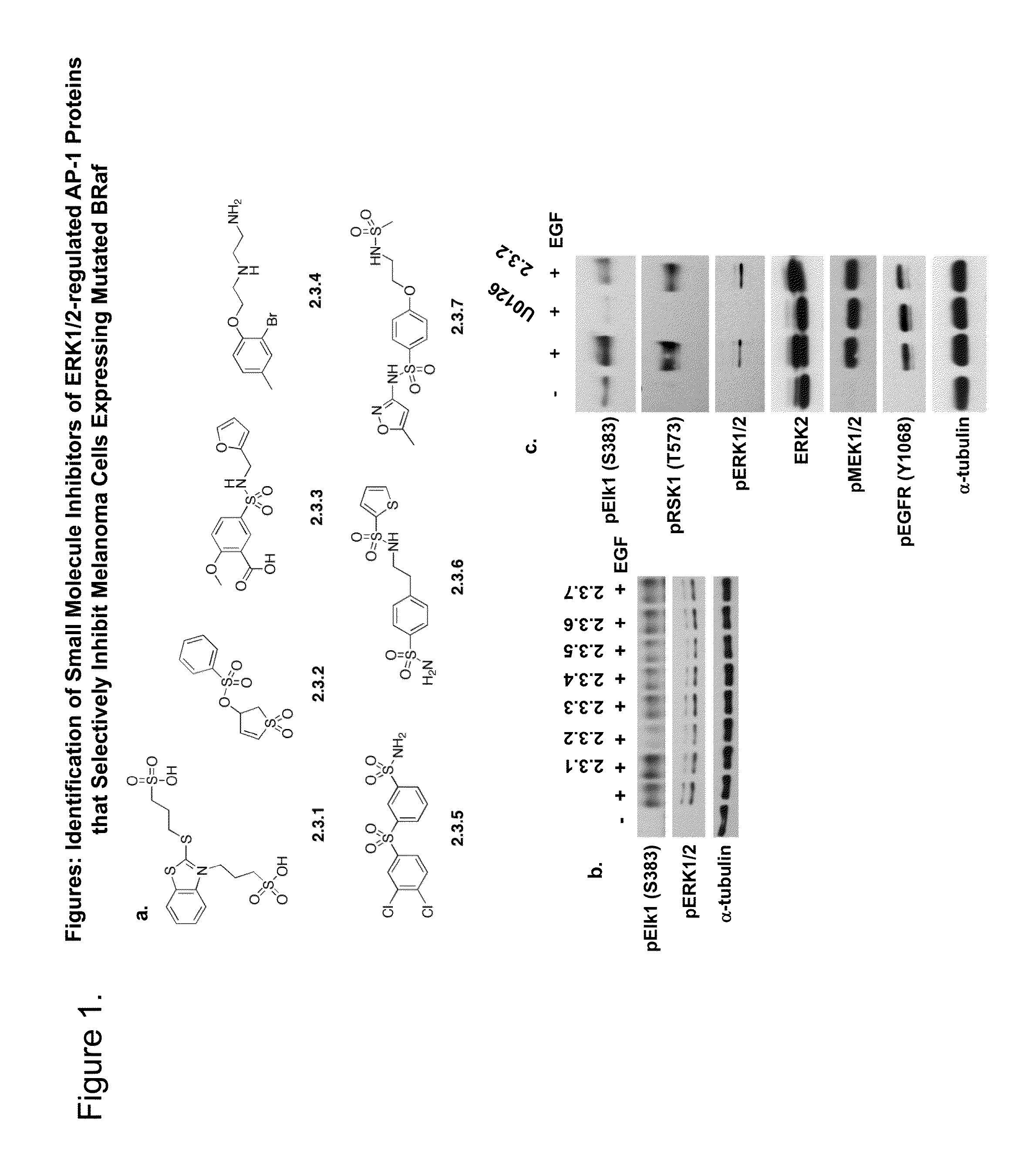
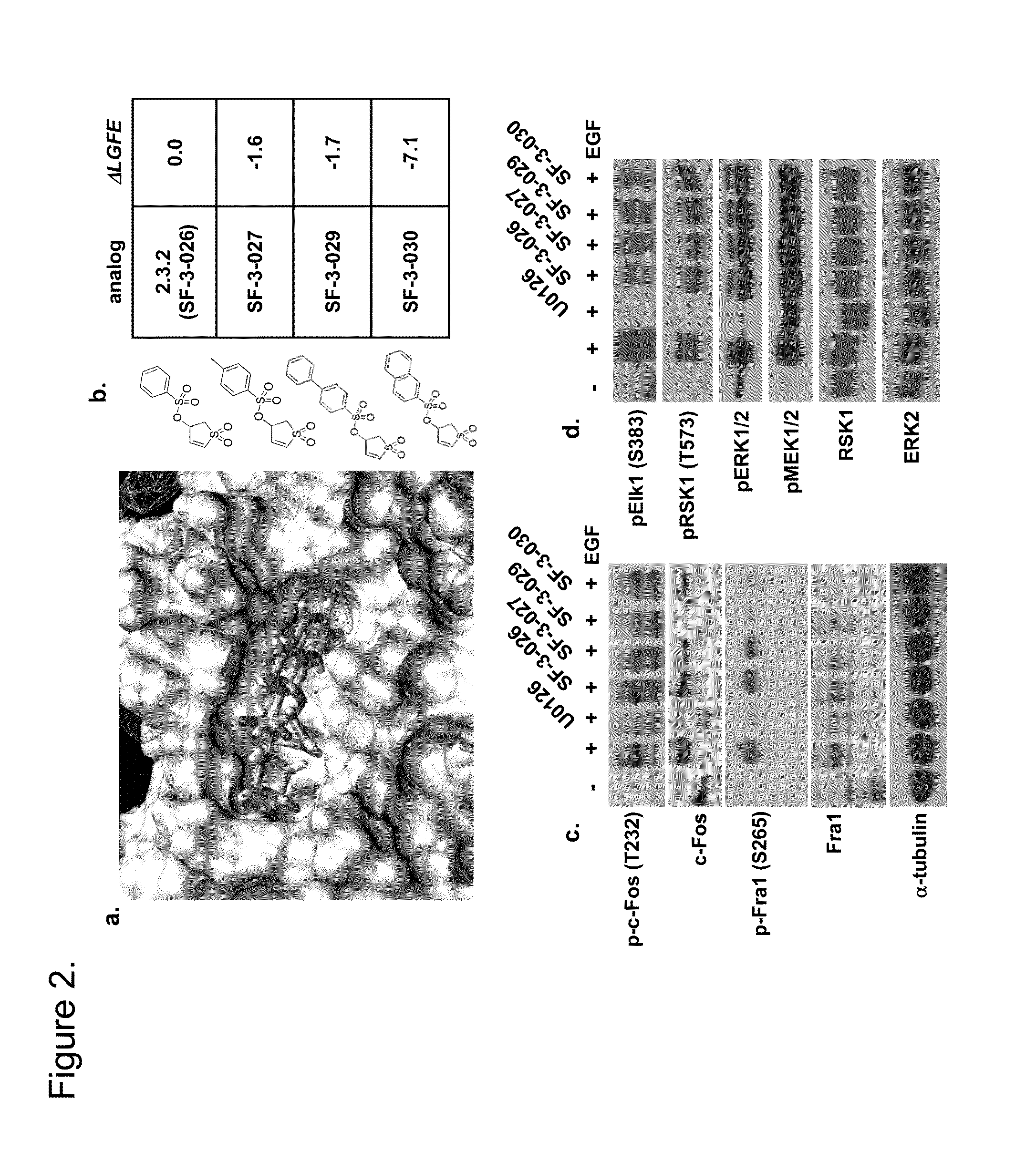
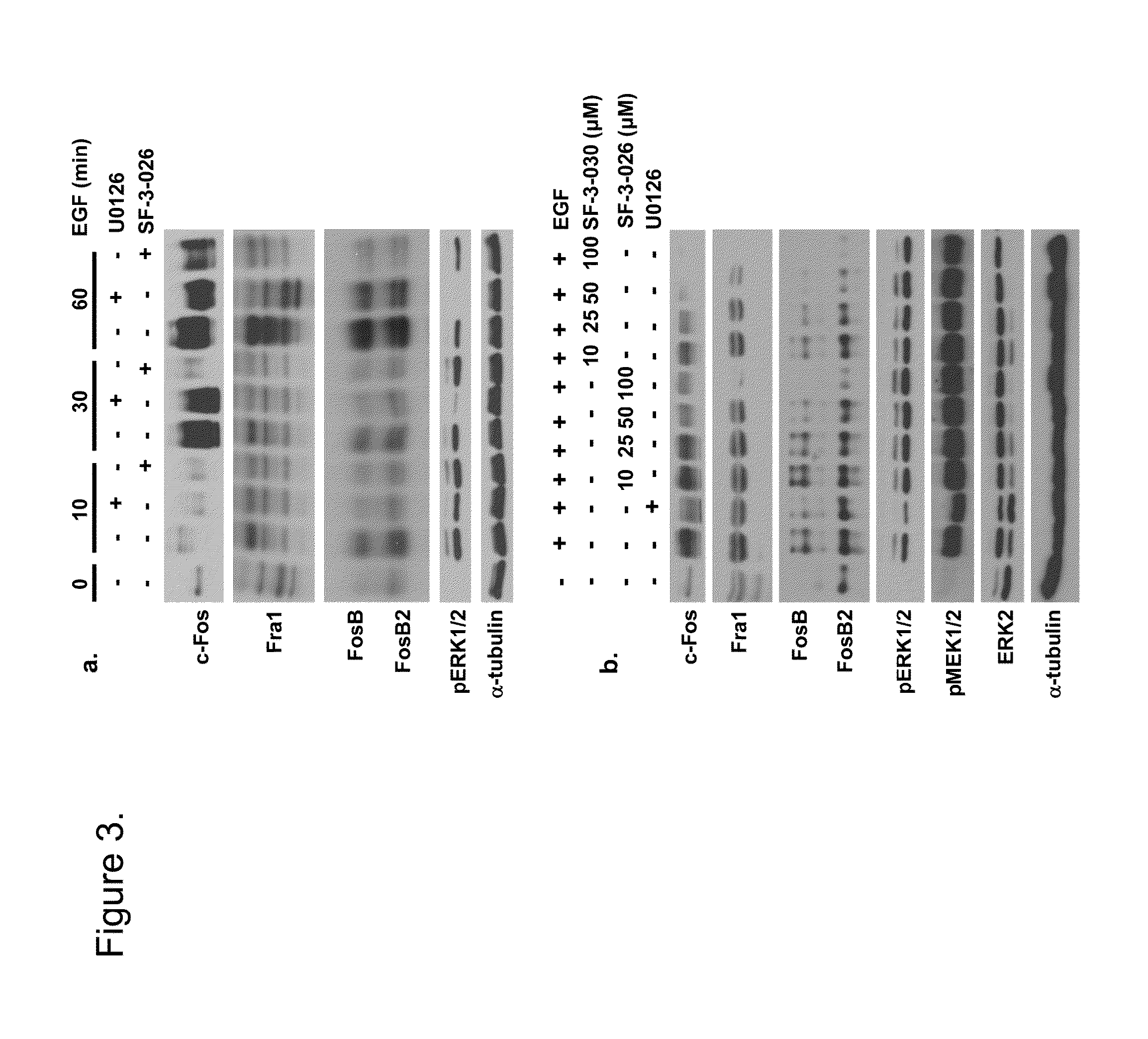
Patents
Literature
53 results about "Extracellular signal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
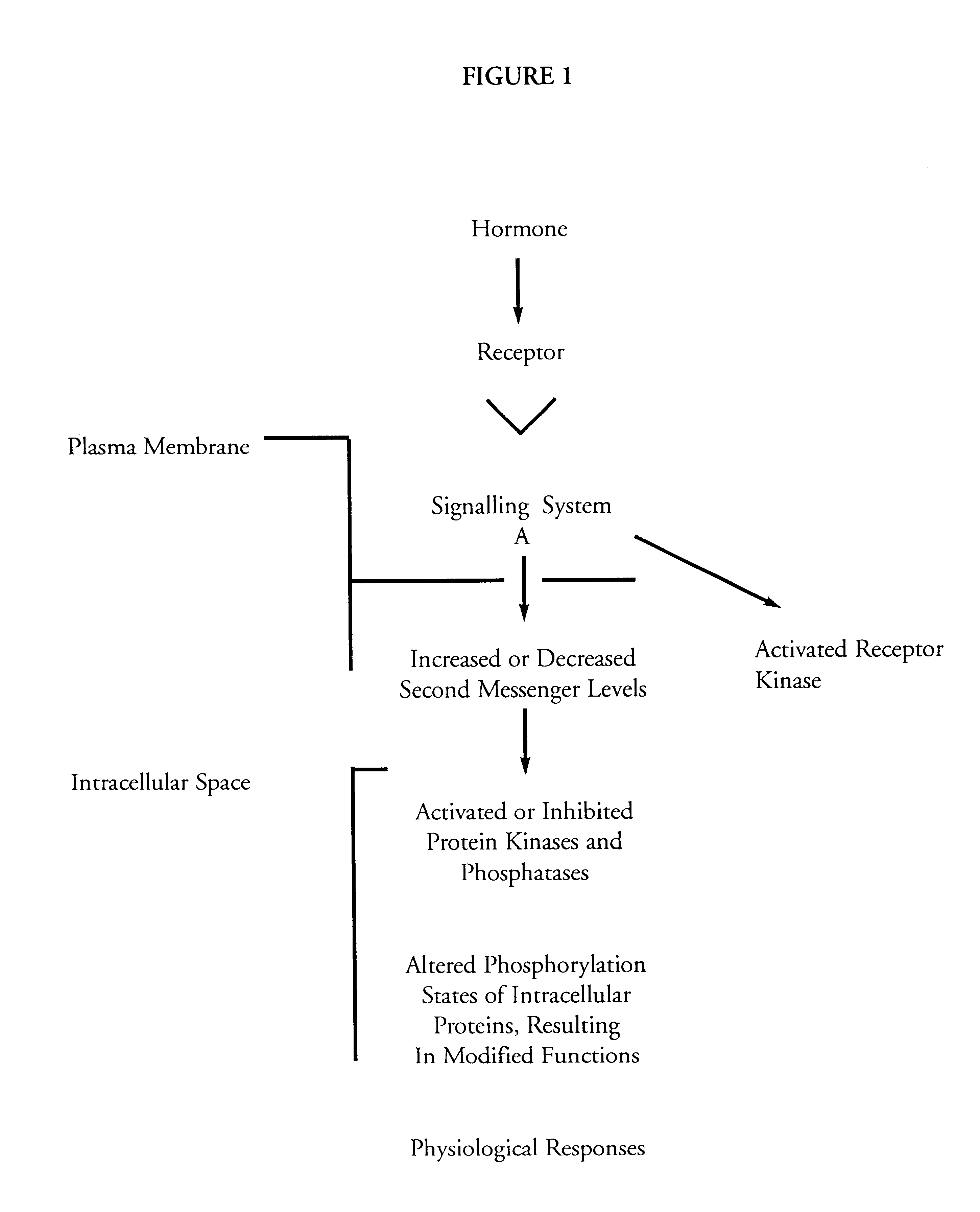
Extracellular signaling molecule: an extracellular signaling molecule is produced by one cell and is at least capable of traveling to neighboring cells. Receptor protein: cells must have cell surface receptor proteins which bind to the signaling molecule and communicate inward into the cell.
