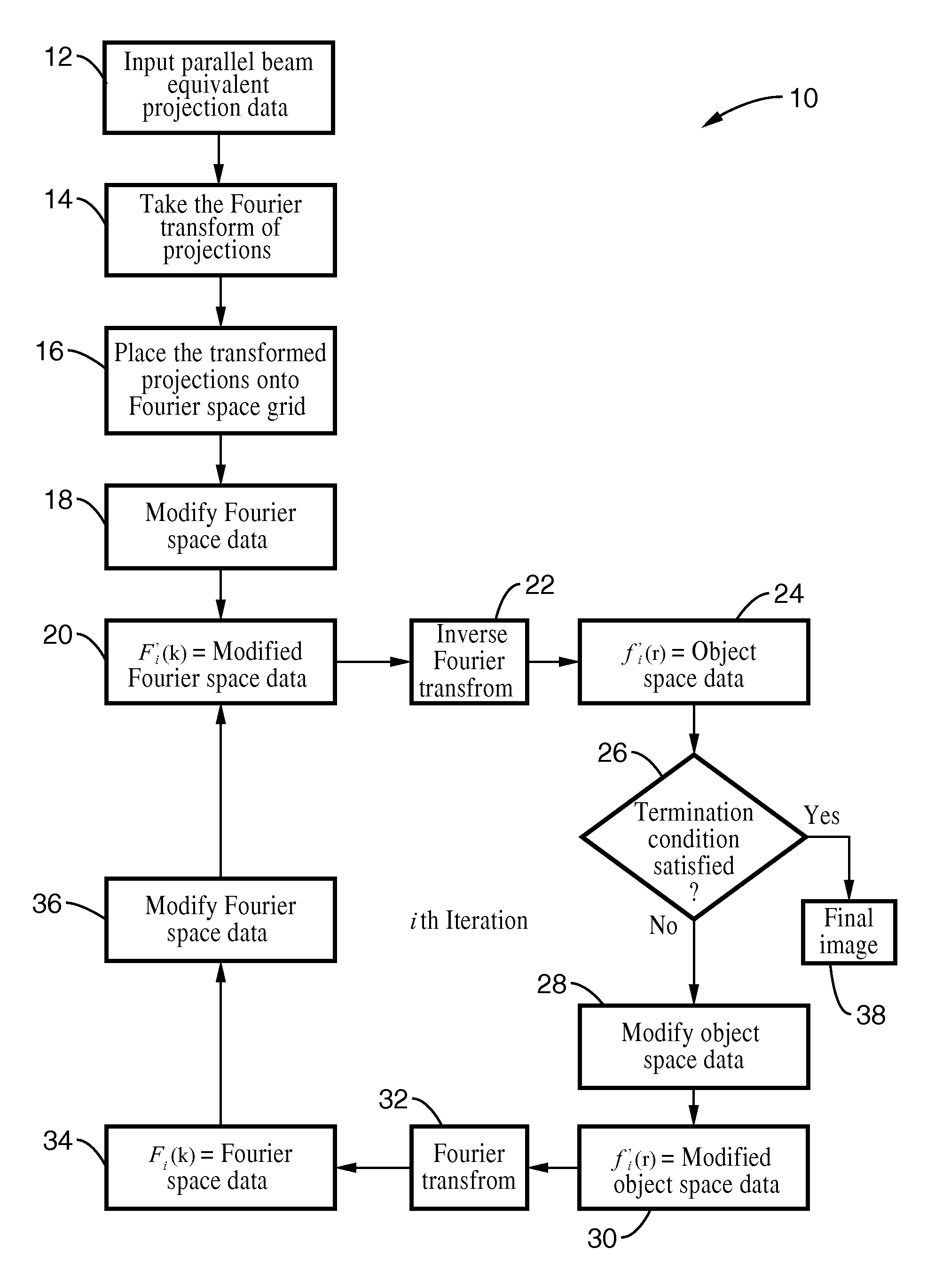
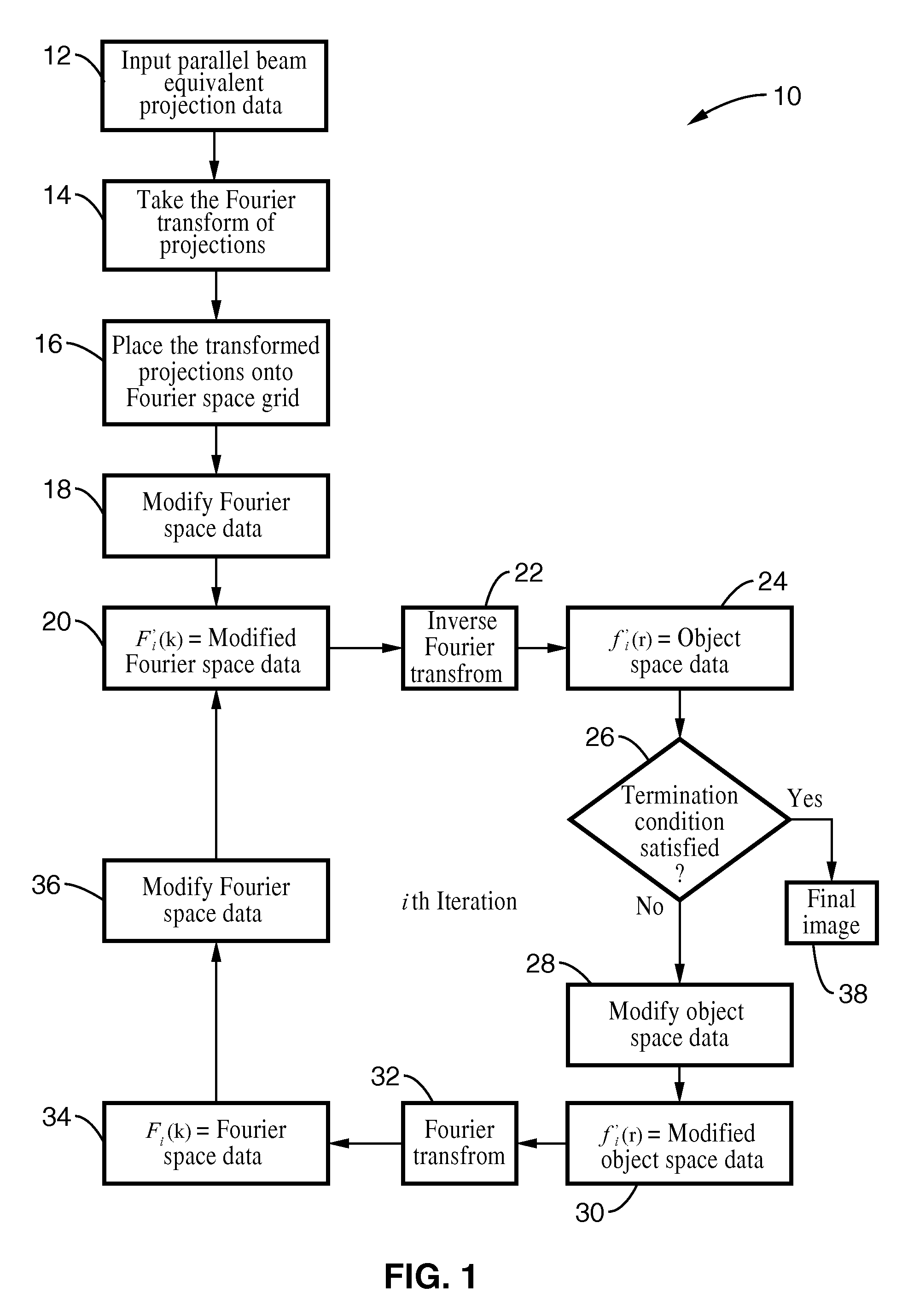
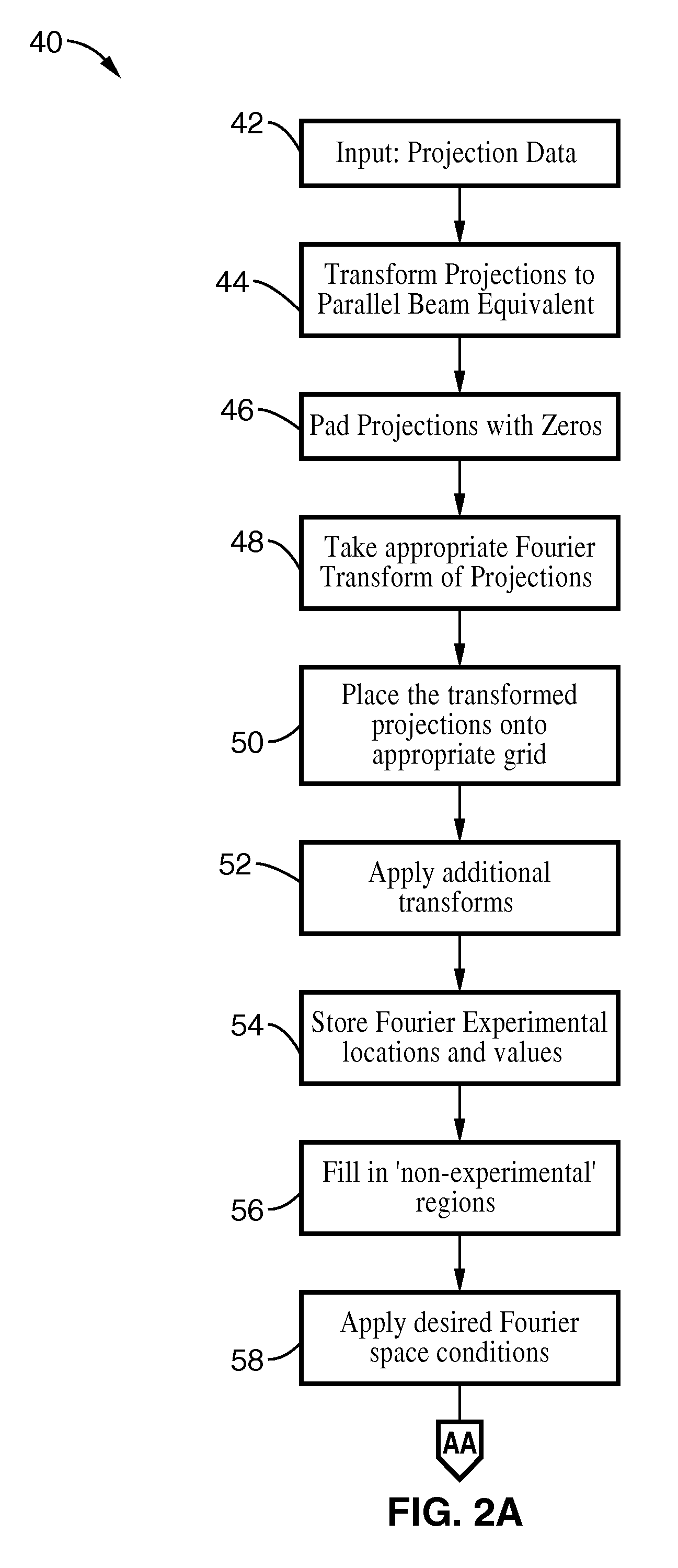
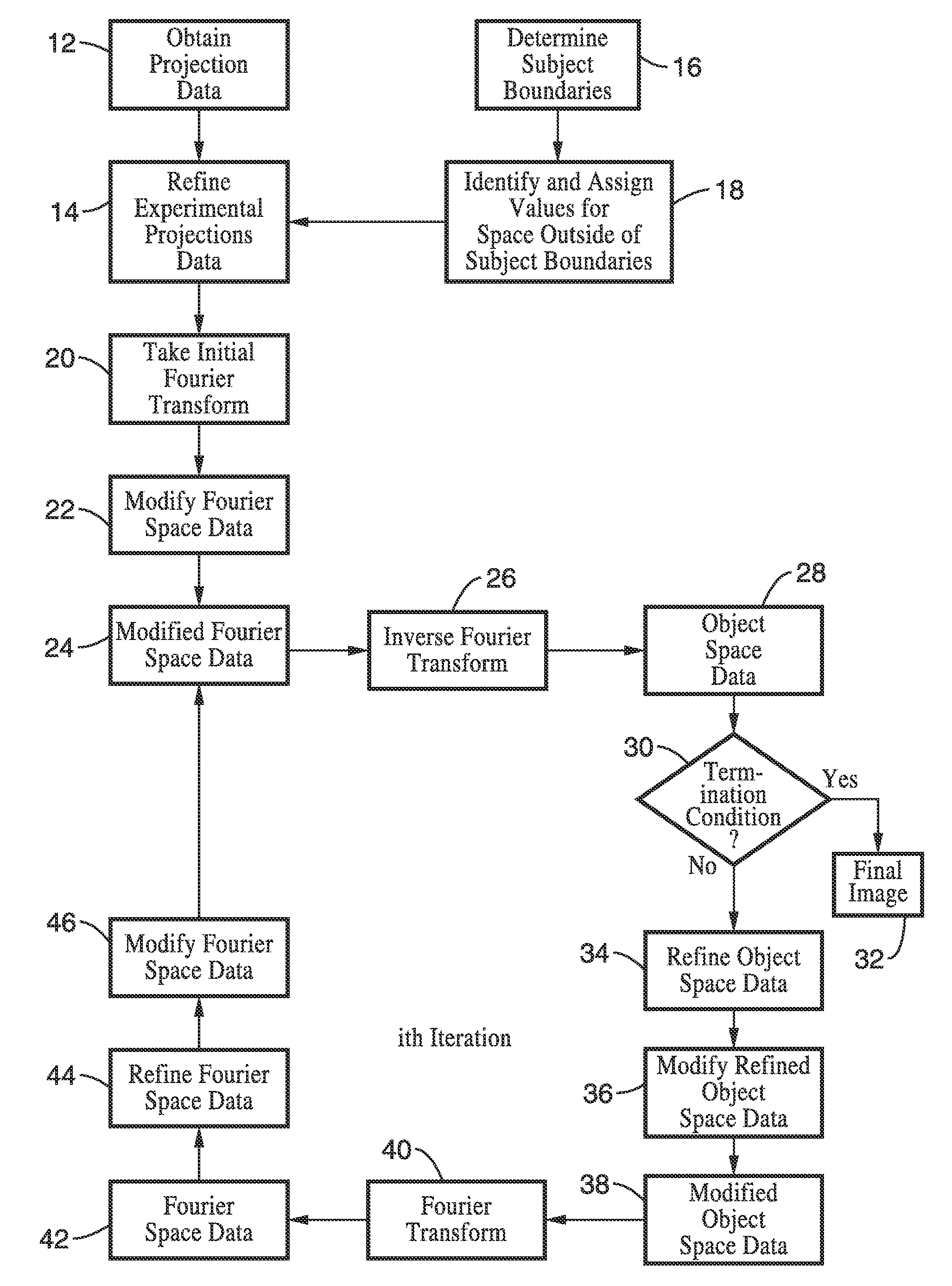
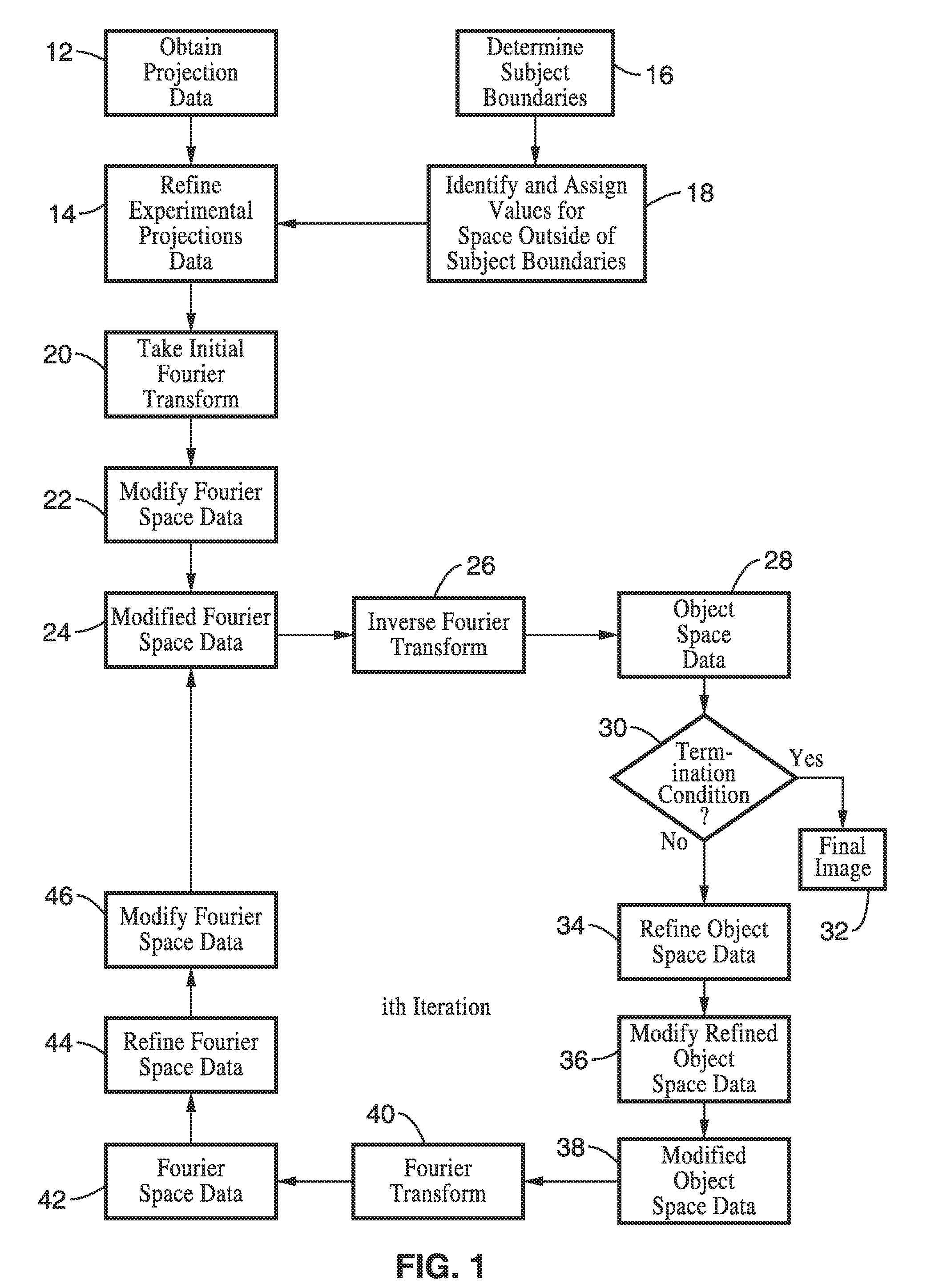
A system and method for creating a three dimensional cross sectional image of an object by the reconstruction of its projections that have been iteratively refined through modification in object space and Fourier space is disclosed. The invention provides systems and methods for use with any tomographic imaging system that reconstructs an object from its projections. In one embodiment, the invention presents a method to eliminate interpolations present in conventional tomography. The method has been experimentally shown to provide higher resolution and improved image quality parameters over existing approaches. A primary benefit of the method is radiation dose reduction since the invention can produce an image of a desired quality with a fewer number projections than seen with conventional methods.
Owner:RGT UNIV OF CALIFORNIA
Apparatus for spatial modulation of an x-ray beam
InactiveUS20050117707A1Accurate measurementAdditive manufacturing apparatusMaterial analysis using wave/particle radiationUltrasound attenuationElectricity
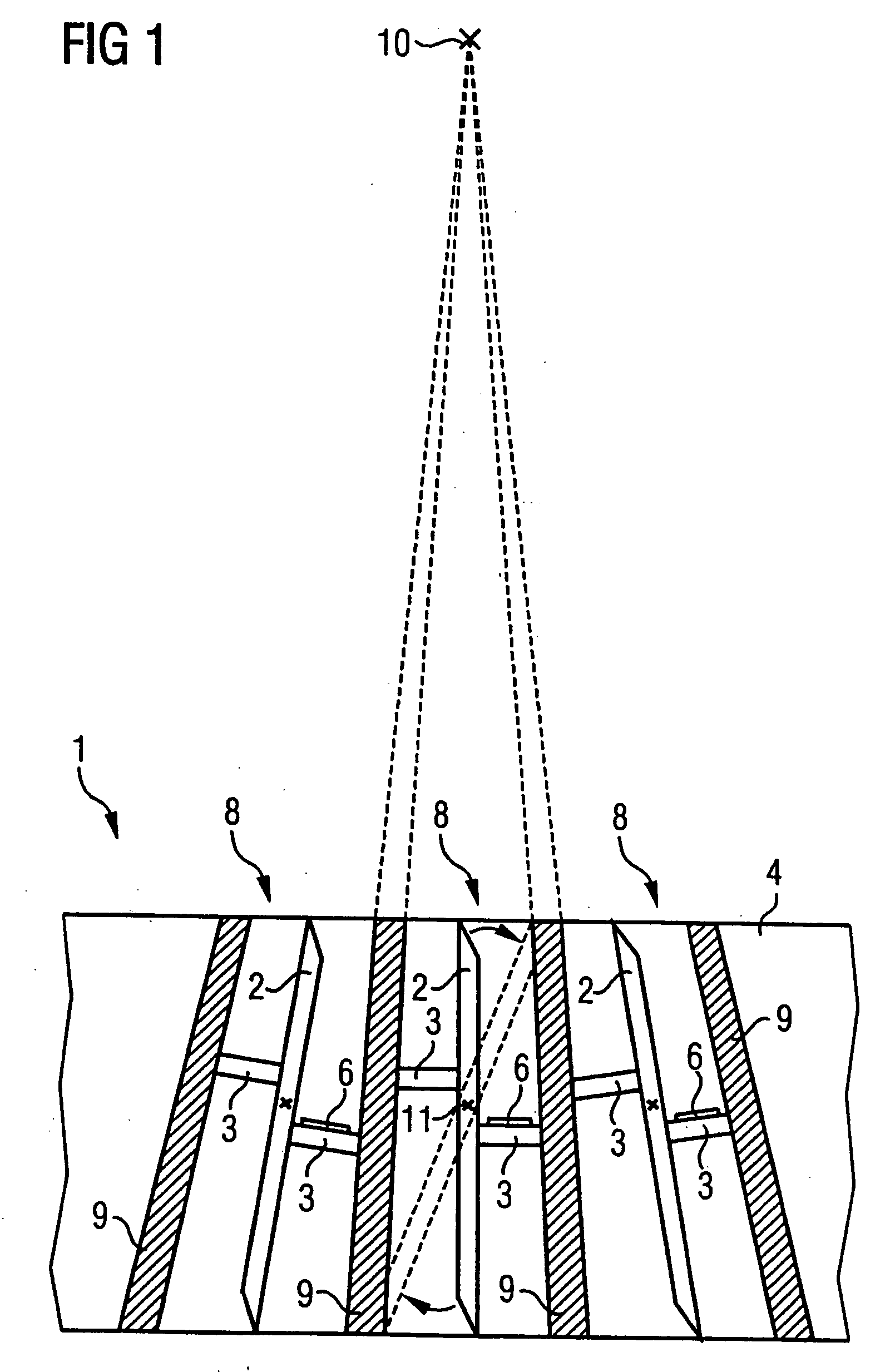
An apparatus for spatial modulation of an x-ray beam has a number of planar attenuation elements for x-ray radiation that are disposed in a grid on a carrier and can be pivoted or tilted by a piezoelectric actuator, independently of one another, between at least two positions. One or more sensors with which a piezoelectrically-caused length and / or width and / or position change of the piezoelectrically influenced regions can be detected, are arranged on piezoelectrically influenced regions of the attenuation elements or the actuators. A significant dose reduction and / or dynamic adjustment thereof can be achieved with the apparatus by image adaptation in many areas of x-ray imaging, since a precise determination of the position of each attenuation element in real time is enabled.
Owner:SIEMENS HEALTHCARE GMBH
Inhibitors for extracellular signal-regulated kinase docking domains and uses therefor
InactiveUS20070066616A1Inhibit cell proliferationReduce cell proliferationBiocideAnimal repellantsExtracellular signalComputer aid
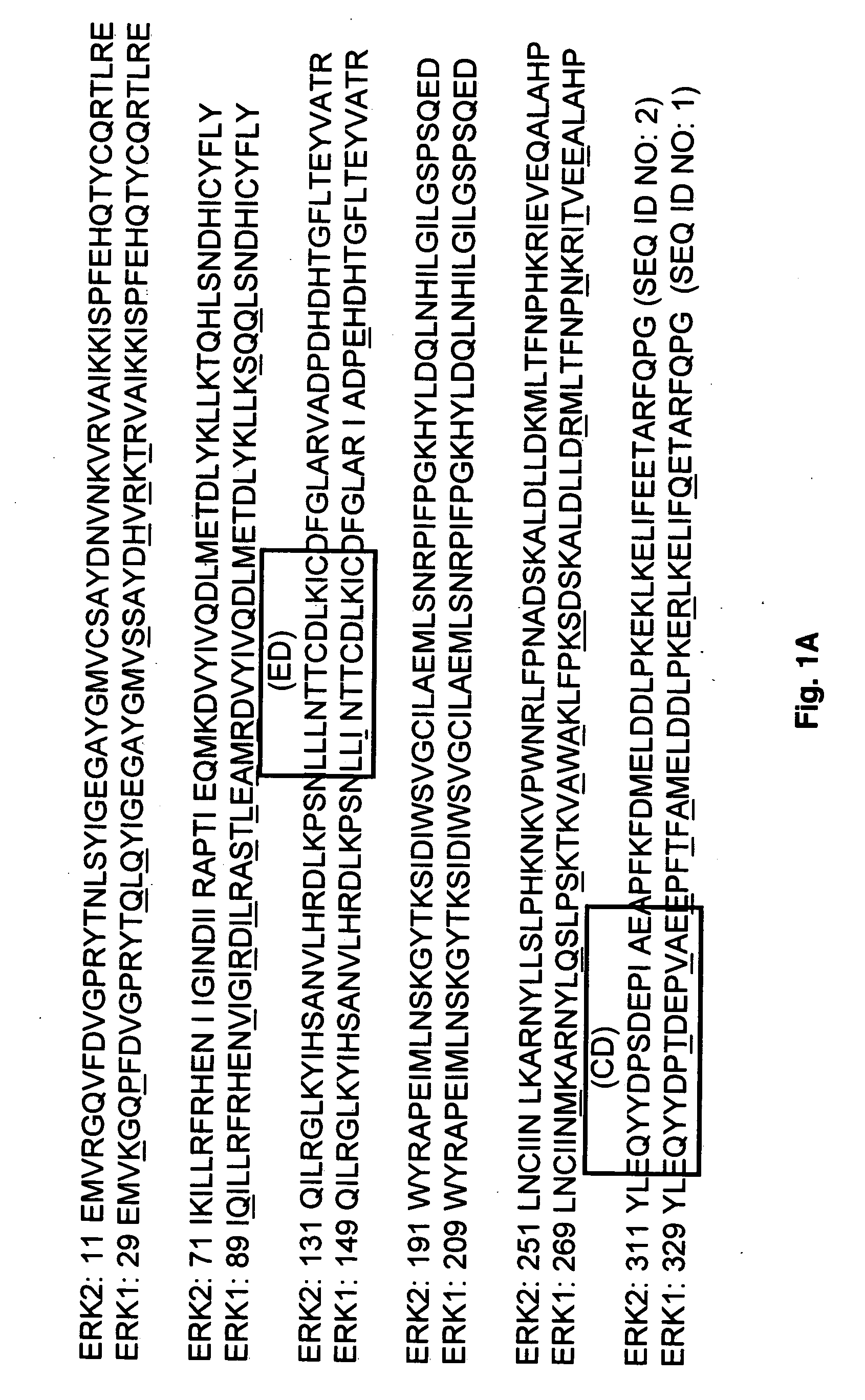
Provided herein are compounds and methods of using compounds that selectively inhibit binding to one or more docking domain regions of an extracellular signal-regulated kinase (ERK) to inhibit in a cell having an extracellular signal-regulated kinase activity. Such methods may be used to inhibit cell proliferation of a neoplastic cell, to treat a cancer and further may be used in conjunction with administration of an anticancer drug at a reduced dosage to treat a cancer with a concomitant reduction in toxicity to an individual receiving the treatment. Also provided is a method to design and screen for compounds to inhibit binding within the extracellular signal-regulated kinase docking domain region, using at least in part computer-aided drug design modeling.
Owner:SHAPIRO PAUL +1
Incorporation of mathematical constraints in methods for dose reduction and image enhancement in tomography
ActiveUS20110164799A1Solve the slow scanning speedReduce in quantityImage enhancementReconstruction from projectionDose reductionTomography
A system and method for creating a three dimensional cross sectional image of an object by the reconstruction of its projections that have been iteratively refined through mathematical transformations and modifications in object space and Fourier space is disclosed. A primary benefit of the method is radiation dose reduction since the invention can produce an image of a desired quality with a fewer number projections than seen with conventional methods.
Owner:RGT UNIV OF CALIFORNIA
Itraconazole temperature-sensitive type gel preparation as well as preparation method and application thereof
InactiveCN104027299AGood slow-release propertiesExtended stayOrganic active ingredientsAntimycoticsGel preparationNasal cavity