Inhibitors for extracellular signal-regulated kinase docking domains and uses therefor
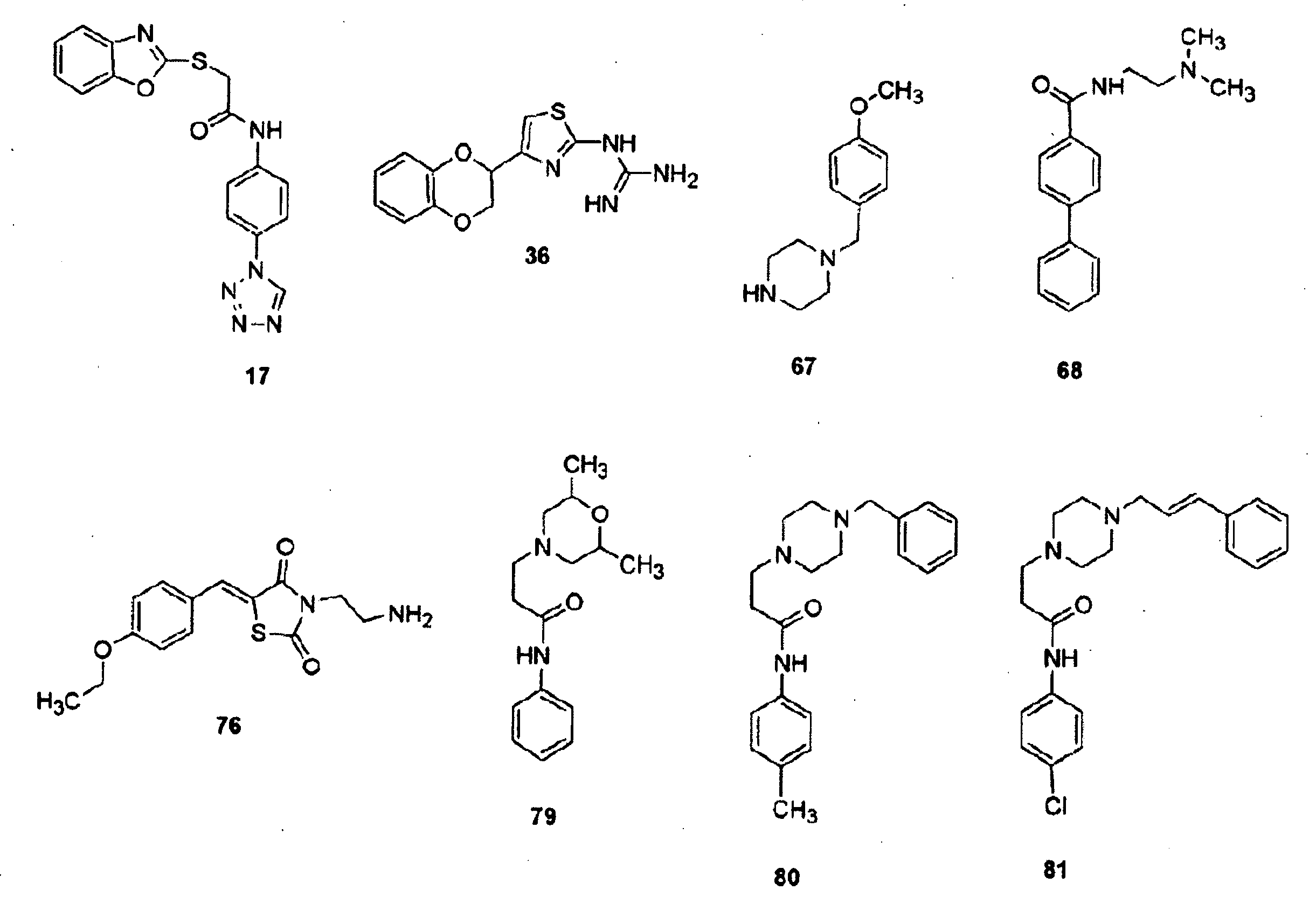
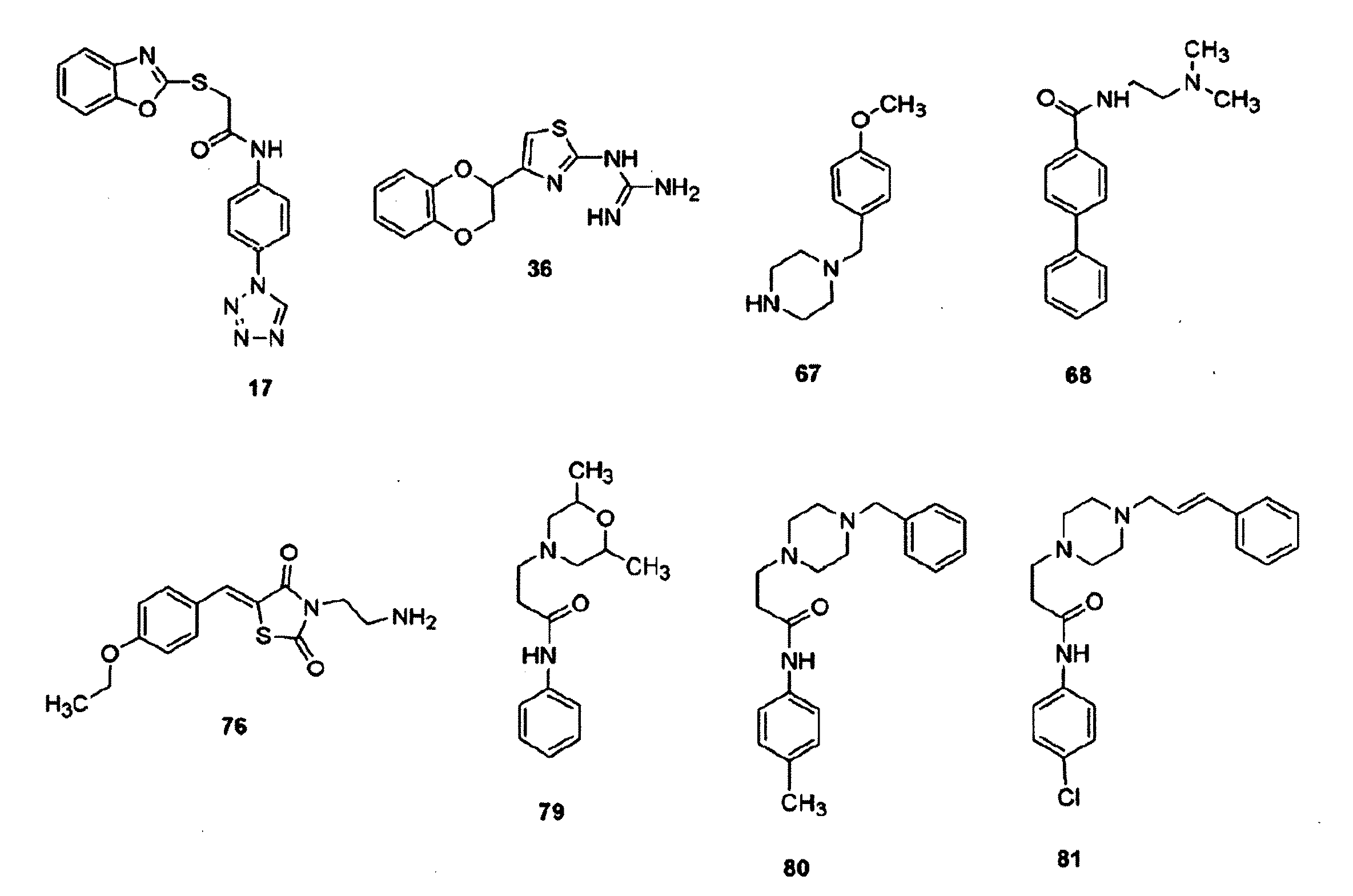
InactiveUS20070066616A1Inhibit cell proliferationReduce cell proliferationBiocideAnimal repellantsExtracellular signalComputer aid
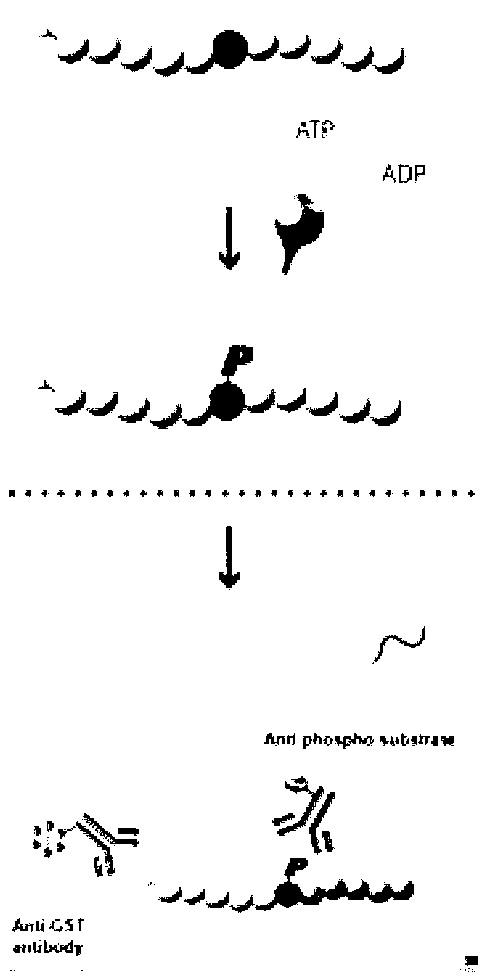
Provided herein are compounds and methods of using compounds that selectively inhibit binding to one or more docking domain regions of an extracellular signal-regulated kinase (ERK) to inhibit in a cell having an extracellular signal-regulated kinase activity. Such methods may be used to inhibit cell proliferation of a neoplastic cell, to treat a cancer and further may be used in conjunction with administration of an anticancer drug at a reduced dosage to treat a cancer with a concomitant reduction in toxicity to an individual receiving the treatment. Also provided is a method to design and screen for compounds to inhibit binding within the extracellular signal-regulated kinase docking domain region, using at least in part computer-aided drug design modeling.
Owner:SHAPIRO PAUL +1
Compound with MEK (Mitogen-activated and Extracellular signal-regulated Kinase) inhibiting function as well as preparation method and application of compound