The invention belongs to the technical field of medicines and discloses an itraconazole temperature-sensitive type gel preparation as well as a preparation method and application thereof. The itraconazole temperature-sensitive type gel preparation is composed of the following components in percentage by weight: 0.02-3% of medicines, 0.05-15% of solubilizing agent, 5-50% of gel matrix, 0-15% of biological adhesive, 0.001-2% of preservative, 0-10% of other additives and the balance of solvent. The itraconazole temperature-sensitive type gel preparation can regulate gelatinization temperature by adjusting composition and control drug release rate and degree, can effectively inhibit growth of candida in vitro and can be used for drug administration on skins, eyes, nasal cavity, oral cavity, straight intestine or vaginal mucosa. The itraconazole temperature-sensitive type gel preparation has obvious slow release and biological adhesion property and can prolong action time of medicines on an infection part, realize long-acting treatment, reduce dosage and toxic and side effects and improve patient compliance.
Owner:JINAN UNIVERSITY
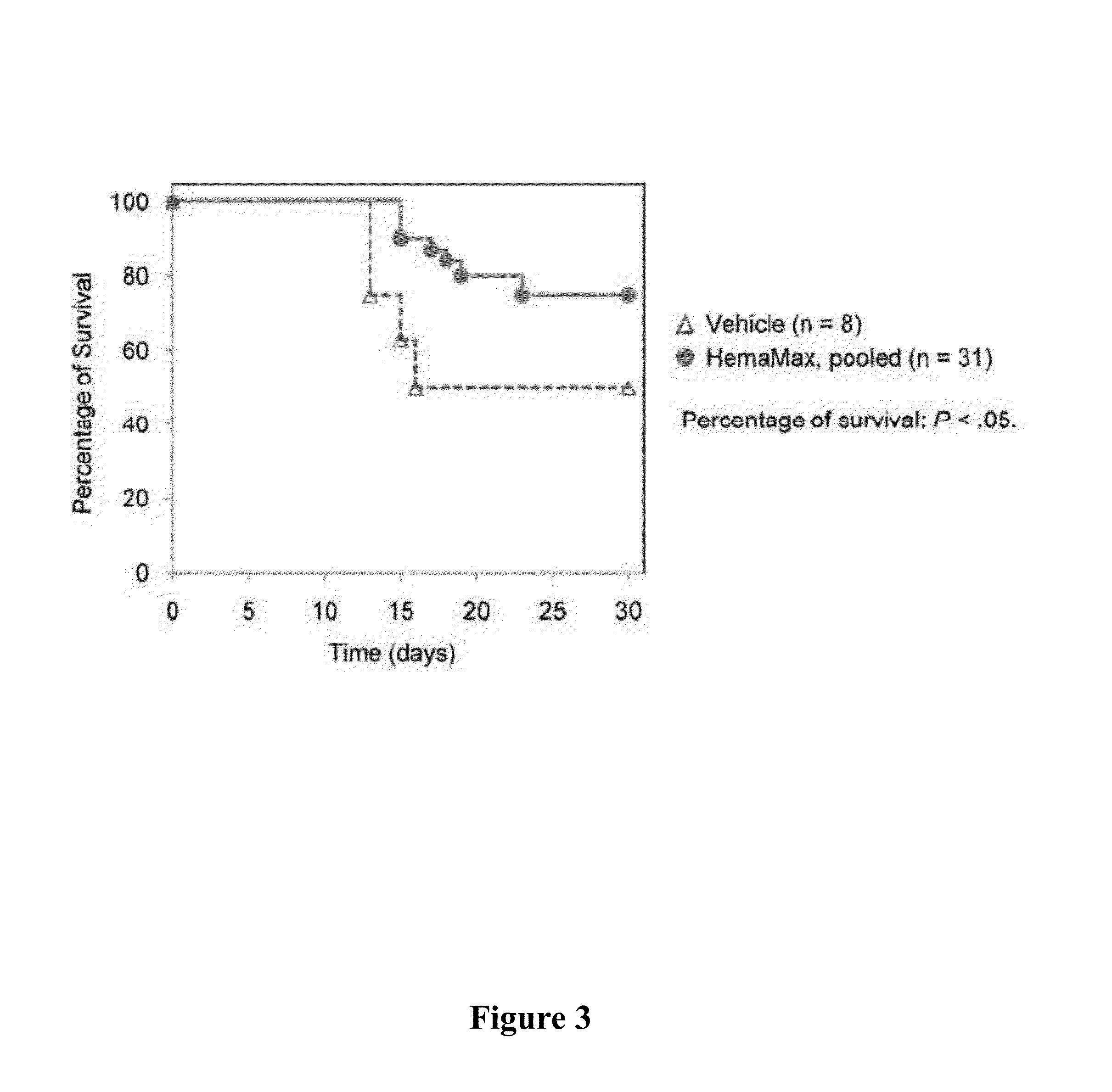
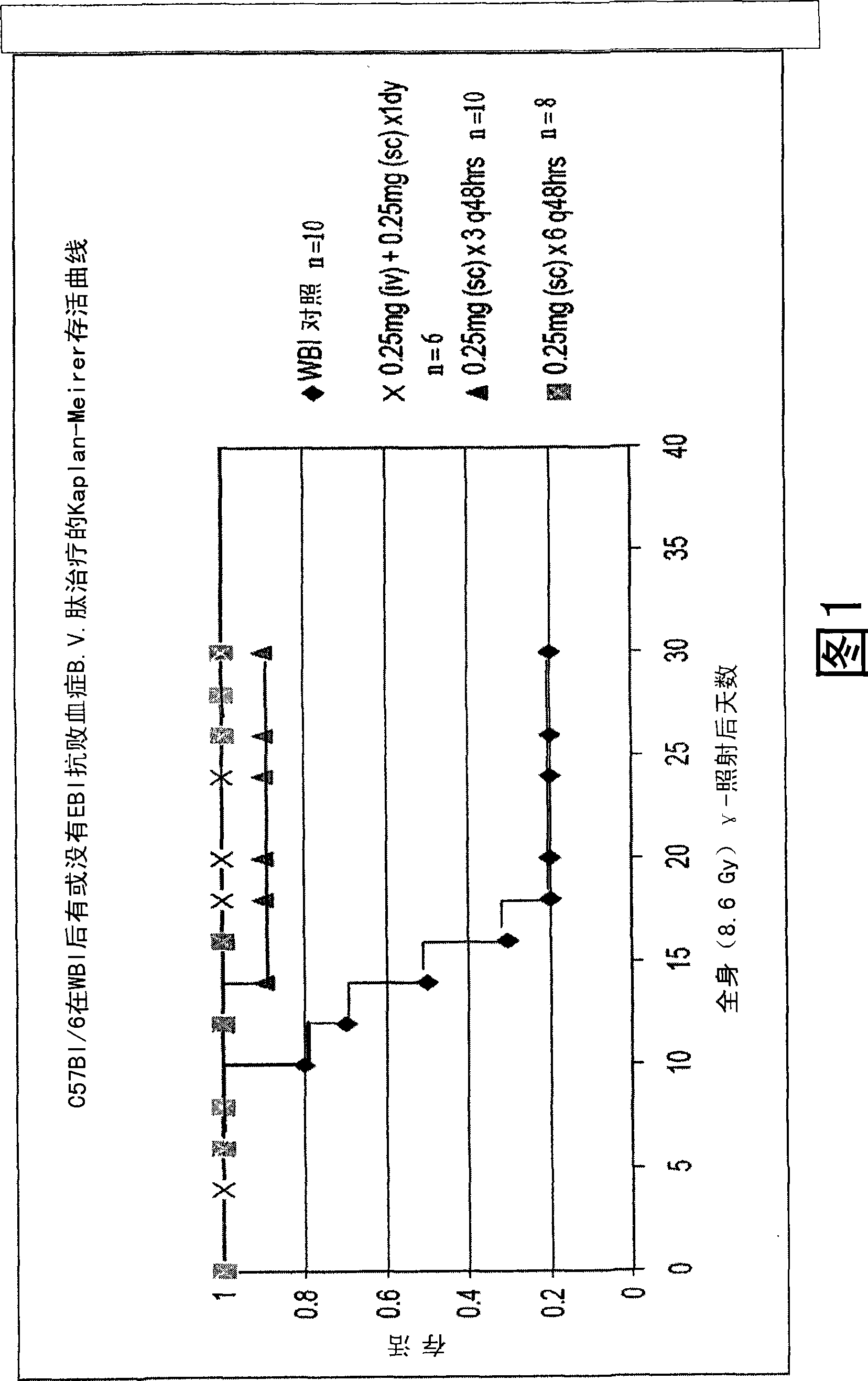
Control of radiation injury
ActiveUS20080027007A1Shorten the counting processBad radiation exposureDigestive systemTetrapeptide ingredientsWhole bodyGamma irradiation
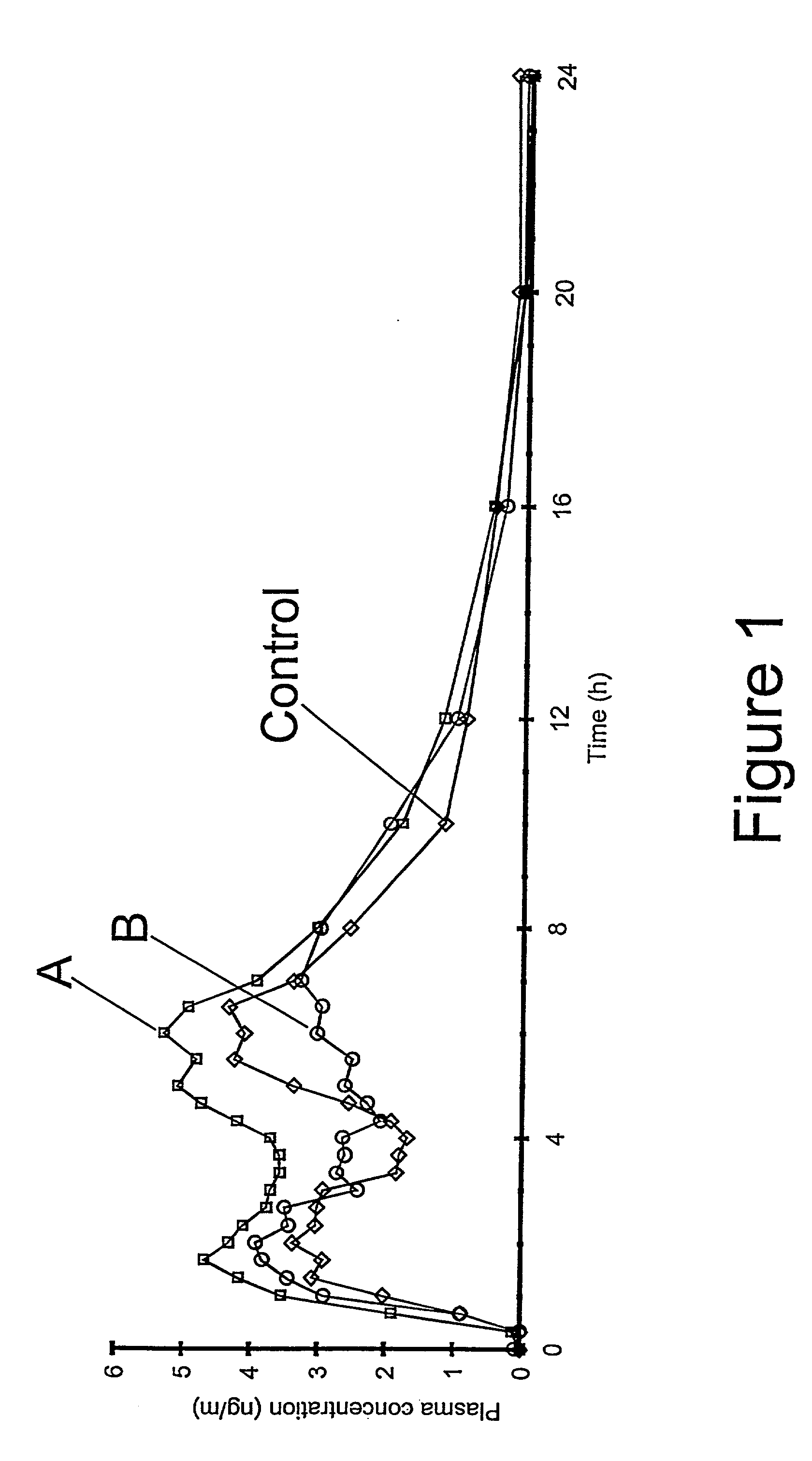
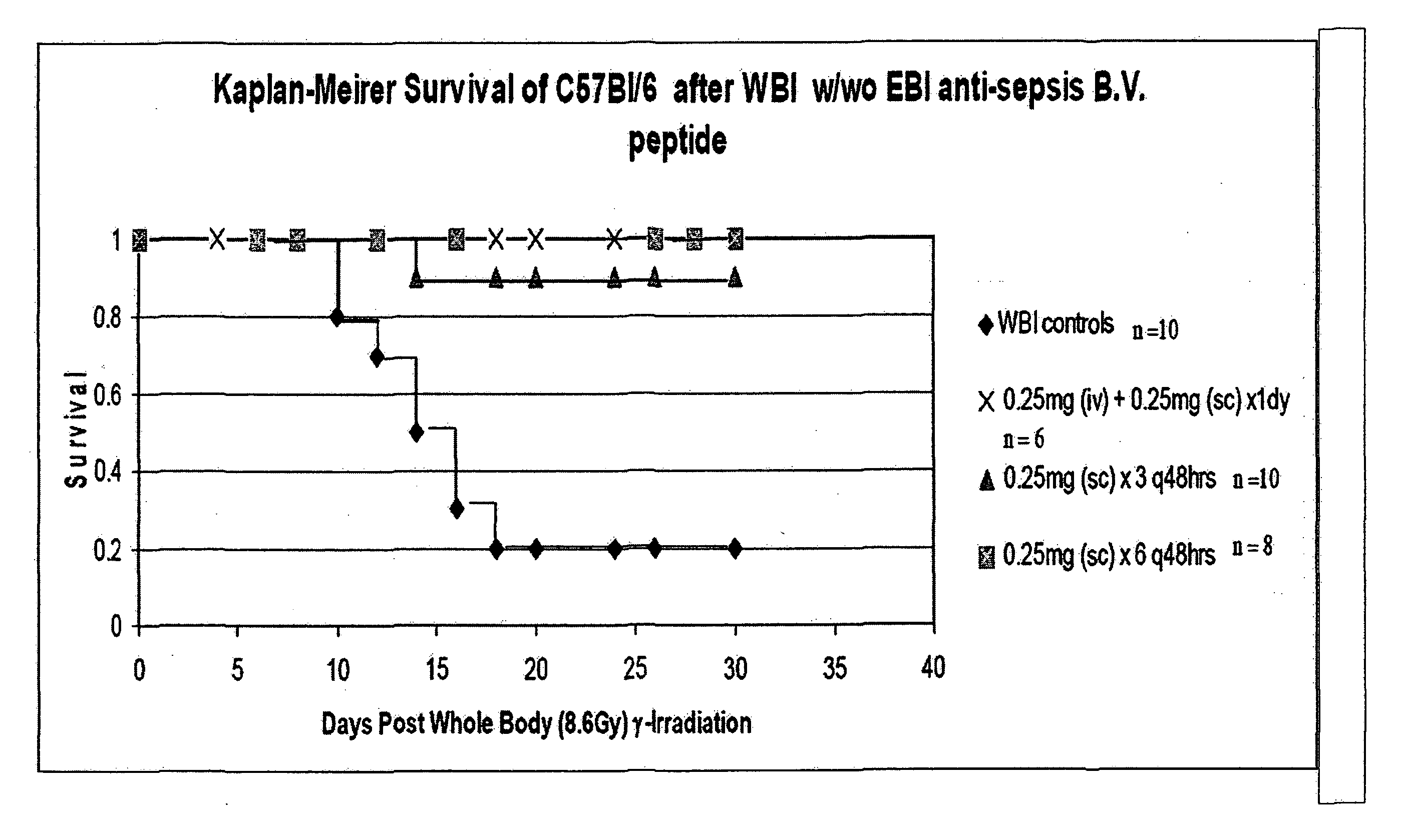
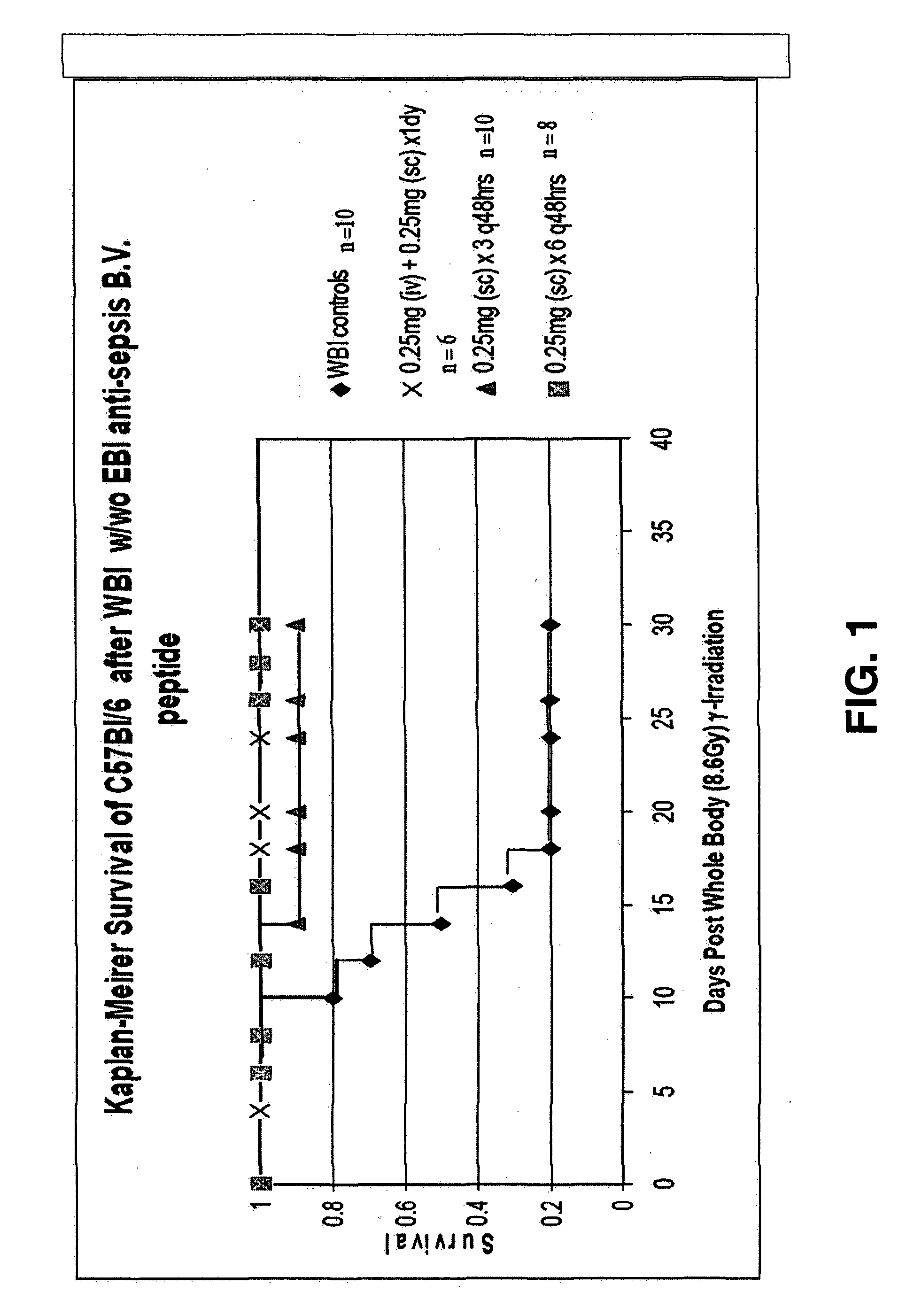
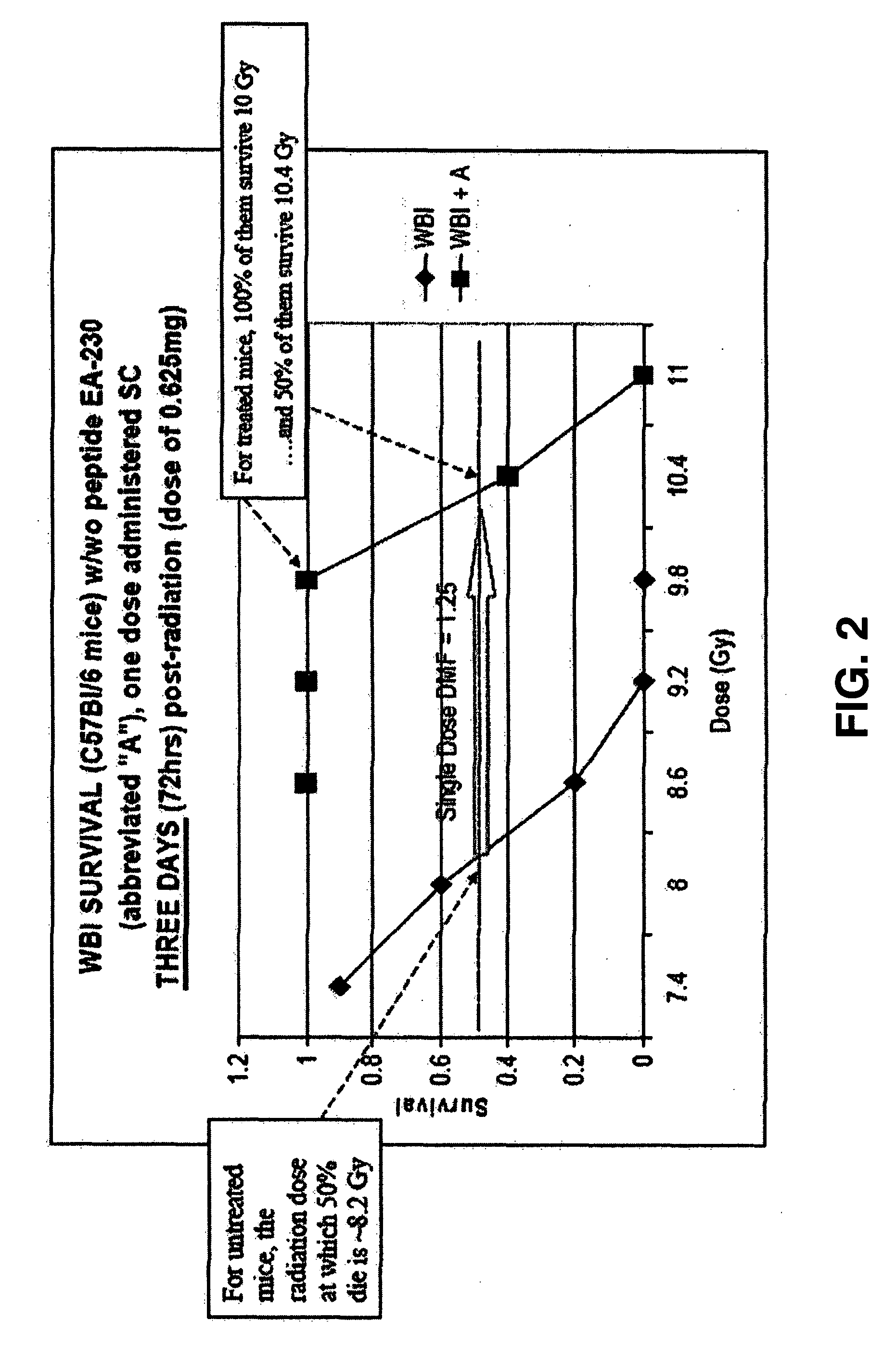
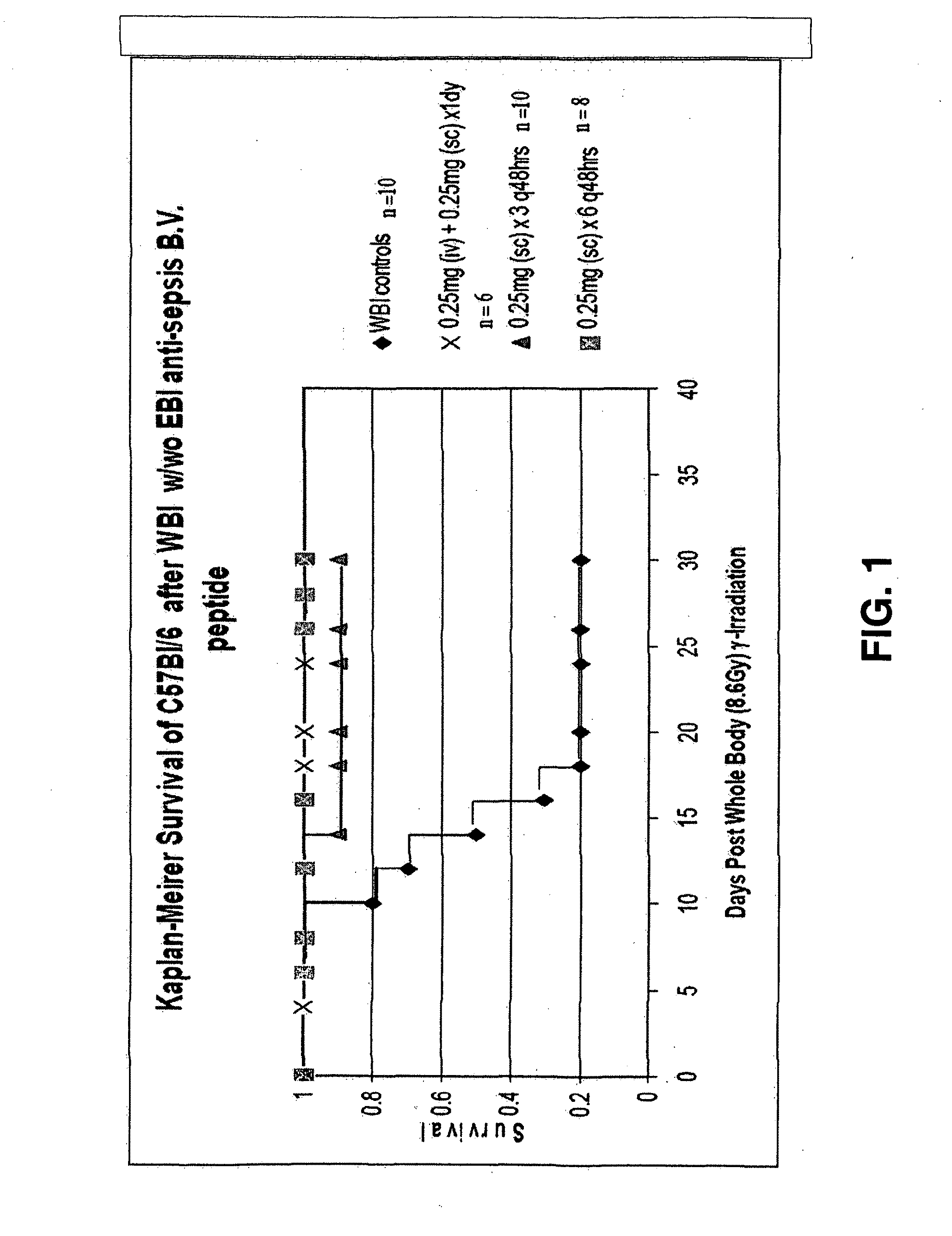
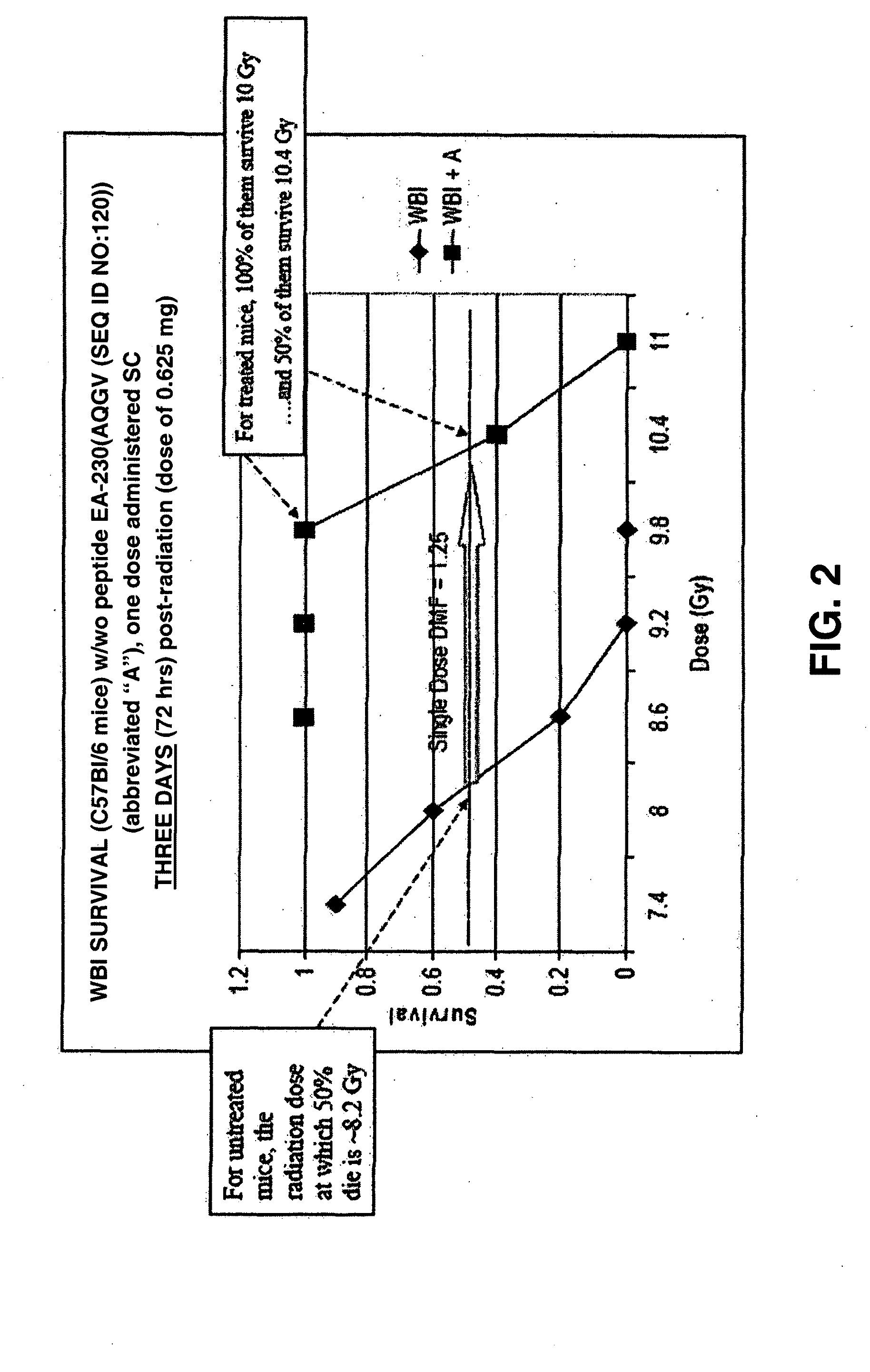
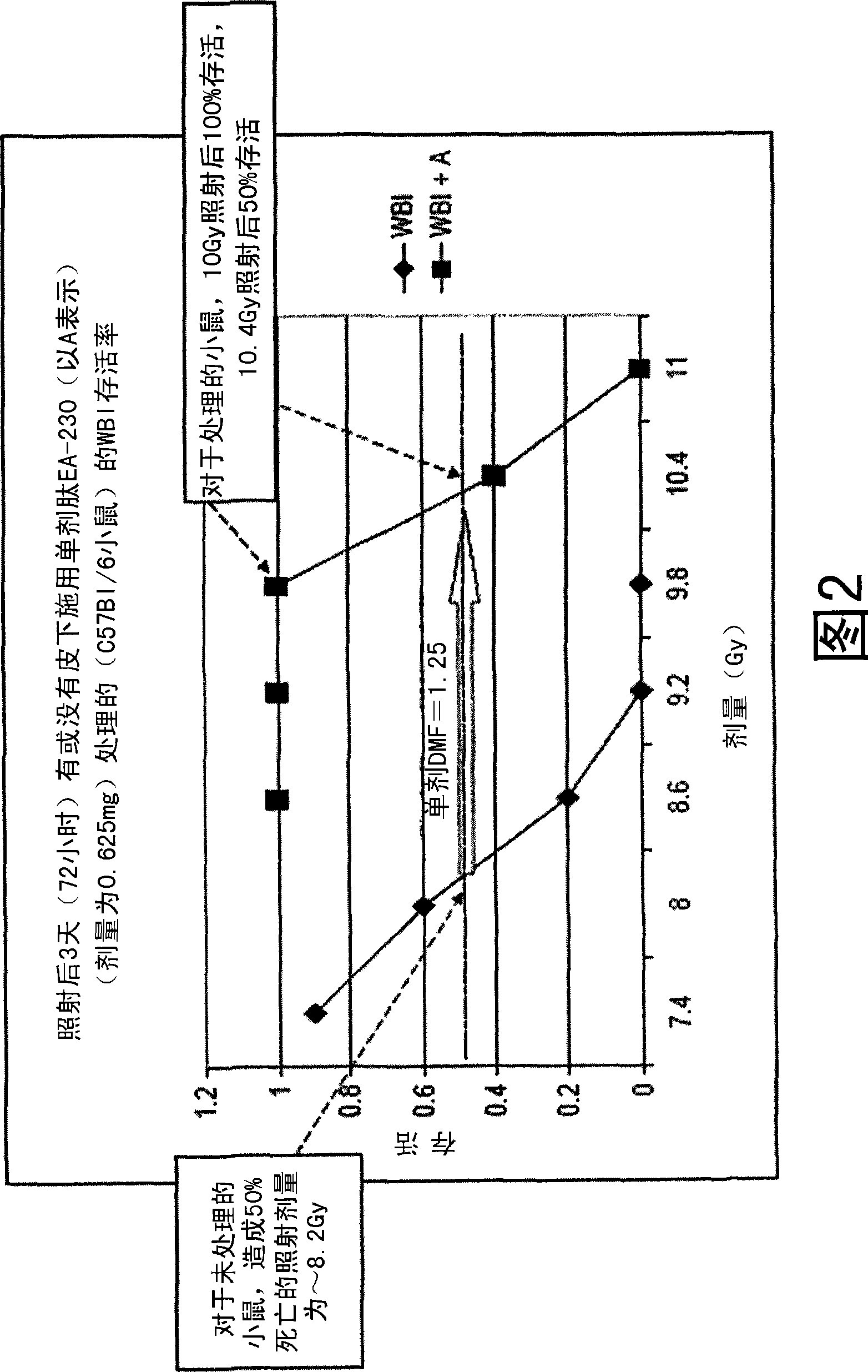
The invention relates to the field of drug development against acute radiation injury caused by exposure to high-energy electromagnetic waves (X-rays, gamma rays) or particles (alpha particles, beta particles, neutrons). To date, there is no effective drug to ameliorate radiation injury after accidental exposure to ionizing irradiation. The invention provides a method of treating radiation injury of a subject in need thereof comprising administering to the subject a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids. Furthermore, the invention provides use of a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids for the production of a pharmaceutical composition for the treatment of a subject suffering from or believed to be suffering from radiation injury. In particular, the invention provides anti-radiation peptides having a dose reduction factor (DRF) against acute gamma irradiation of at least 1.10, said DRF determinable by testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a test group of mice treated with said peptide at 72 hours after WBI and, testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a control group of mice treated only with the vehicle of said peptide at 72 hours after WBI and wherein the DRF is calculated by dividing the LD50 / 30 of the peptide-treated animals by the LD50 / 30 of the vehicle-treated animals.
Owner:BIOTEMPT
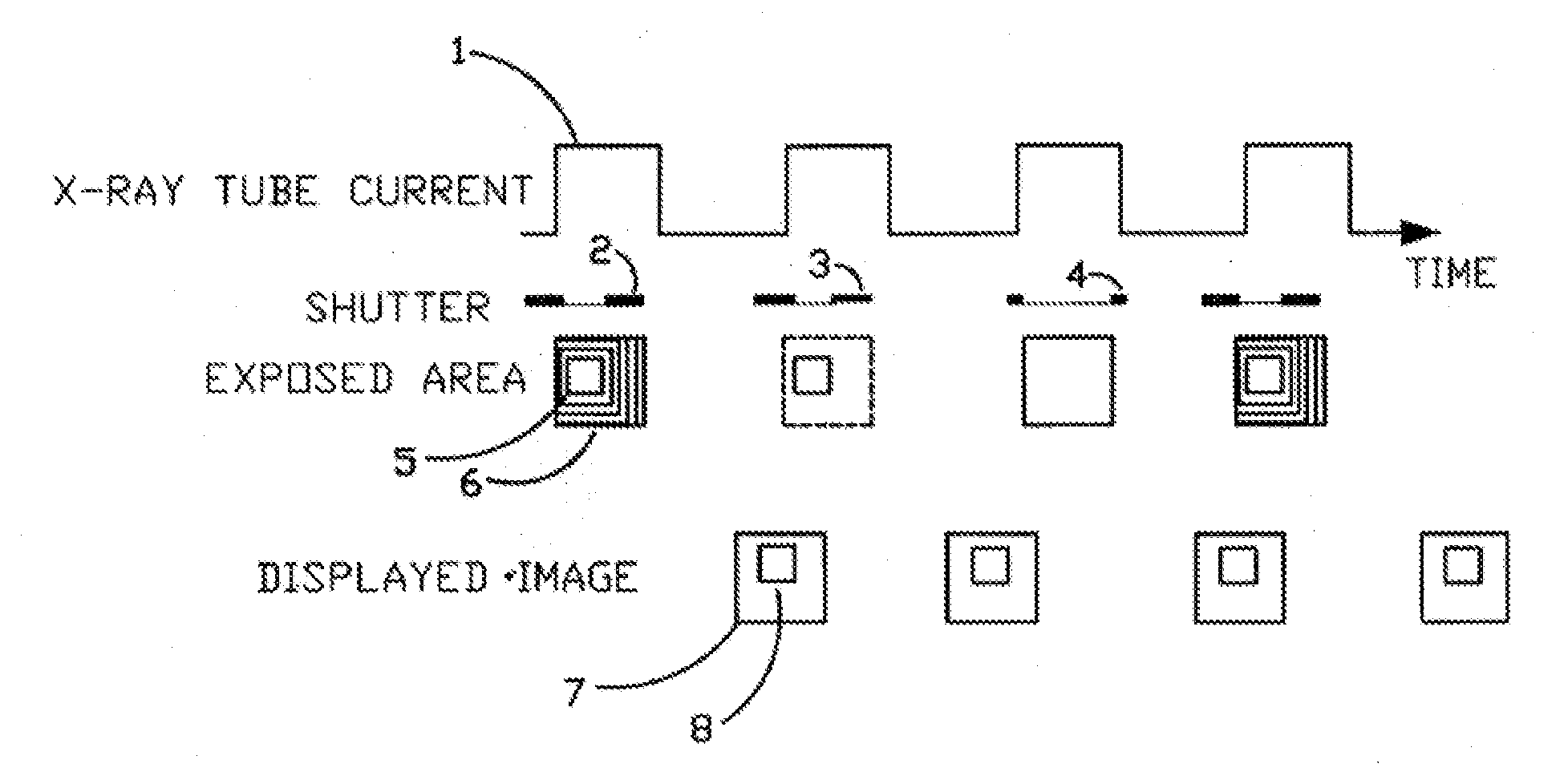
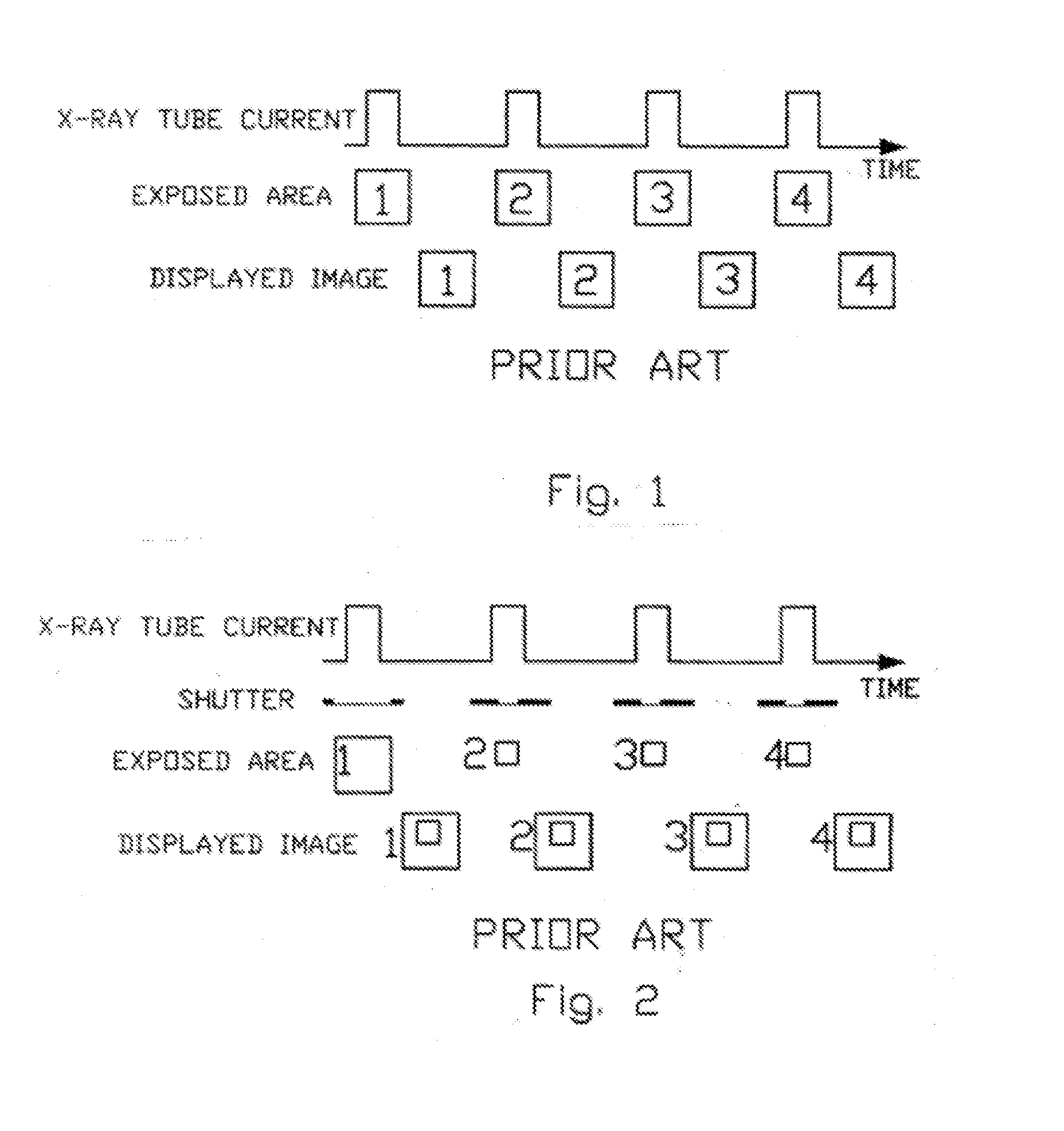
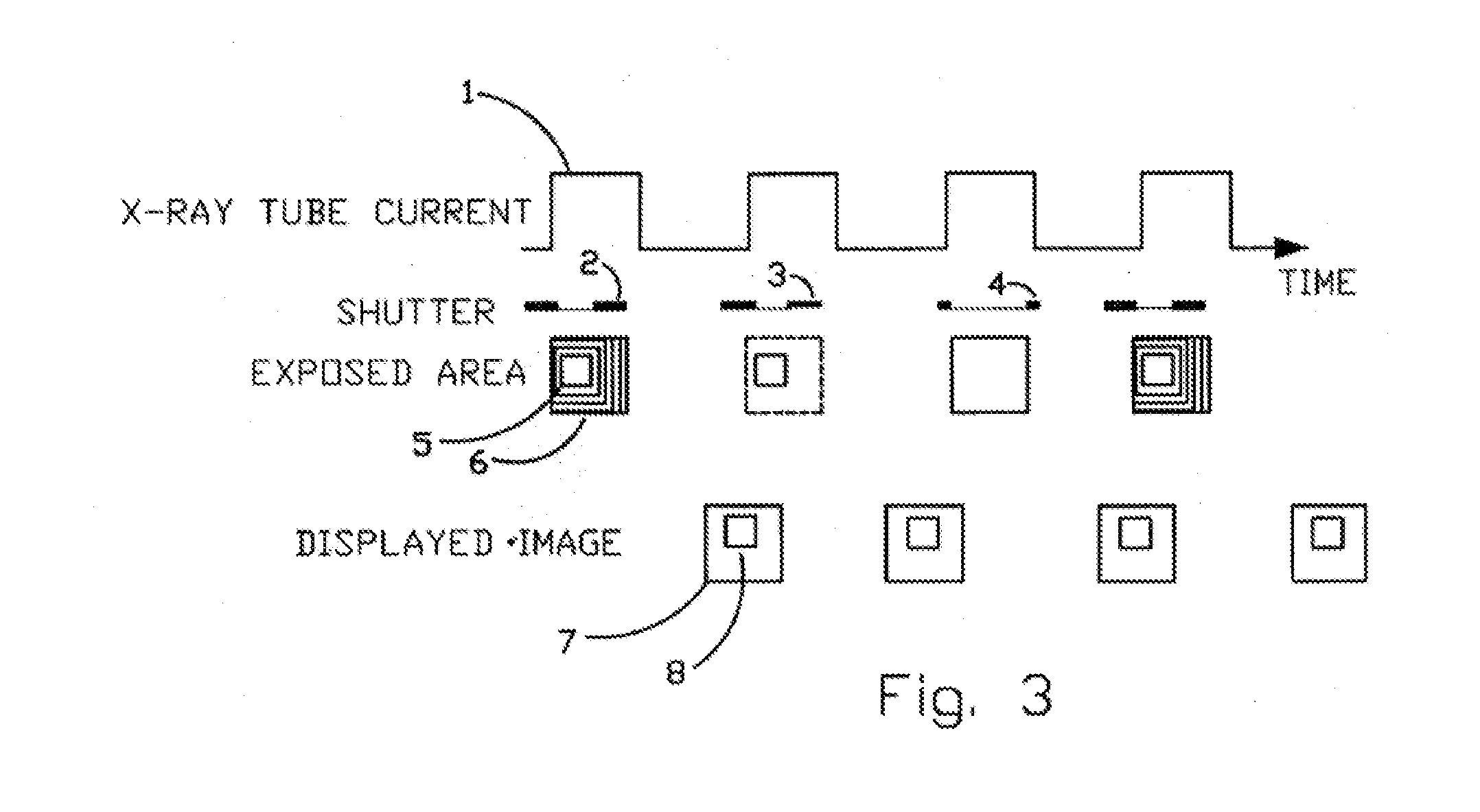
X-Ray Dose Reduction by Controlled Shutter Speed
InactiveUS20130272504A1High resolutionReduce resolutionHandling using diaphragms/collimetersTomographyFluorescenceX-ray
A fast moving X-Ray shutter has four independently controlled blades defining a Region of Interest (ROI). The ROI area receives the normal X-Ray dose and is exposed at the machine's X-ray pulse frame rate. The ROI can be automatically controlled based on image contents using computer vision techniques. Periodically the shutter blades retract at a controlled speed, exposing the area outside the ROI to a lower, non-uniform, exposure. The non-uniformity in this background exposure is dynamically corrected by a look-up table. The displayed image is a seamless combination of the ROI image and the corrected background image. The displayed image has slightly lower resolution outside the ROI but better resolution (as compared to standard fluoroscopy practices) in the ROI because of reduced scattering.
Owner:DEUT MEIR
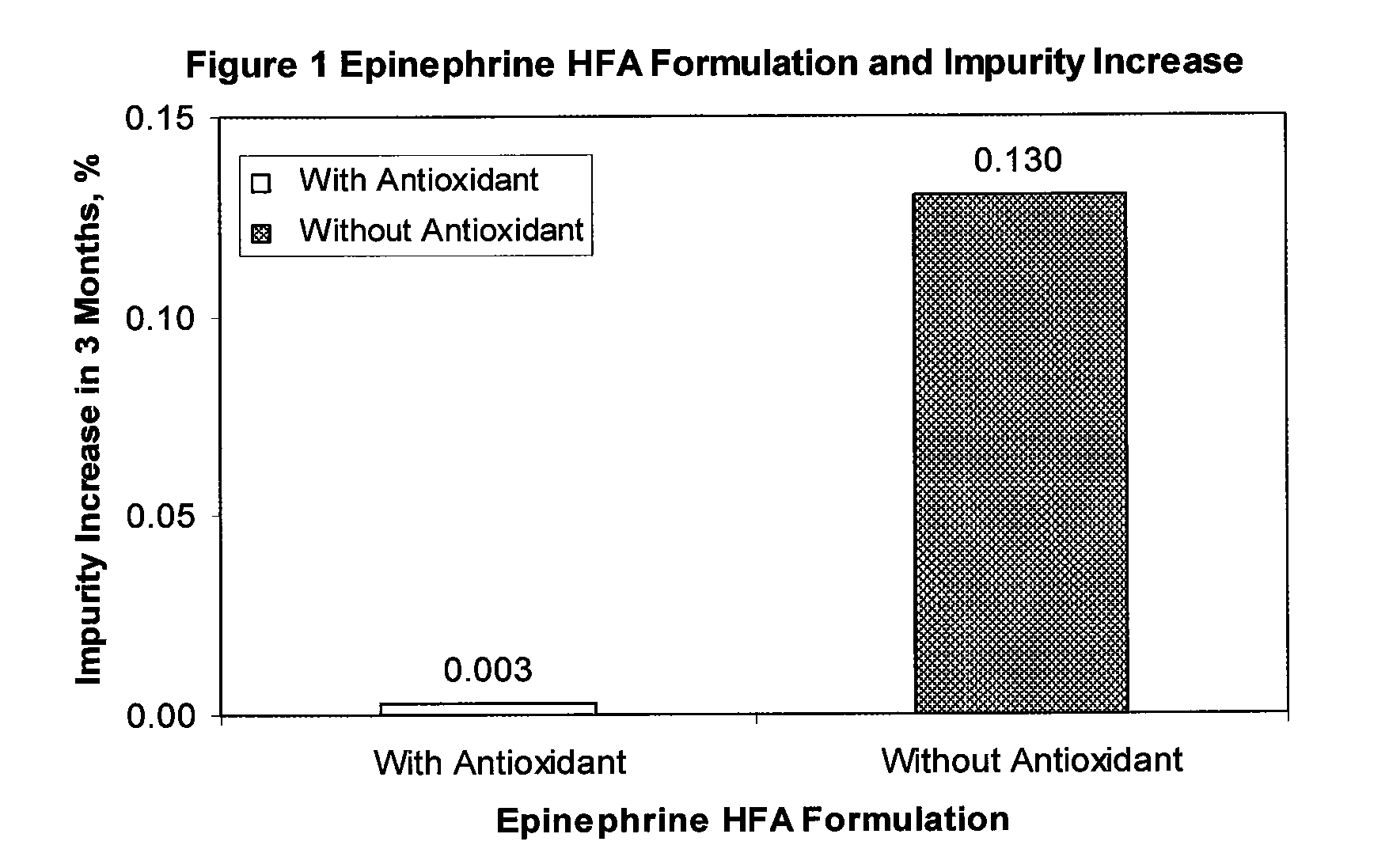
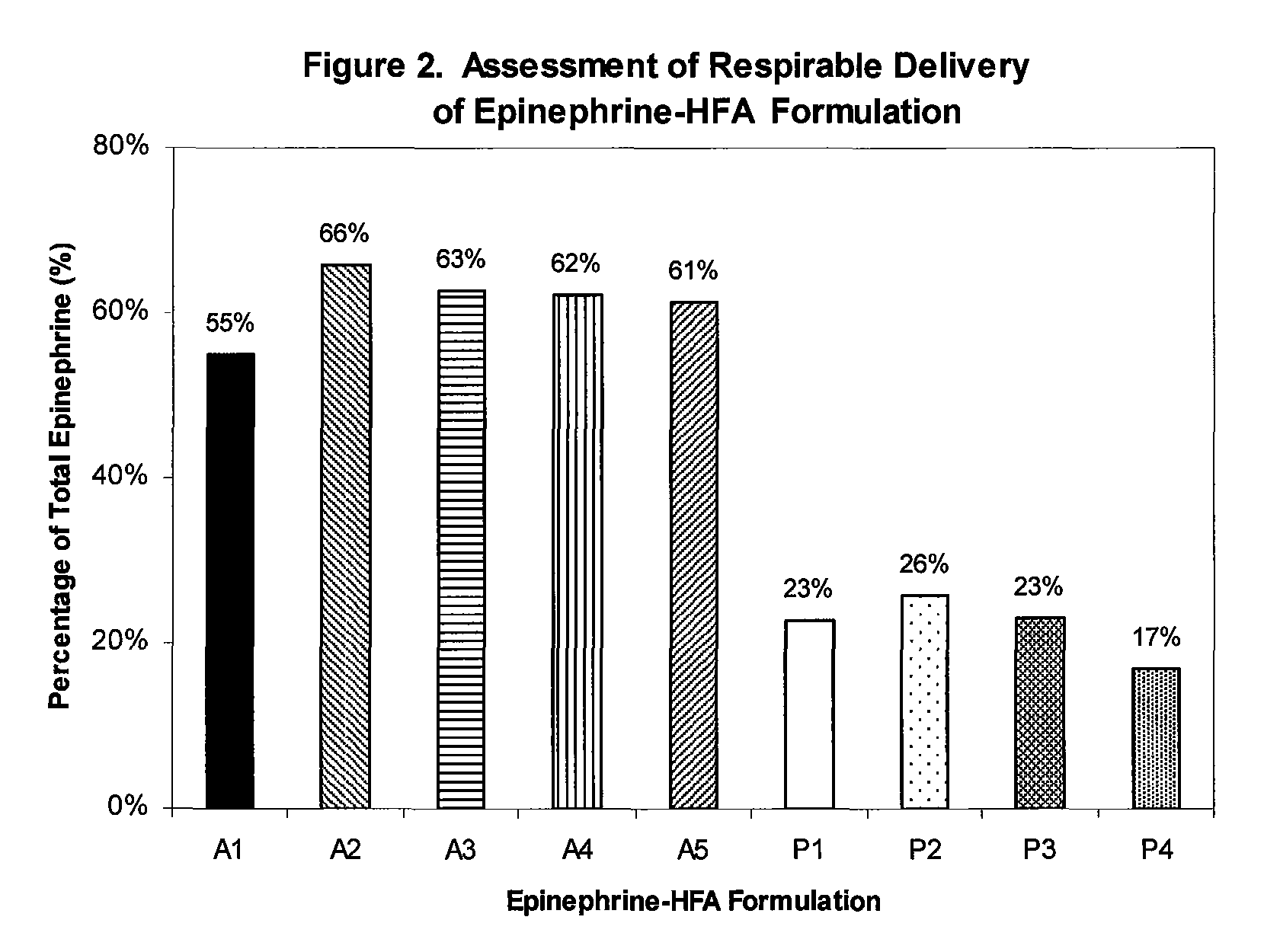
Stable epinephrine suspension formulation with high inhalation delivery efficiency
ActiveUS8367734B1Efficient deliveryImprove efficacyBiocidePowder deliveryAntioxidantChlorofluorocarbon
A stable suspension aerosol formulation of epinephrine is suitable for administration through inhalation comprising a therapeutically effective amount of epinephrine, hydrofluorocarbon propellant, co-solvent, surfactant, and antioxidant. The suspension aerosol formulation further comprises [pre-] pre-micronized epinephrine suspended in an alcohol / surfactant solution with hydrofluoroalkane propellant. The suspension formulation provides a highly efficient delivery of drug microparticles into the respirable region of patients' lungs and has the following advantages: lower dosage requirement, minimum alcohol content, with less impurities generated during storage, improved efficacy and safety, and exhibits no ozone depleting potential compared to a formulation containing chlorofluorocarbon.