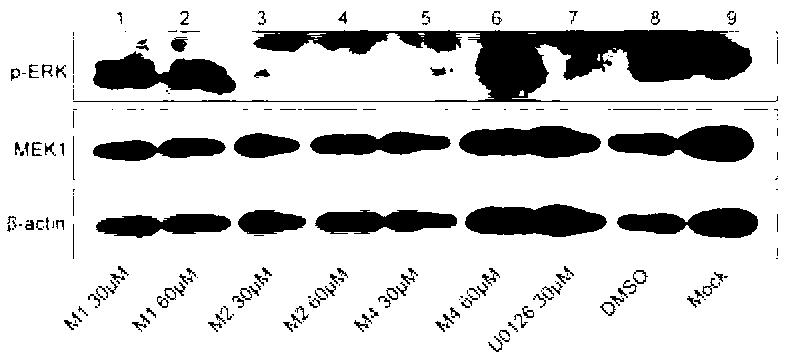
The invention discloses a compound with an MEK (Mitogen-activated and Extracellular signal-regulated Kinase) inhibiting function as well as a preparation method and application of the compound. The structure of the compound disclosed by the invention is as shown in a formula (I). The preparation method of the compound disclosed by the invention comprises the steps of forming a coumarin ring by adopting a Pechmann reaction mainly and carrying out structural modification of different sites. In the binding experiment of the compound disclosed by the invention and MEK, the binding activity is up to 54.57nM; the anti-proliferation effect IC50 (half maximal inhibitory concentration) value on the melanoma cell A375 is up to 1.23 micrometers; the anti-proliferation effect IC50 value on the colon cancer cell HT-29 is up to 2.13 micrometers; and the activity is higher than that of a positive control U0126. The novel structure type coumarins compound with the MEK inhibiting function shows good MEK binding activity, MEK inhibiting activity, ERK (Extracellular signal Regulated Kinase) pathway inhibiting activity, anti-tumor effect and antiviral effect and has broad application value. The formula (I) is as shown in the specification.
Owner:PEKING UNIV
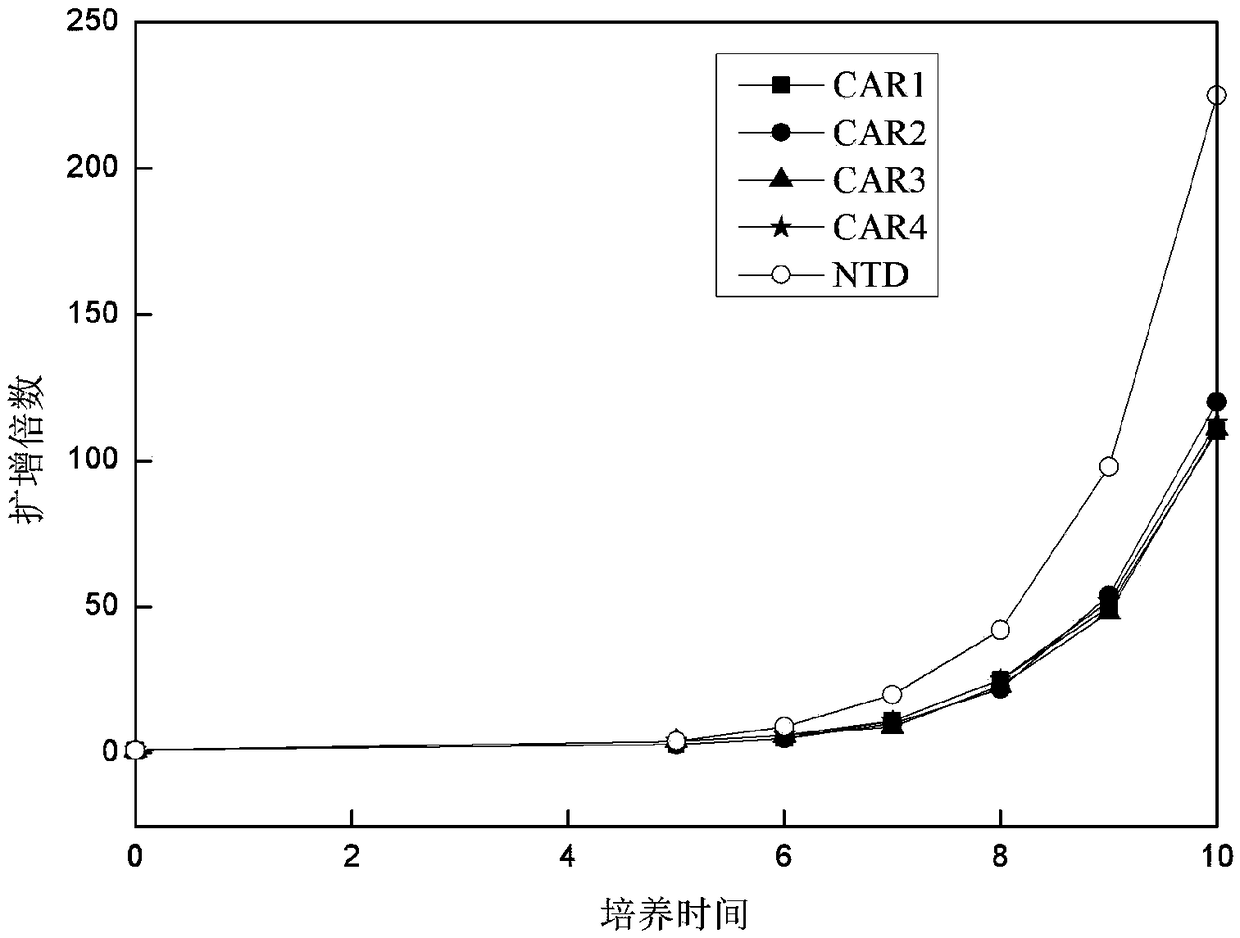
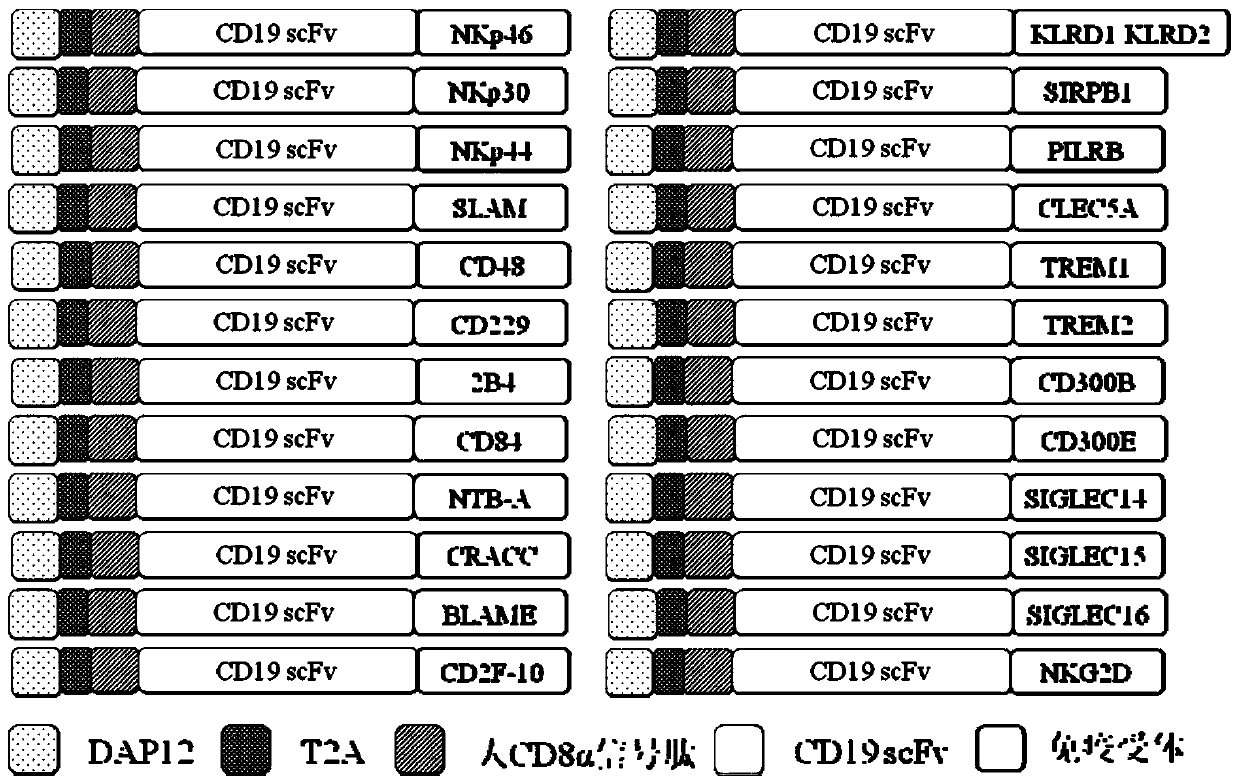
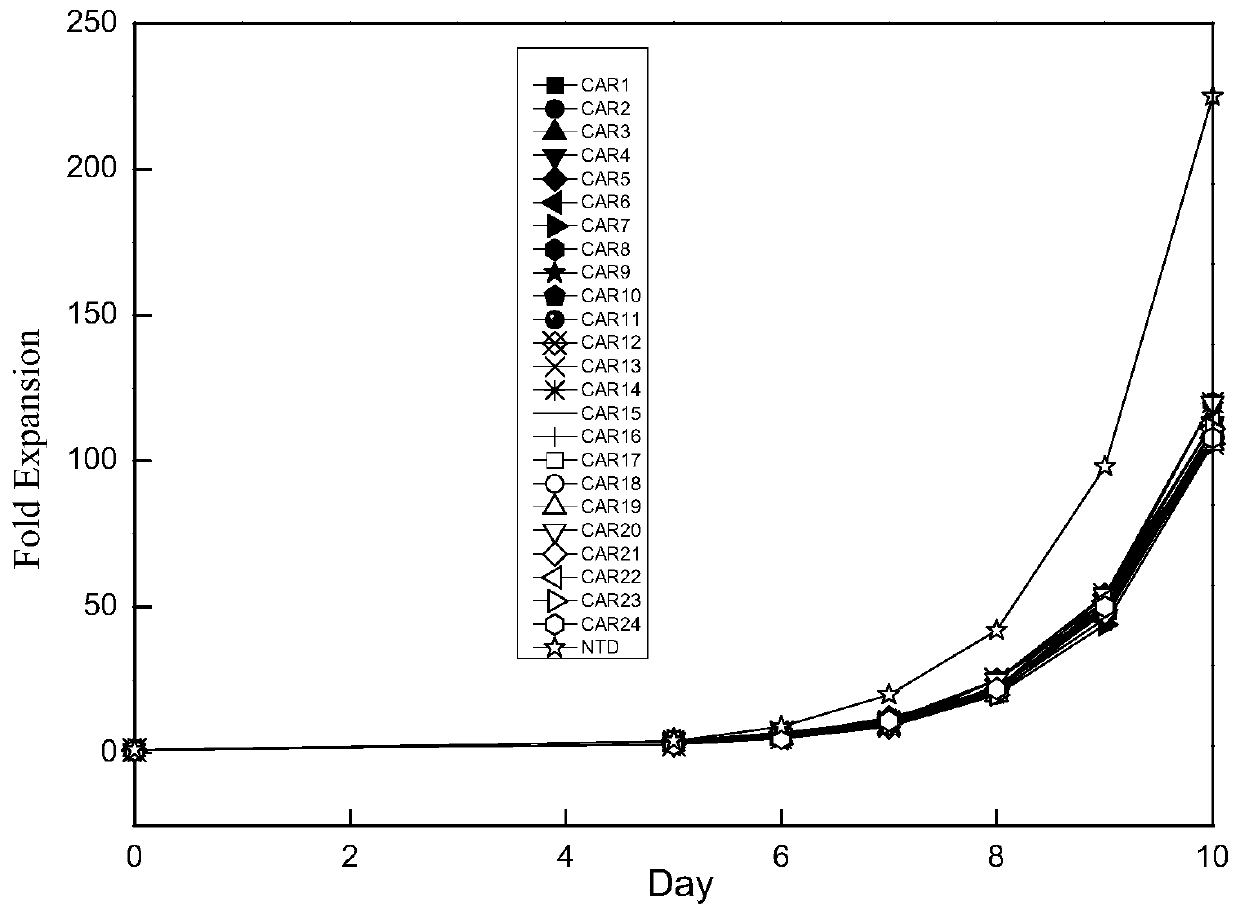
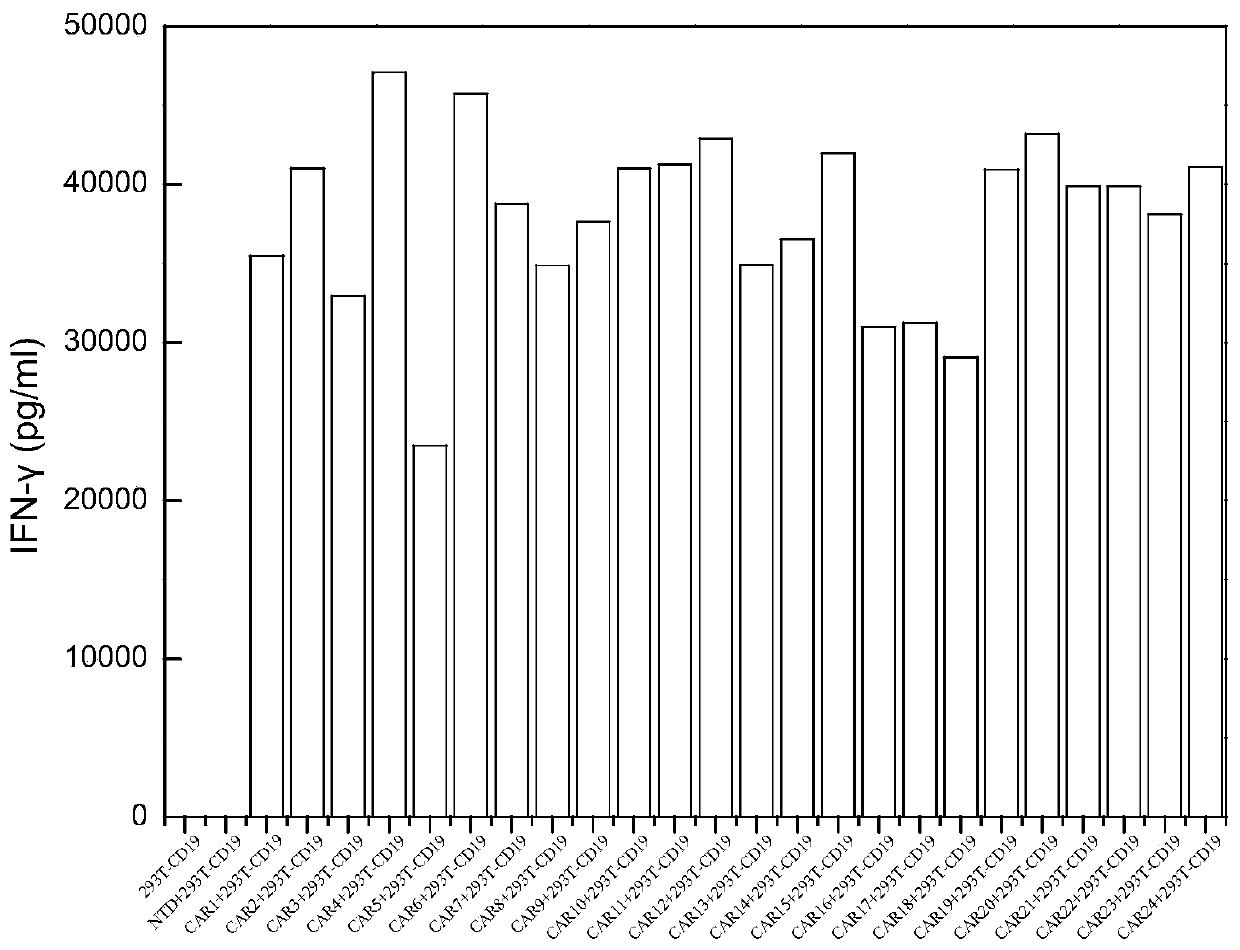
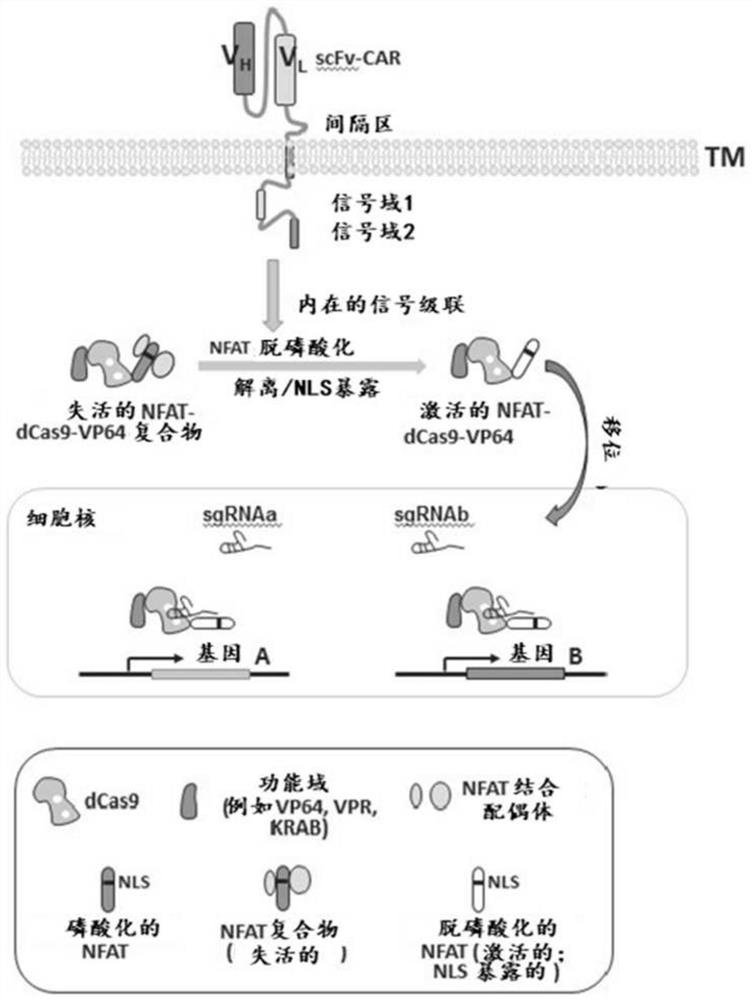
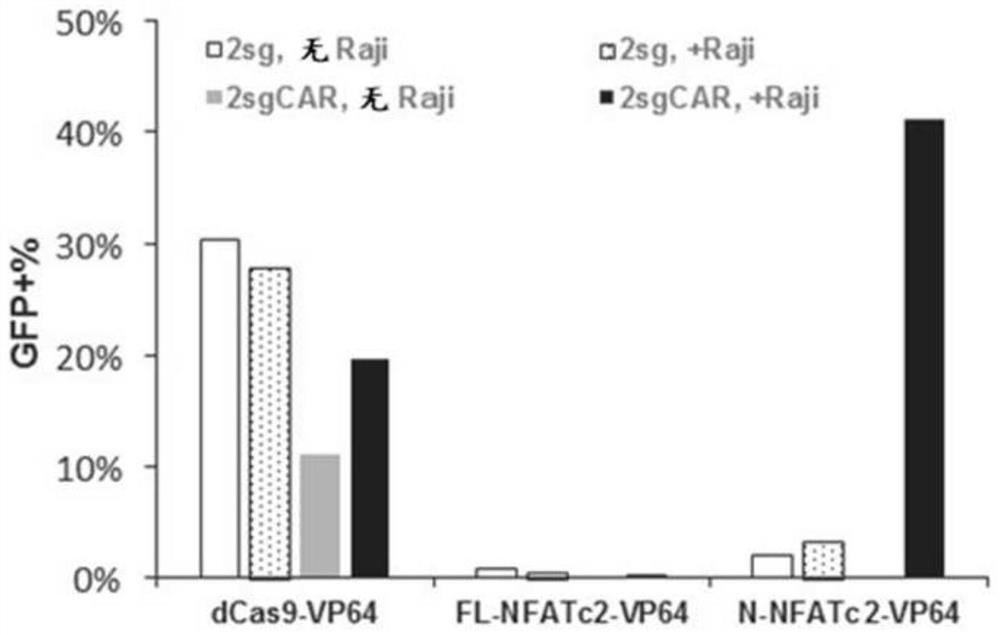
Application of novel CAR (Chimeric Antigen Receptor) modified T cells for treating cancer