Owner:AMPHASTAR PHARMA INC
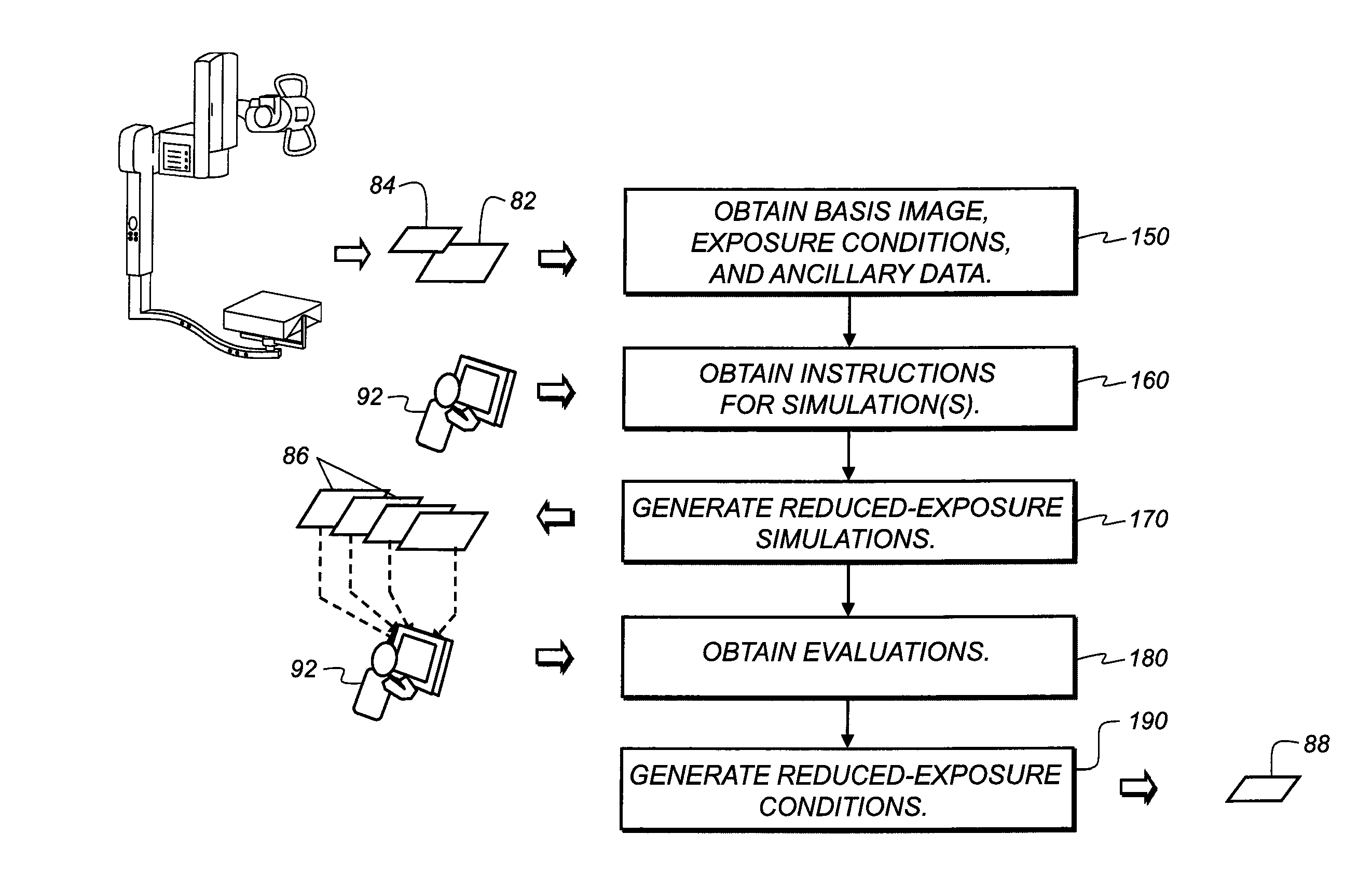
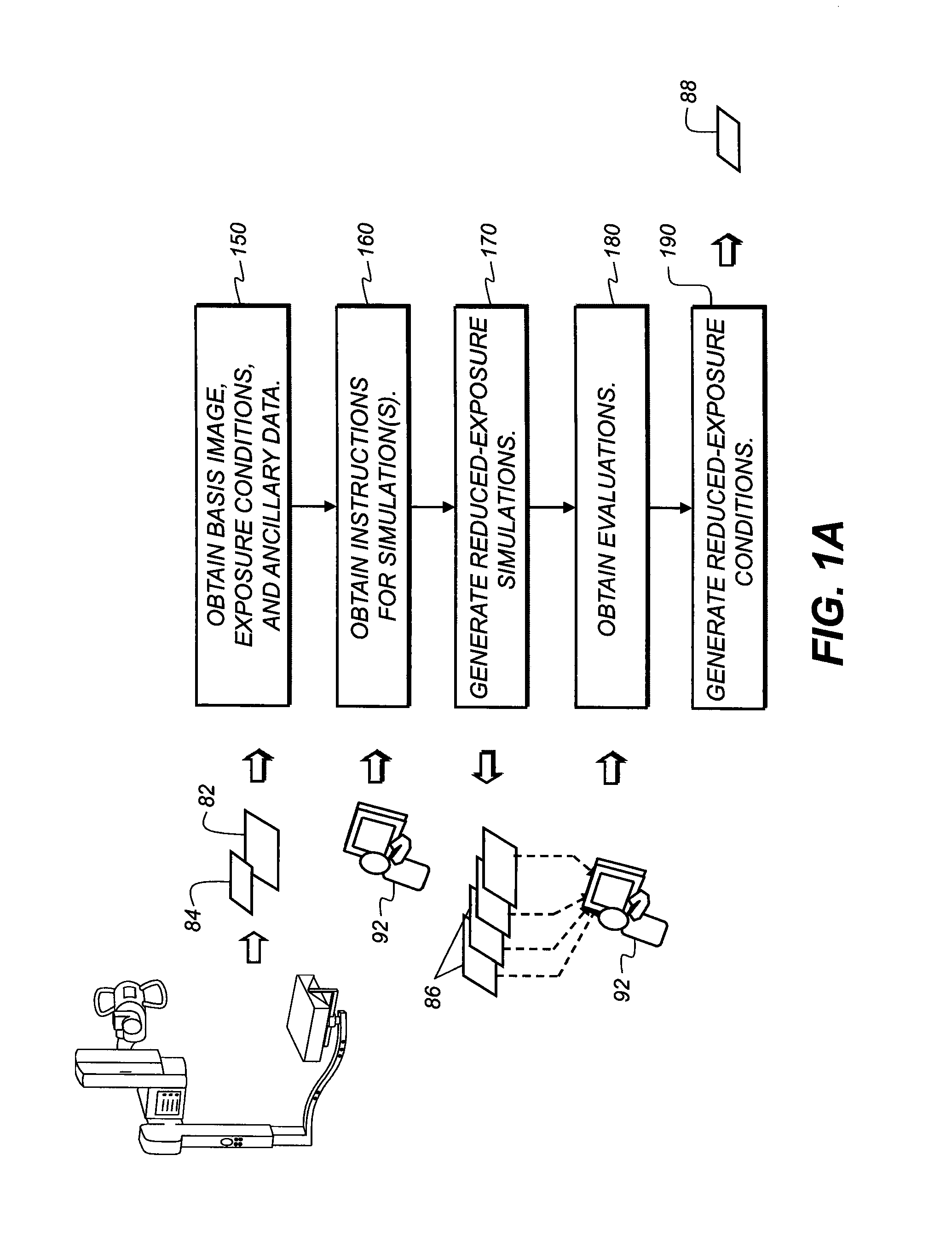
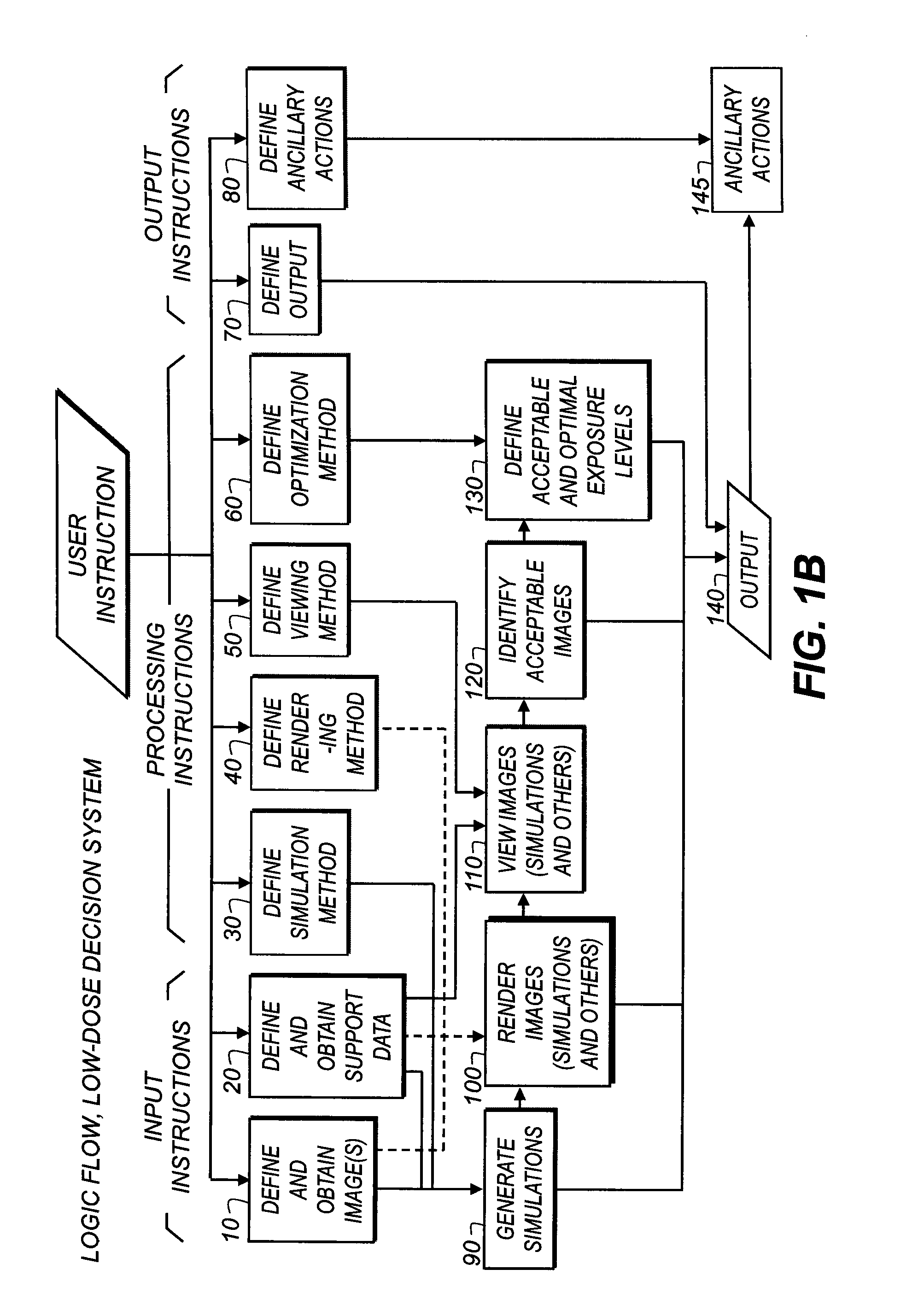
Dose-reduction decision system for medical images
InactiveUS20100091950A1Reducing x-ray exposure levelLow level of exposureCharacter and pattern recognitionX-ray apparatusProcessing InstructionDecision system
A method of obtaining recommendations for lowered radiation dose for a type of radiological image, executed at least in part by a computer system, obtains at least one clinical image of at least one patient, taken under a baseline set of exposure conditions, as a basis image. Processing instructions related to image simulation under one or more reduced exposure conditions are obtained. The basis image is processed according to the processing instructions to generate a set of one or more simulation images, each simulation image representative of corresponding reduced exposure conditions. One or more simulation images are displayed to one or more diagnostic practitioners and an evaluation obtained from the one or more practitioners related to at least the quality of the one or more simulation images. At least one recommended reduced exposure condition is generated and electronically stored according to the practitioner evaluation.
Owner:CARESTREAM HEALTH INC
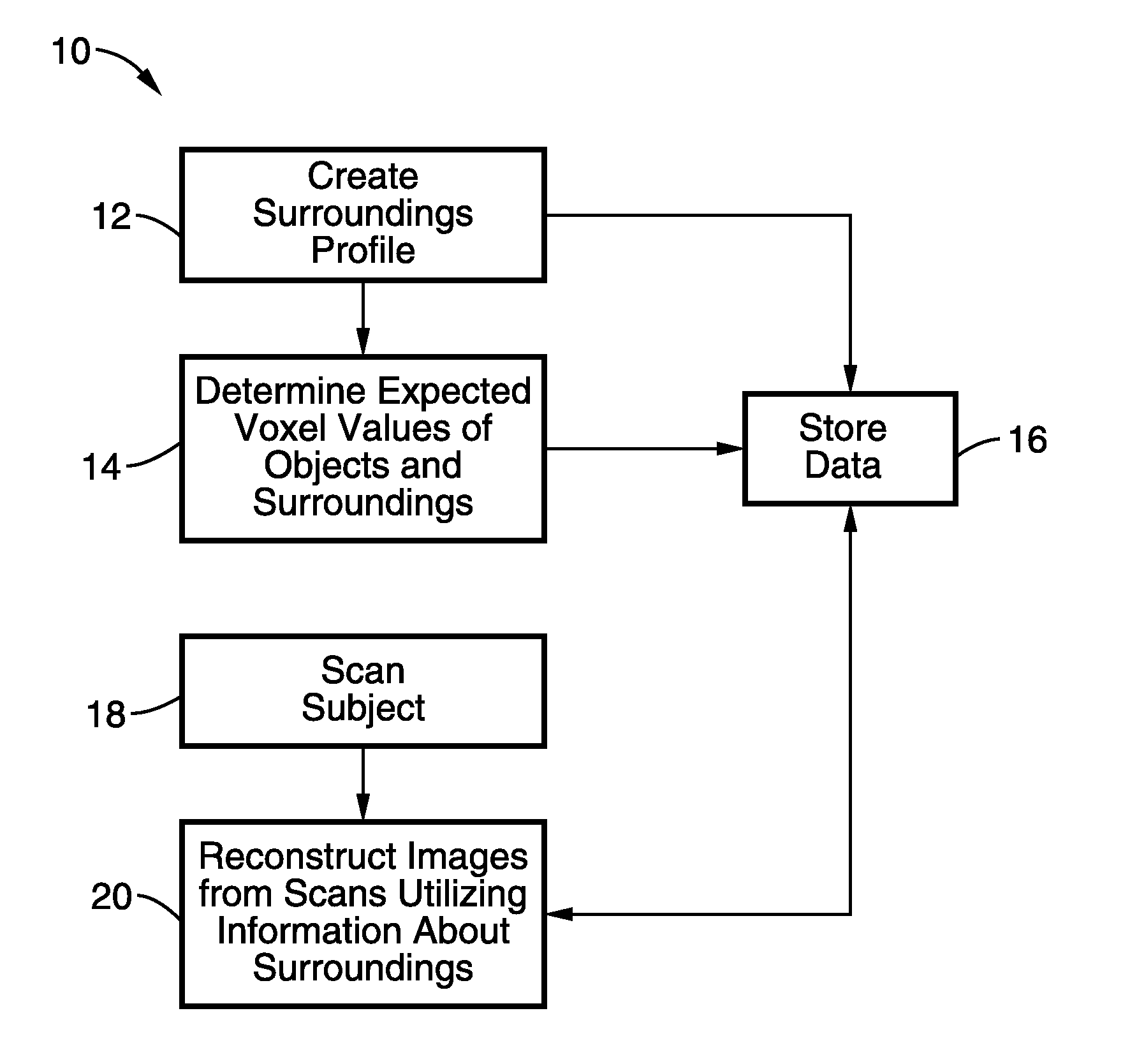
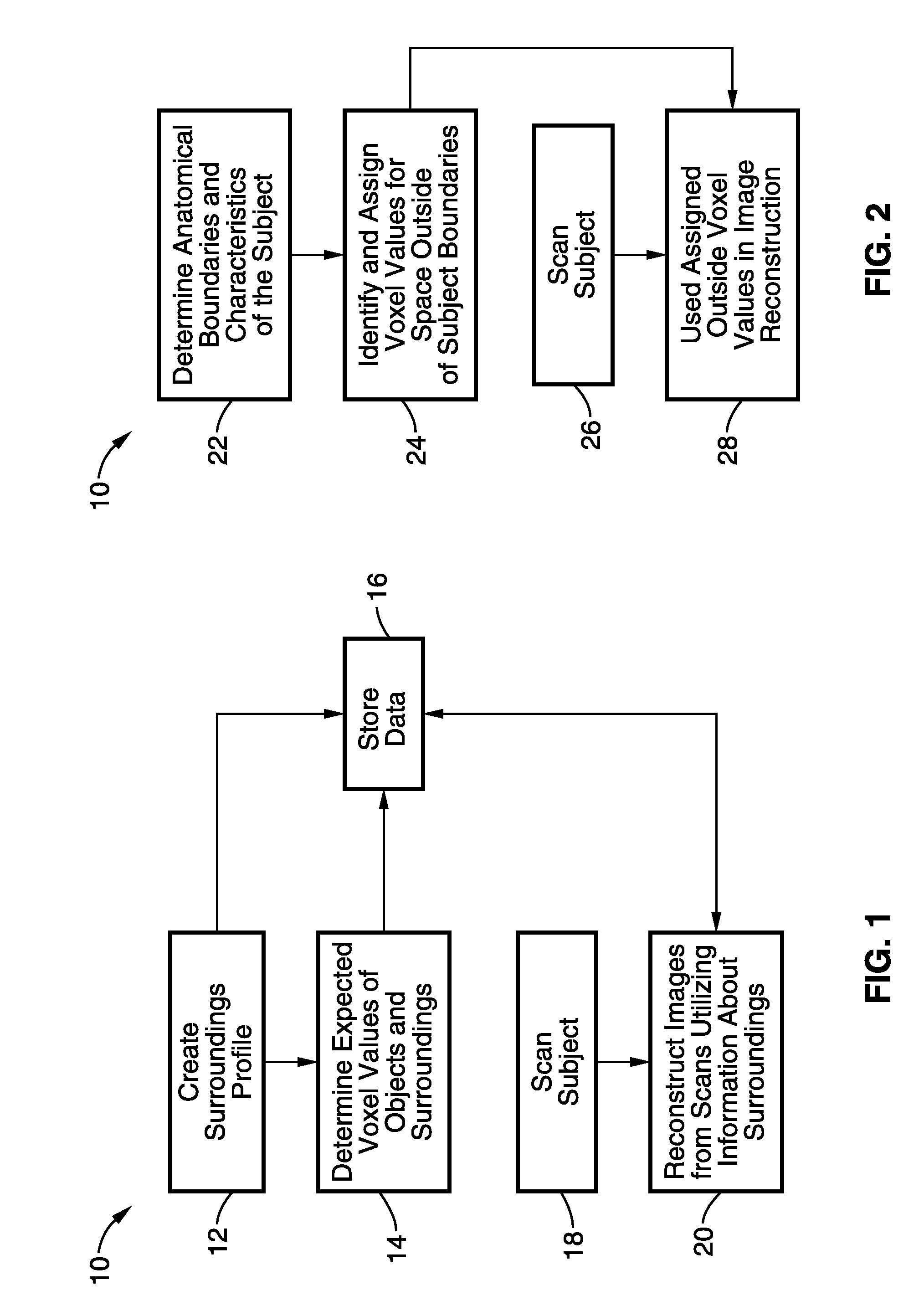
Dose reduction and image enhancement in tomography through the utilization of the objects surroundings as dynamic constraints
InactiveUS20110007980A1Solve the slow scanning speedReduce in quantityReconstruction from projectionMaterial analysis using wave/particle radiationImaging qualityReconstruction method
Image reconstruction methods are described where the characteristics of the surroundings of the patient in the scanner image field are used as supplemental information in the reconstruction process to reduce the dose, reduce scan times, scan number and / or improve the image quality of the reconstructed image.
Owner:RGT UNIV OF CALIFORNIA
Treatment methods with low-dose, longer-acting formulations of local anesthetics and other agents
InactiveUS20050119340A1Prolong the action timeProlonged durationBiocidePowder deliveryAnesthetic AgentDose reduction
Drug formulations that provide sustained action and / or reduced dosage requirements are provided. In the formulations the drugs (particularly local anesthetics) are associated with reversed cubic phase and reversed hexagonal phase lyotropic liquid crystalline material.
Owner:LYOTROPICS THERAPEUTICS INC
Method of dose reduction for CT imaging and apparatus for implementing same
ActiveUS8942341B2Material analysis using wave/particle radiationRadiation/particle handlingDose ReducedFiltration
A CT system includes an x-ray source configured to project an x-ray beam toward an object, a detector array, and a bowtie filter. The bowtie filter includes a first x-ray filtration region positioned to attenuate x-rays that pass through an isochannel of the detector array, a second x-ray filtration region positioned to attenuate x-rays that pass through channels of the detector array that are offcenter in a channel direction from the isochannel, and an x-ray attenuation material positionable to attenuate the x-rays that pass through the channels of the detector array that are offcenter in the channel direction from the isochannel. The CT system also includes a data acquisition system (DAS) connected to the detector array and configured to receive outputs from the detector array, and a computer programmed to acquire projections of imaging data of the object, and generate an image of the object using the imaging data.
Owner:GENERAL ELECTRIC CO
Supervised machine learning technique for reduction of radiation dose in computed tomography imaging
ActiveUS9332953B2Low-doseQuality improvementImage enhancementReconstruction from projectionDose ReducedComputed tomography
Substantial reduction of the radiation dose in computed tomography (CT) imaging is shown using a machine-learning dose-reduction technique. Techniques are provided that (1) enhance low-radiation dosage images, beyond just reducing noise, and (2) may be combined with other approaches, such as adaptive exposure techniques and iterative reconstruction, for radiation dose reduction.
Owner:UNIVERSITY OF CHICAGO
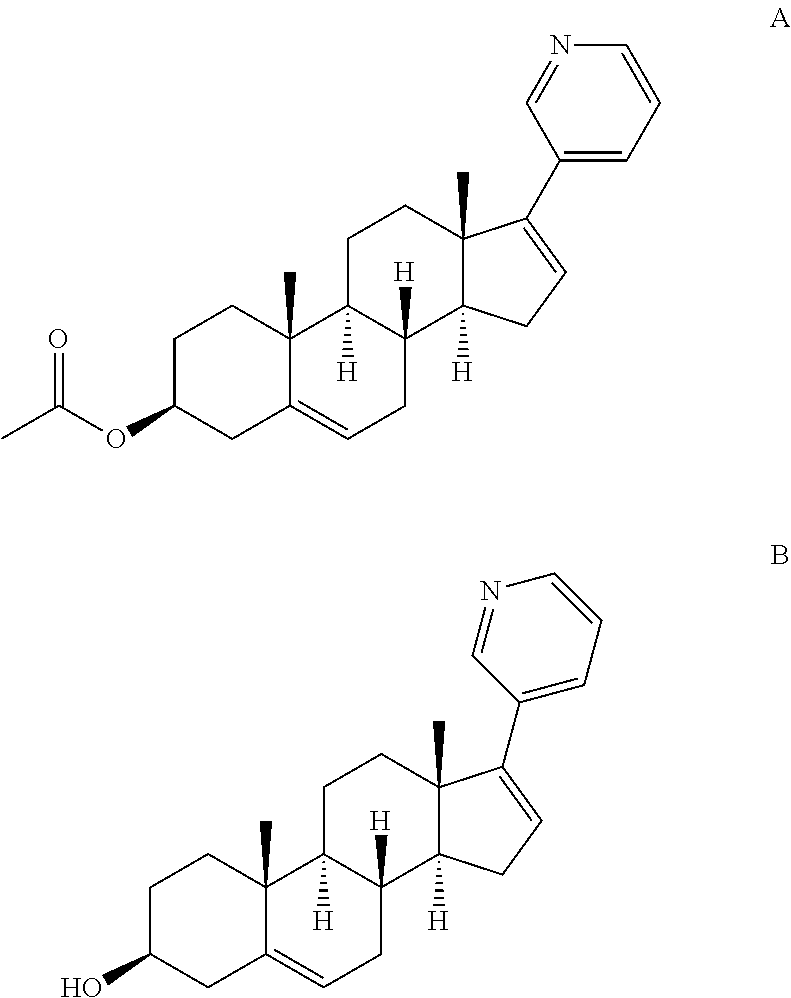
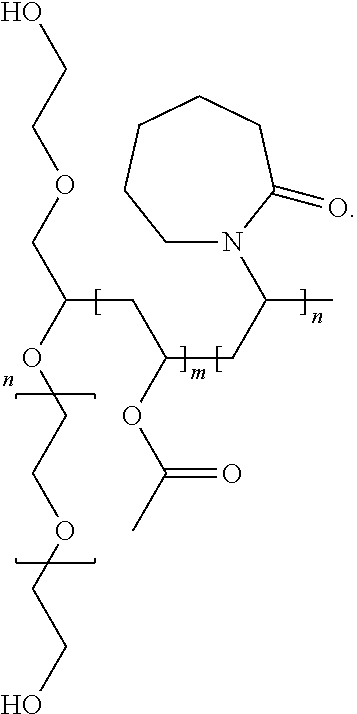
Complexes of abiraterone acetate, process for the preparation thereof and pharmaceutical compositions containing them
ActiveUS20160228455A1Improved physicochemical characteristicImprove biological performanceOrganic active ingredientsPowder deliveryFOOD EFFECTDose reduction
The present disclosure relates to pharmaceutically acceptable complex formulae comprising complexes of Abiraterone acetate and pharmaceutically acceptable excipients, process for the preparation thereof and pharmaceutical compositions containing them. The complex formulae of the present disclosure have improved physicochemical properties which results in reduced food effect which allows significant dose reduction and the abandoning of the requirement of taking the drug on an empty stomach.
Owner:TAVANTA THERAPEUTICS HUNGARY INC
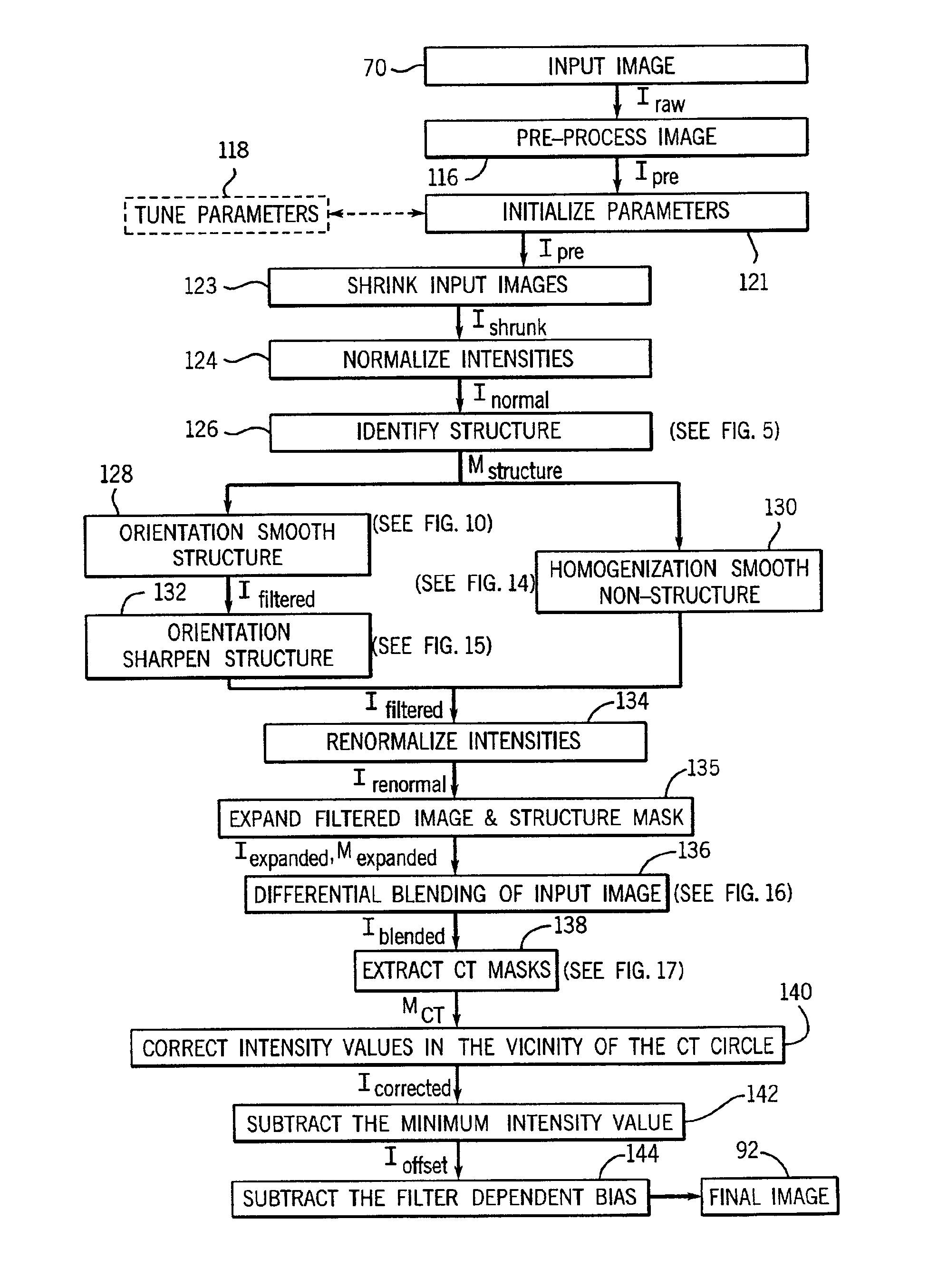
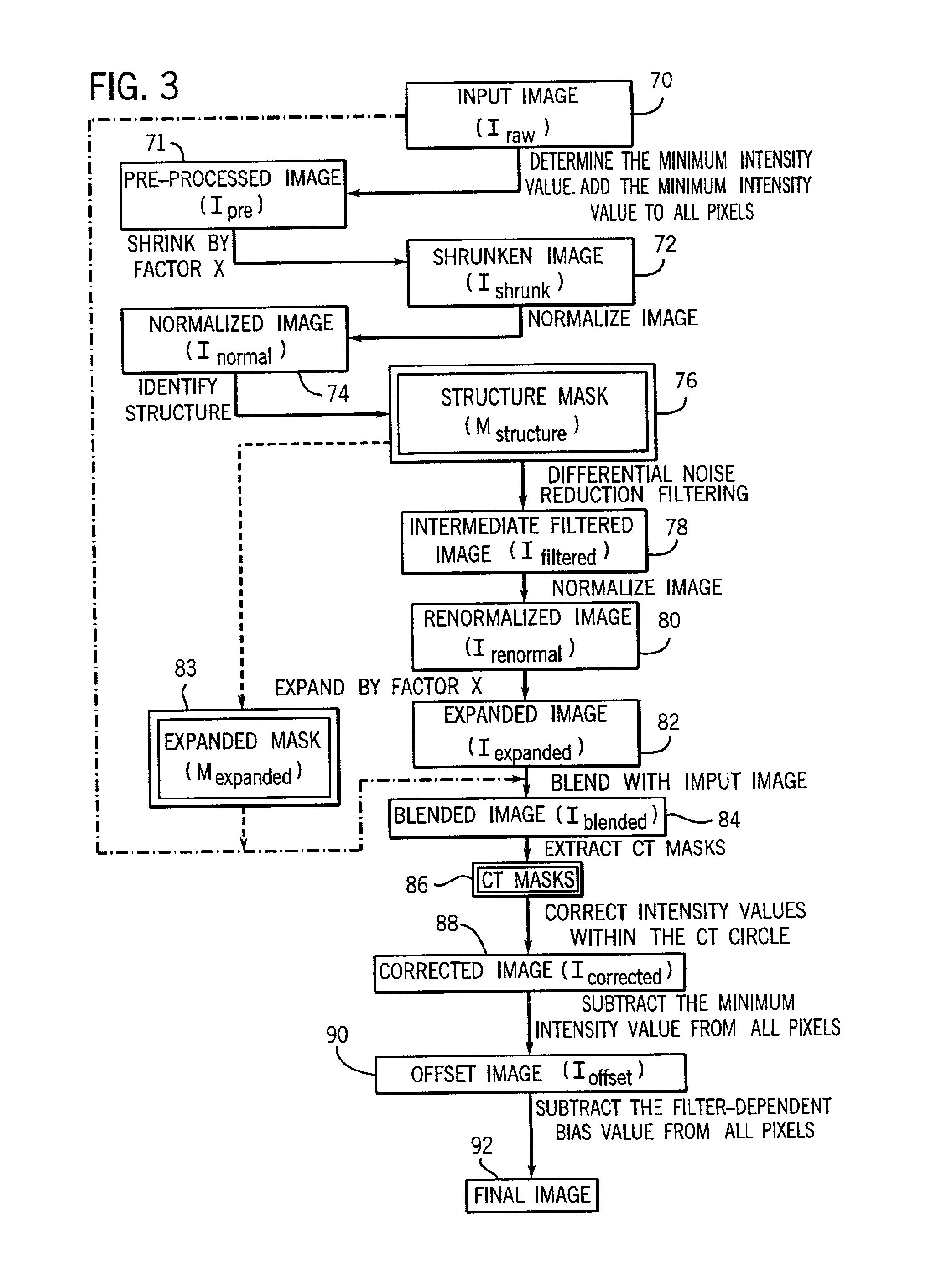
CT dose reduction filter with a computationally efficient implementation
InactiveUS6963670B2Improve computing efficiencyMaintain image qualityImage enhancementImage analysisImaging qualityDose reduction
A technique for reducing noise in pixel images includes modifying initial image data to prevent subsequent image data loss, shrinking the modified image, processing the shrunken image with known segmentation-based filtering techniques which identify and differentially processing structures within the image. After processing, the shrunken image is enlarged to the dimensions of the initial data and subsequently processed to correct intensity irregularities and to preserve CT numbers. The resulting technique is versatile and provides greatly improved computational efficiency while maintaining image quality, allowing for dose reduction during imaging.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
Dose reduced digital medical image simulations
A method for providing a reduced exposure value for radiographic imaging obtains a set of flat-field images at two or more exposure values and measures the noise power spectra using the flat field images. At least one noise table is generated according to interpolated noise power spectra for a set of predetermined exposure values. Values from the at least one noise table are applied to a clinical image to form a reduced exposure simulation image. A noise mask is generated according to at least one noise table and the exposure values of the reduced exposure simulation image and added to the reduced exposure simulation image. The reduced exposure simulation image is assessed to generate a desired dose reduction factor.
Owner:CARESTREAM HEALTH INC
Method of dose reduction for CT imaging and apparatus for implementing same
ActiveUS20130058451A1Material analysis using wave/particle radiationRadiation/particle handlingSoft x rayFiltration
A CT system includes an x-ray source configured to project an x-ray beam toward an object, a detector array, and a bowtie filter. The bowtie filter includes a first x-ray filtration region positioned to attenuate x-rays that pass through an isochannel of the detector array, a second x-ray filtration region positioned to attenuate x-rays that pass through channels of the detector array that are offcenter in a channel direction from the isochannel, and an x-ray attenuation material positionable to attenuate the x-rays that pass through the channels of the detector array that are offcenter in the channel direction from the isochannel. The CT system also includes a data acquisition system (DAS) connected to the detector array and configured to receive outputs from the detector array, and a computer programmed to acquire projections of imaging data of the object, and generate an image of the object using the imaging data.
Owner:GENERAL ELECTRIC CO
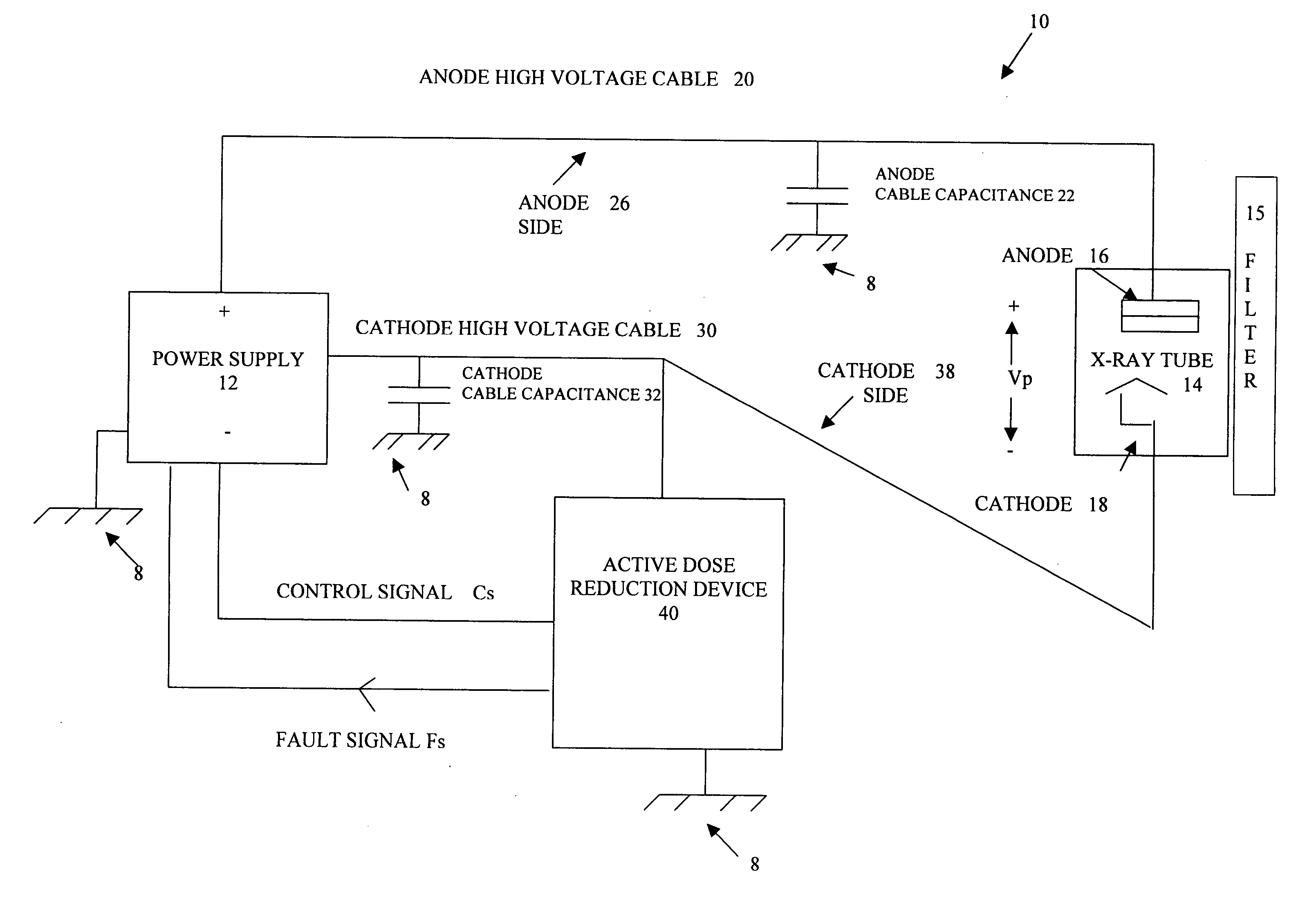
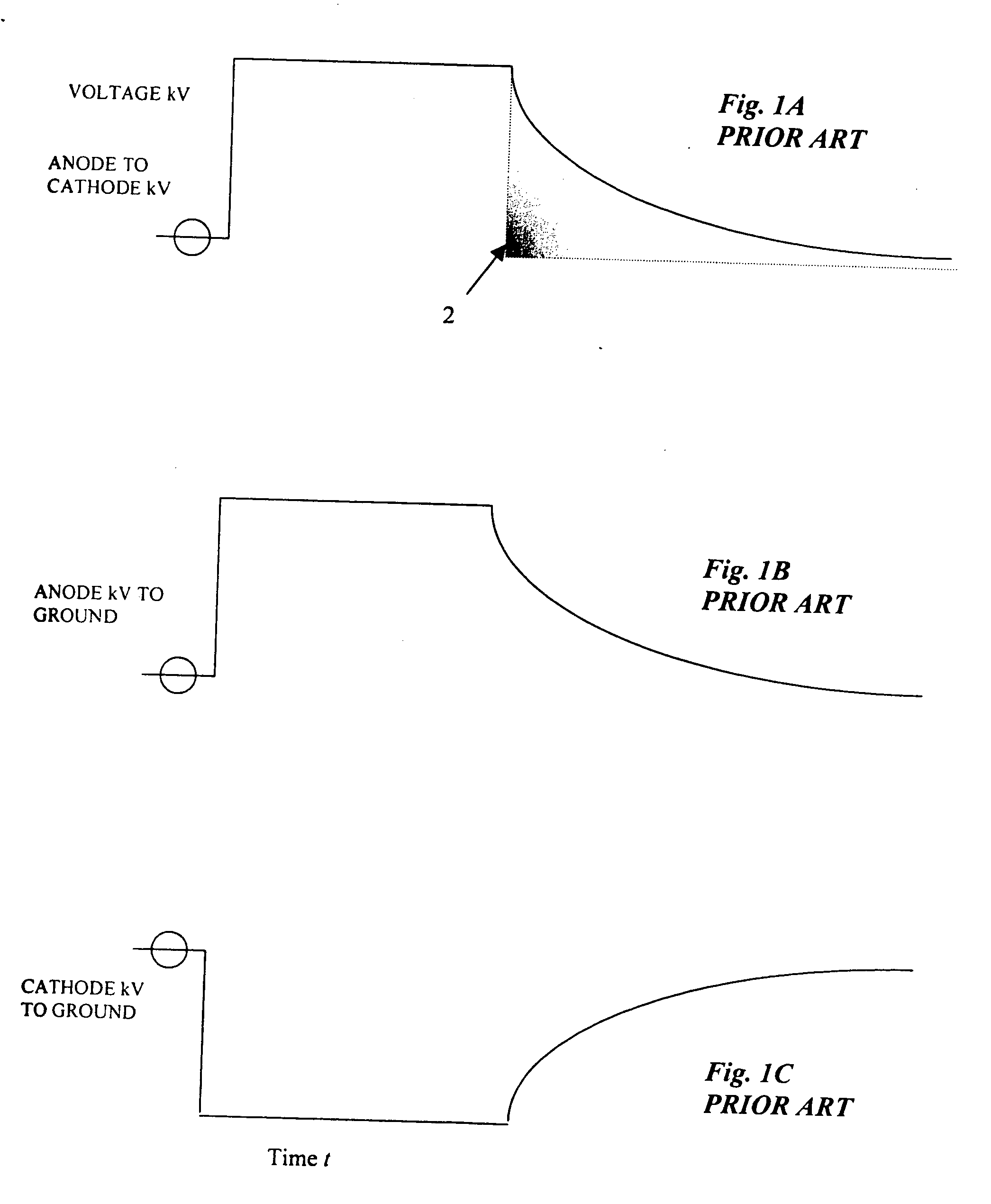
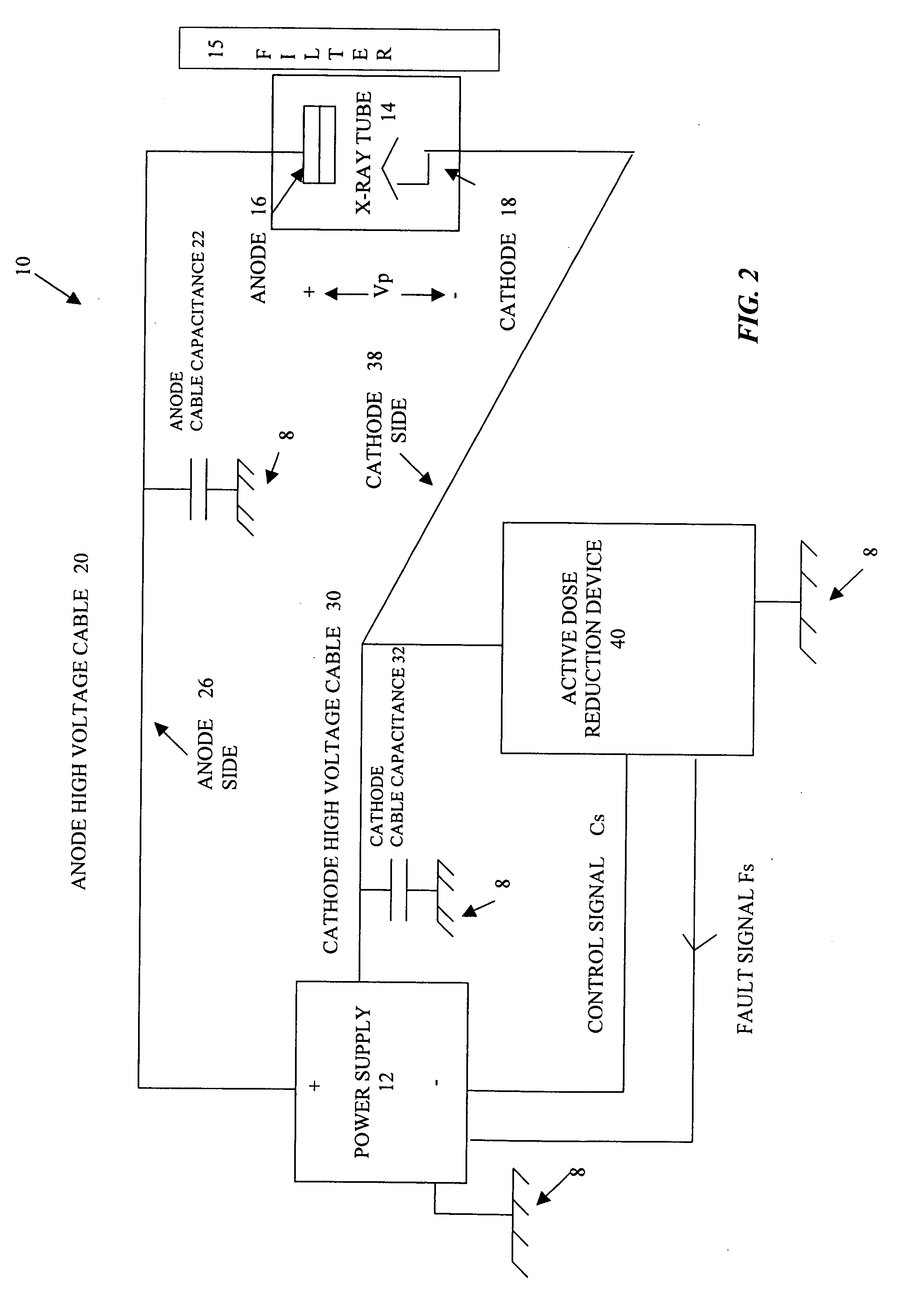
Active dose reduction device and method
InactiveUS20060018430A1Consume energyLow costX-ray apparatusRadiation diagnosticsOvervoltageVoltage pulse
An active dose radiation device and method decreases the amount of x-rays which may be generated by an x-ray tube following termination of the x-ray exposure. One component of the x-ray system is connected to the device which has a plurality of electronic cells that can be overvoltaged from a first state, where they are less conductive, to a second state, where they are highly conductive. A voltage pulse generator overvoltages a first cell in the plurality of cells at termination of an x-ray exposure. Overvoltaging the first cell causes a cascading effect which eventually overvoltages all of the cells and changes the state of the cells from the first less conductive state to the second highly conductive state. In the second highly conductive state, the cells can conduct current from at least one component of the x-ray system to ground thereby reducing x-rays generated by the x-ray tube and reducing the x-ray dosage from the imaging system after the x-ray exposure has been completed. The plurality of cells automatically reverts to the first state once current passing through the cells decreases below a threshold current level. The plurality of cells are connected to only the anode side 26 or the cathode side 38 of the x-ray imaging device in order to discharge energy from only one side to ground which results in a sufficient decrease in the voltage across the x-ray tube to prevent generation of active dosage of x-rays which may penetrate the patient.
Owner:COMM & POWER IND CANADA
Clindamycin palmitate hydrochloride particle and preparation method thereof
The invention provides a clindamycin palmitate hydrochloride particle and a preparation method thereof. The clindamycin palmitate hydrochloride particle provided by the invention consists of a drug pill core and a coating layer covering the outside of the drug pill core, wherein the drug pill core comprises clindamycin palmitate hydrochloride, a carrier and a water-soluble pore-forming agent; andthe coating layer comprises a pH-dependent coating material. According to the clindamycin palmitate hydrochloride particle provided by the invention, a soft material is prepared by virtue of a wet process / the drug pill core is prepared by virtue of an extrusion-spheronisation process firstly, and then the soft material or the drug pill core is coated by coating liquid by virtue of a spray coatingprocess. The clindamycin palmitate hydrochloride particle provided by the invention is applicable to children; the integrity of the particle can be kept before the particle is taken, and the particle,after entering human bodies, can be slowly released, so that a stable and lasting blood concentration can be provided, and clinical demands of reducing the times of administration, reducing dose-related toxicity and improving patient compliance can be satisfied; and meanwhile, the bad taste of the medicine (the particle) can be masked without adding a great amount of flavoring agents.
Owner:GUANGZHOU DAGUANG PHARMA
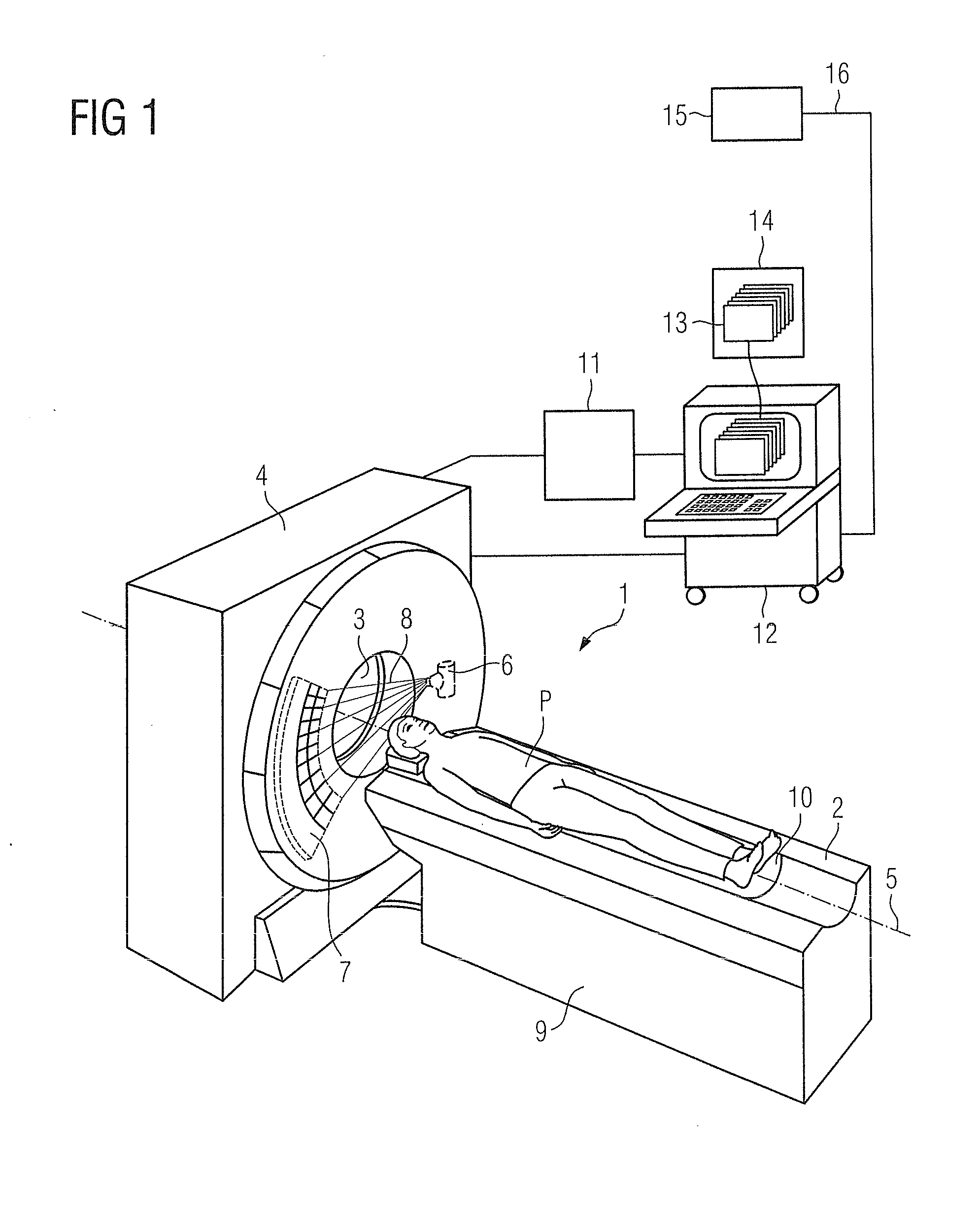
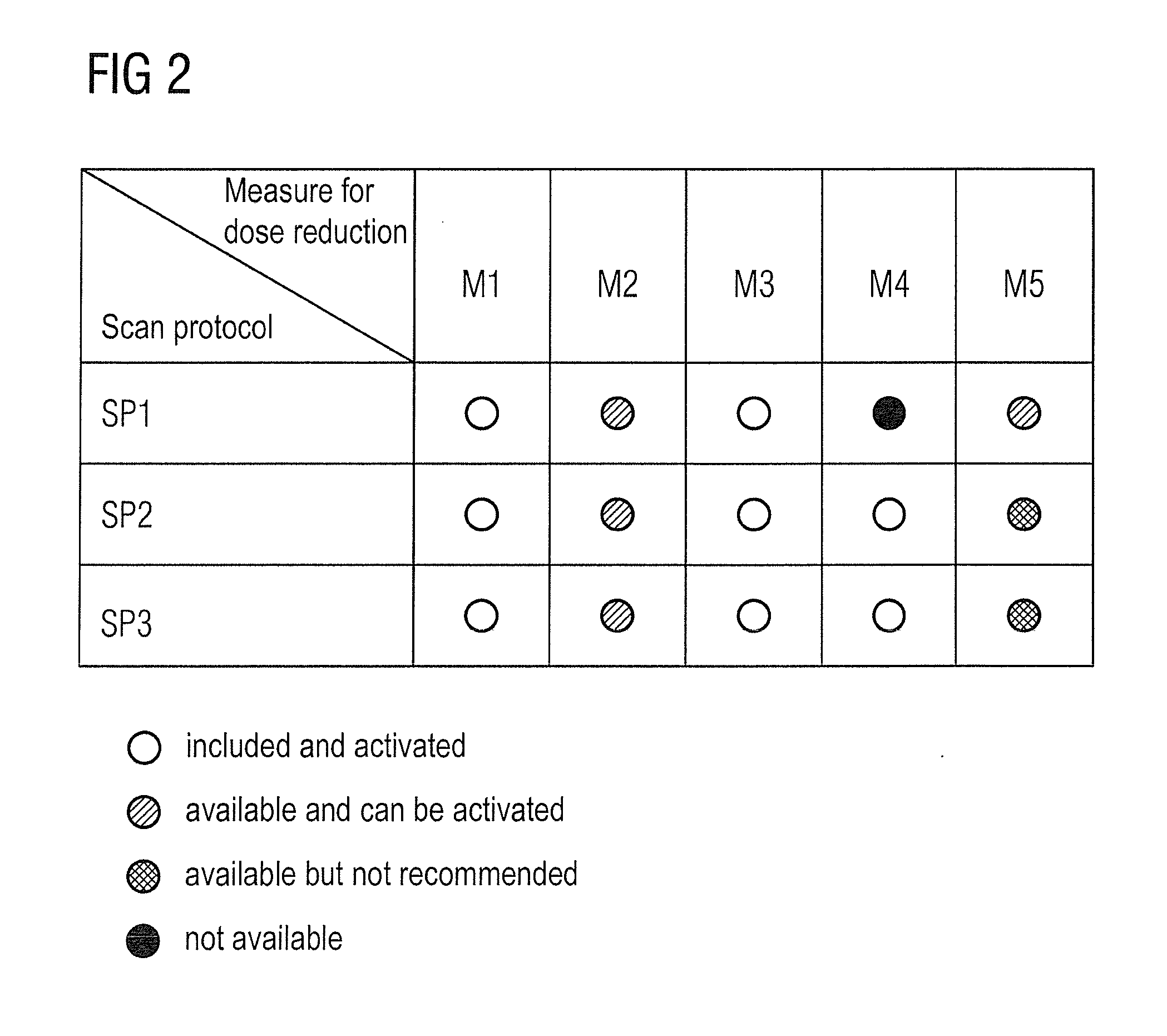
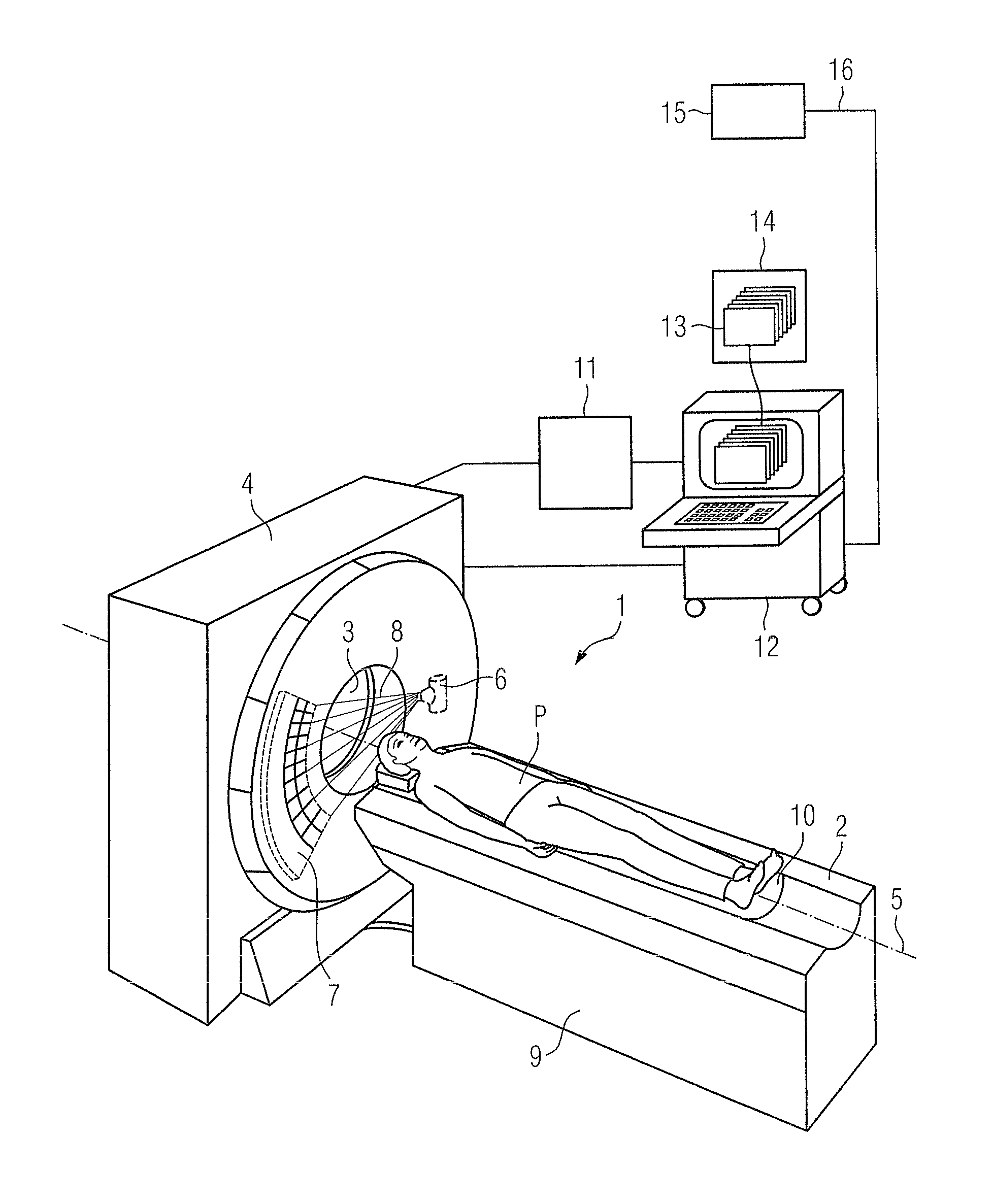
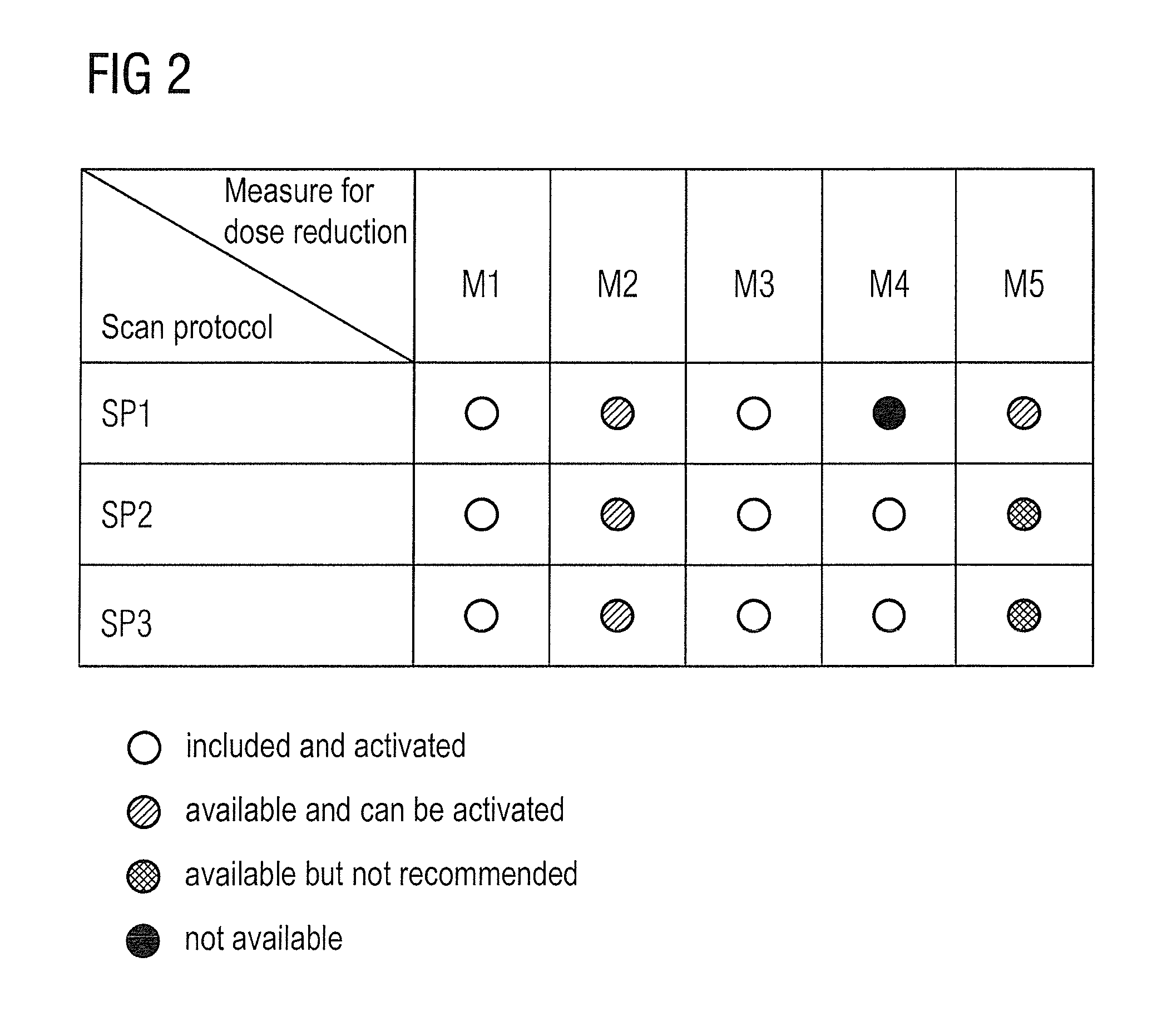
Method and device to assist in dose reduction of x-ray radiation applied to a patient
ActiveUS20120128121A1Simple working processReduce stepsMaterial analysis using wave/particle radiationRadiation/particle handlingSoft x rayX-ray
In a method and x-ray device to assist in the reduction of the dose of x-ray radiation applied to the patient in the acquisition of at least one x-ray projection of the patient, a selected protocol is entered by a user into an x-ray device, the selected protocol being defined for the acquisition of the at least one x-ray projection and including a measure or measures for reduction of the dose of x-ray radiation to be applied to the patient in the acquisition of the at least one x-ray projection. The does reduction measure or measures in the selected protocol are automatically compared in a processor of the x-ray device, with the dose reduction measures that are installed at the x-ray device for reduction of the dose of x-ray radiation to be applied to a patient in the acquisition of the at least one x-ray projection. If the result of the comparison indicates a discrepancy between the dose reduction measure or measures in the selected, defined protocol and those installed in the x-ray device, at least one such measure installed at the x-ray device is visualized as a measure that can be set or activated by the user, that is not yet included in the selected protocol.
Owner:SIEMENS HEALTHCARE GMBH
Control of radiation injury
The invention relates to the field of drug development against acute radiation injury caused by exposure to high-energy electromagnetic waves (X-rays, gamma rays) or particles (alpha particles, beta particles, neutrons). To date, there is no effective drug to ameliorate radiation injury after accidental exposure to ionizing irradiation. The invention provides a method of treating radiation injury of a subject in need thereof comprising administering to the subject a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids. Furthermore, the invention provides use of a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids for the production of a pharmaceutical composition for the treatment of a subject suffering from or believed to be suffering from radiation injury. In particular, the invention provides anti-radiation peptides having a dose reduction factor (DRF) against acute gamma irradiation of at least 1.10, said DRF determinable by testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a test group of mice treated with said peptide at 72 hours after WBI and, testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a control group of mice treated only with the vehicle of said peptide at 72 hours after WBI and wherein the DRF is calculated by dividing the LD50 / 30 of the peptide-treated animals by the LD50 / 30 of the vehicle-treated animals.
Owner:BIOTEMPT
Method and device to assist in dose reduction of X-ray radiation applied to a patient
ActiveUS8804900B2Simple working processReduce stepsMaterial analysis using wave/particle radiationRadiation/particle handlingX-rayDose reduction
In a method and x-ray device to assist in the reduction of the dose of x-ray radiation applied to the patient in the acquisition of at least one x-ray projection of the patient, a selected protocol is entered by a user into an x-ray device, the selected protocol being defined for the acquisition of the at least one x-ray projection and including a measure or measures for reduction of the dose of x-ray radiation to be applied to the patient in the acquisition of the at least one x-ray projection. The does reduction measure or measures in the selected protocol are automatically compared in a processor of the x-ray device, with the dose reduction measures that are installed at the x-ray device for reduction of the dose of x-ray radiation to be applied to a patient in the acquisition of the at least one x-ray projection. If the result of the comparison indicates a discrepancy between the dose reduction measure or measures in the selected, defined protocol and those installed in the x-ray device, at least one such measure installed at the x-ray device is visualized as a measure that can be set or activated by the user, that is not yet included in the selected protocol.
Owner:SIEMENS HEALTHCARE GMBH
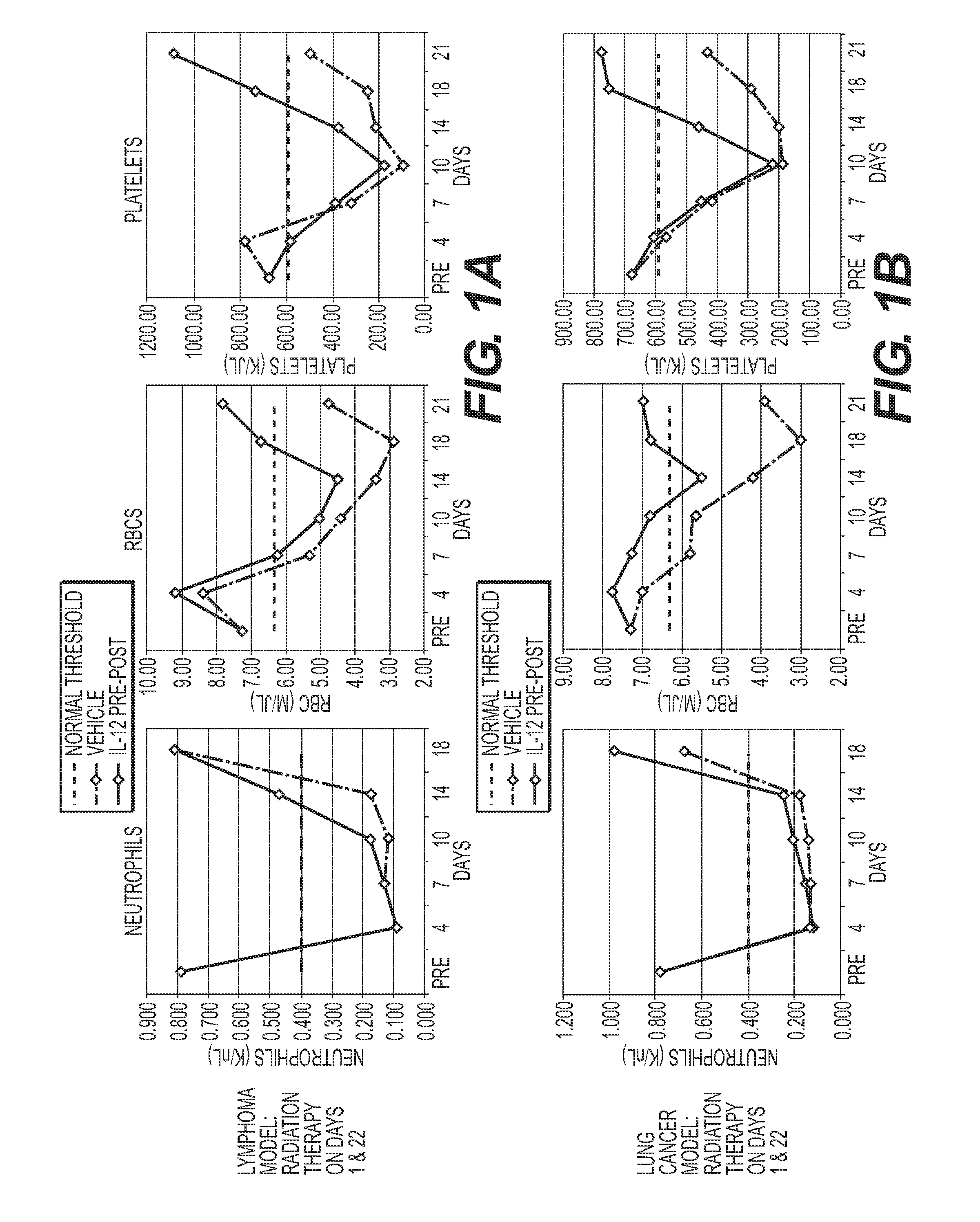
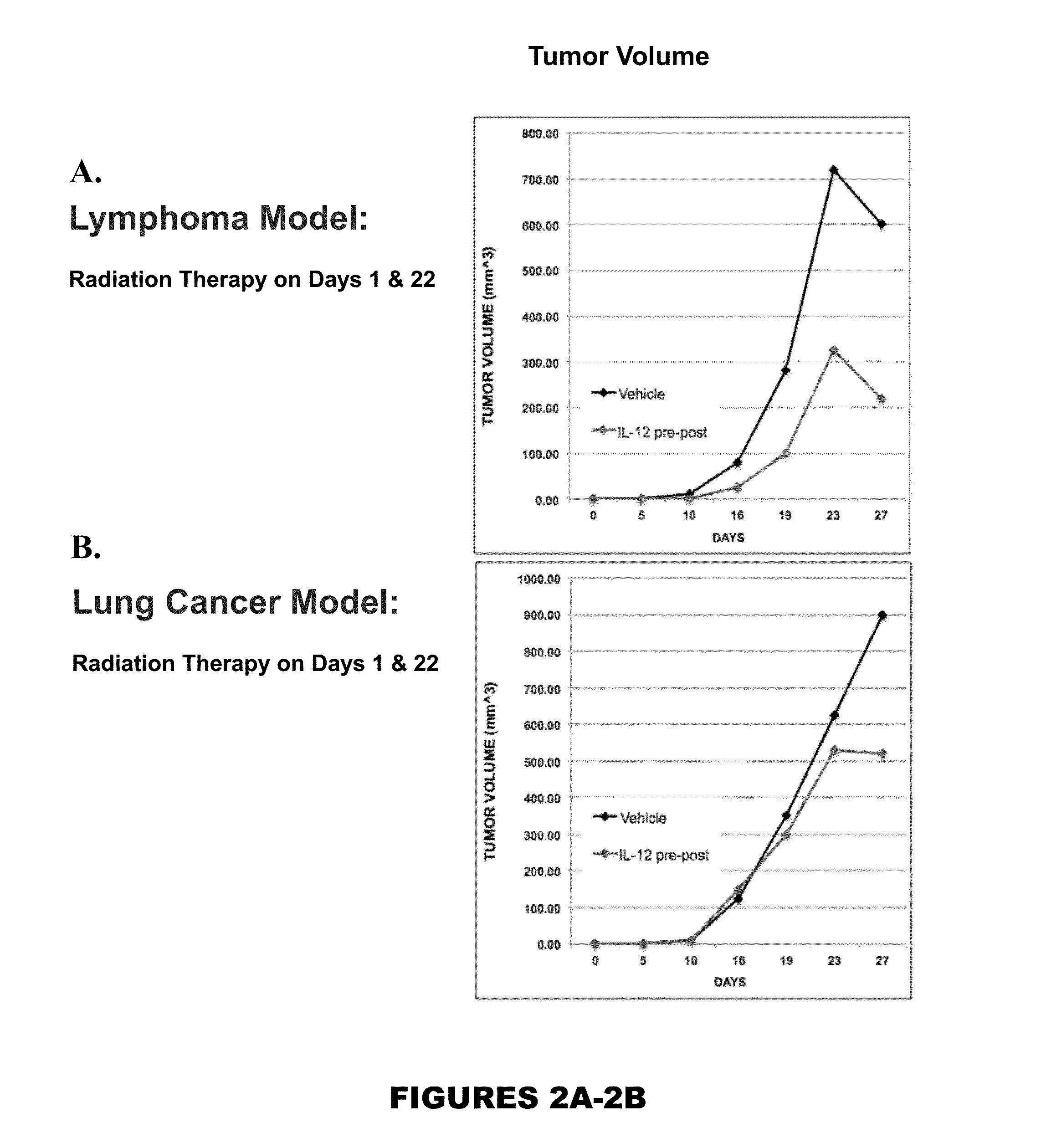
Uses of il-12 in hematopoietic immunotherapy (HIT)
InactiveUS20160120949A1Reduce tumor burdenSurvival time is longOrganic active ingredientsPeptide/protein ingredientsCancer preventionDose reduction
Aspects and embodiments of the instant disclosure provide therapeutic methods and compositions comprising interleukin 12 (IL-12) as a hematopoietic immunotherapy (HIT) useful for treating or preventing a cancer patient from chemotherapy-induced cytopenias necessitating a dose reduction and / or dose delay. following exposure of the patient to chemotherapeutic agents, the method comprising: administering a dose of therapeutically effective amount of a pharmaceutical composition comprising substantially isolated IL-12 to the subject, whereby cytopenias are reduced and leading to increases responses to the chemotherapy agent(s).diminished.
Owner:NEUMEDICINES INC
Use of peptides for the control of radiation injury
ActiveCN101443080AConvenience to workPeptide/protein ingredientsMedical devicesWhole bodyGamma irradiation
The invention relates to the field of drug development against acute radiation injury caused by exposure to high-energy electromagnetic waves (X-rays, gamma rays) or particles (alpha particles, beta particles, neutrons). To date, there is no effective drug to ameliorate radiation injury after accidental exposure to ionizing irradiation. The invention provides a method of treating radiation injury of a subject in need thereof comprising administering to the subject a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids. Furthermore, the invention provides use of a peptide, or functional analogue or derivative thereof, of smaller than 30 amino acids for the production of a pharmaceutical composition for the treatment of a subject suffering from or believed to be suffering from radiation injury. In particular, the invention provides anti-radiation peptides having a dose reduction factor (DRF) against acute gamma irradiation of at least 1.10, said DRF determinable by testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a test group of mice treated with said peptide at 72 hours after WBI and, testing which dose of radiation results in 50% mortality at 30 days (LD50 / 30) after whole body radiation (WBI) in a control group of mice treated only with the vehicle of said peptide at 72 hours after WBI and wherein the DRF is calculated by dividing the LD5O / 3O of the peptide-treated animals by the LD50 / 30 of the vehicle-treated animals.
Owner:BIOTEMPT
Compound composition containing eugenol and mustard essential oil and application
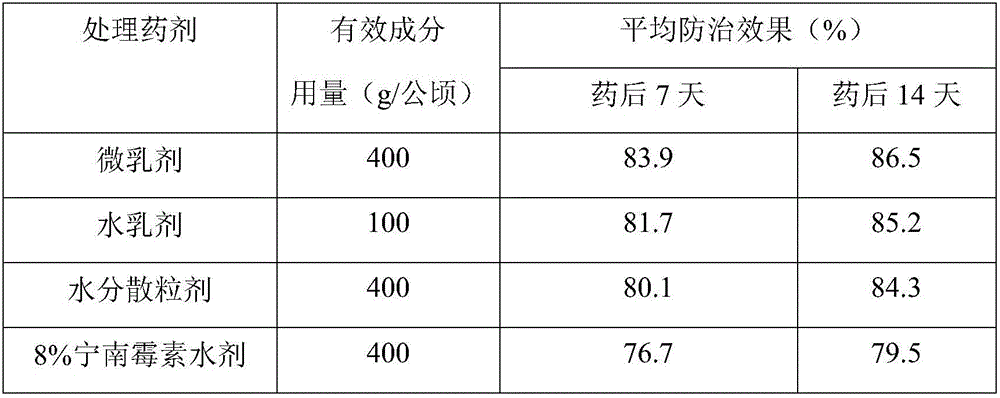
ActiveCN106070343ASynergisticGood control effectBiocideDead animal preservationWater dispersibleEugenol
The invention provides compound composition containing eugenol and mustard essential oil which are plant-sourced active substances with environmental compatibility and mixed in a weight ratio being (1:10)-(10:1), preferably in a weight ratio being (1:4)-(4:1). The compound composition has an obvious synergic effect, can be prepared in a form such as microemulsion, emulsion in water, water dispersible granules and the like and is used for controlling plant fungal diseases, bacteriosis, virus diseases and the like, so that the control effect is improved, the dosage is reduced, the application times are reduced, the control range is enlarged, the resistance is delayed, the requirement of a conventional dose reduction application technology is met, and the prepared preparation is environmentally friendly and has good application prospect.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com