ActiveCN109400713AMild release responseSignificant effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyExtracellular signalAbnormal tissue growth
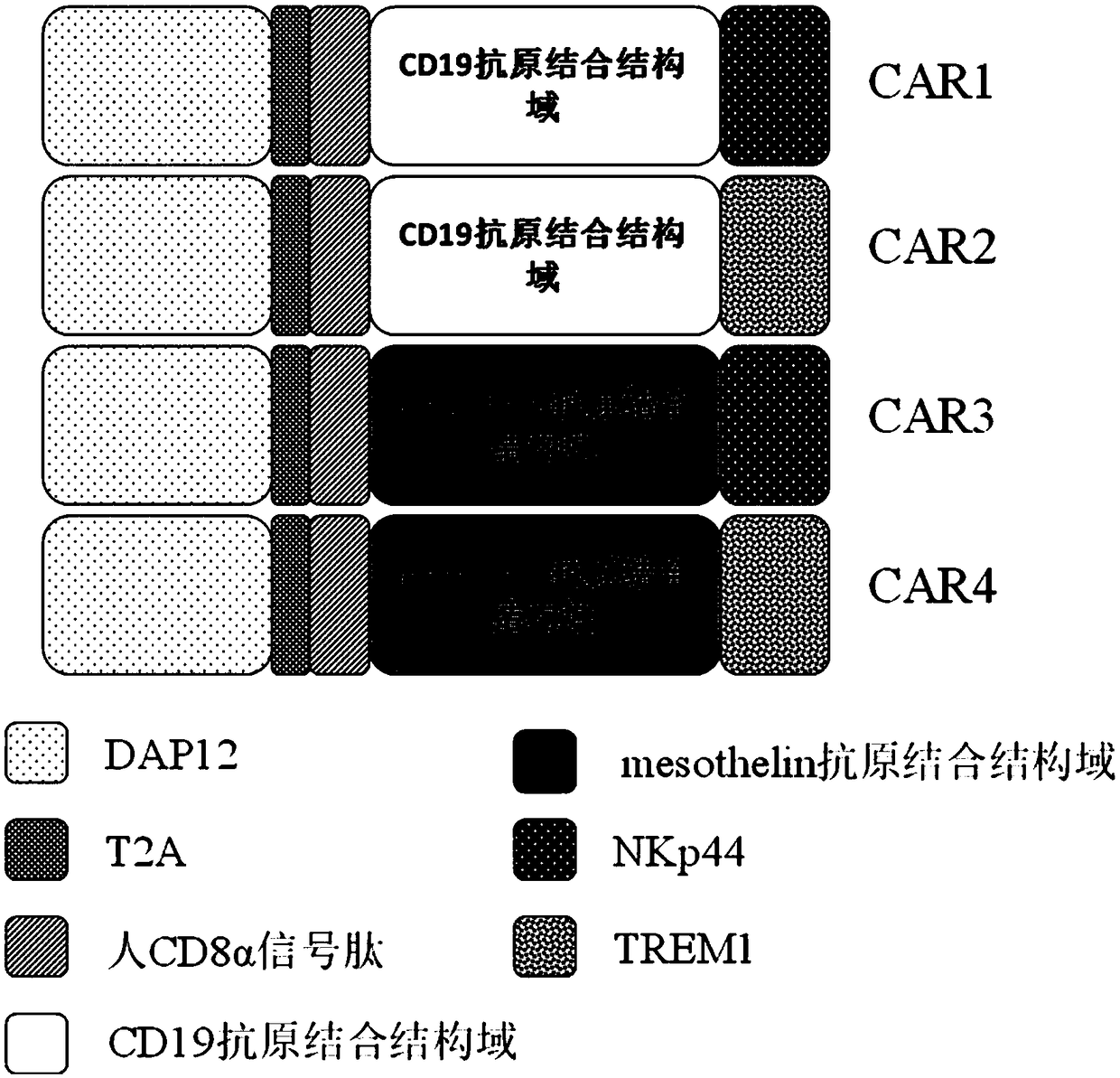
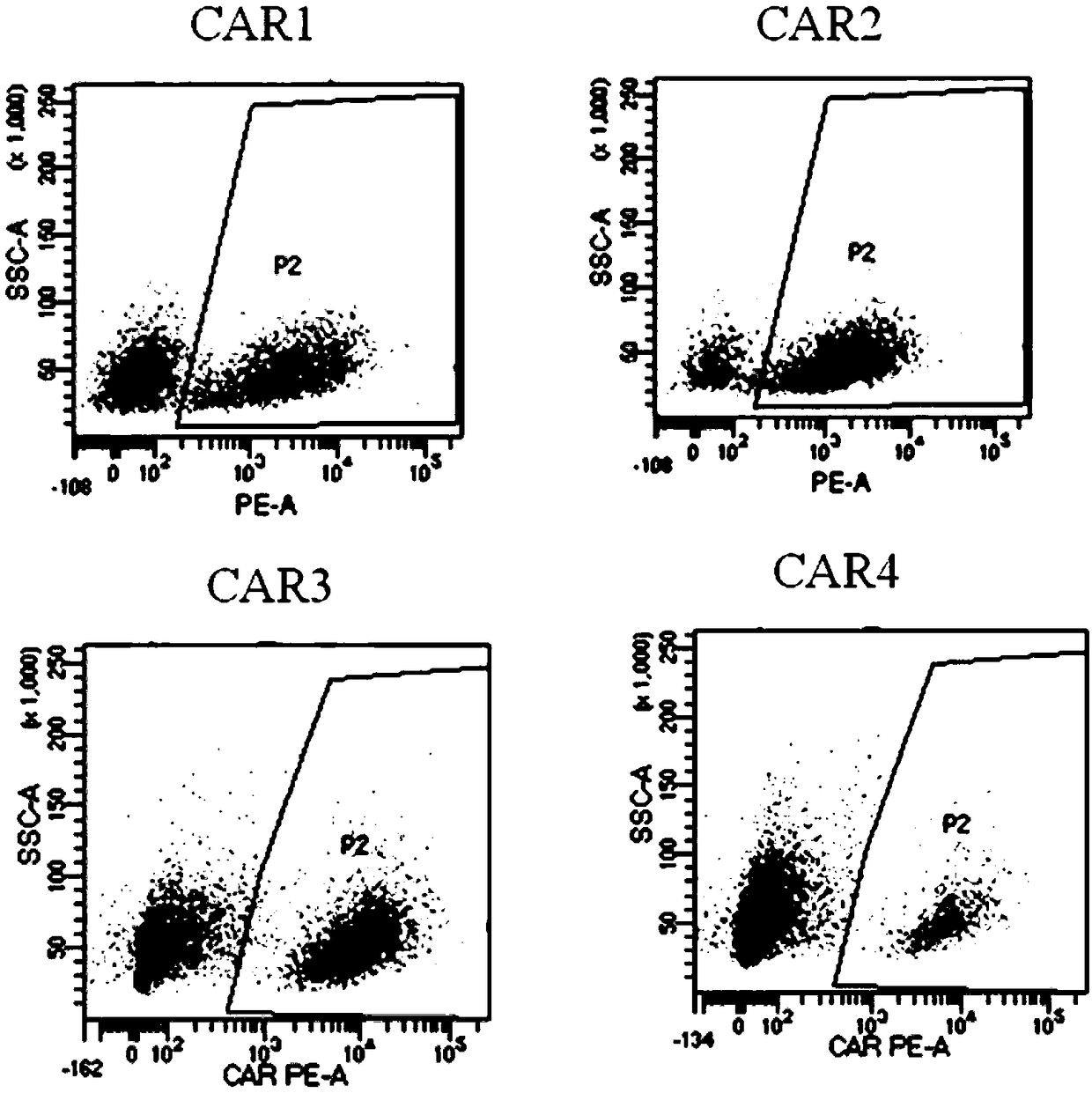
The invention discloses a novel CAR (Chimeric Antigen Receptor) and application of novel CAR modified T cells for treating cancer. The novel CAR is formed by an extracellular signal peptide, an antigen binding structural domain, an intracellular first conduction structural domain and an intracellular second conduction structural domain, and contains an intracellular first conduction structural domain NKp44 or an intracellular first conduction structural domain TREM1. According to the novel CAR disclosed by the invention, multiple CAR nucleotide sequences are separated and purified, and the CARand CAR-T cells which specifically aims at CD19 malignant hematologic tumor and mesothelin malignant solid tumor antigens. In a hematologic tumor and solid tumor killing test, the killing capacity ofthe CAR-T cells to tumor cells is obviously enhanced, and good safety and good anti-tumor activity in clinic application are expressed.
Owner:NANJING CART MEDICAL TECH LTD
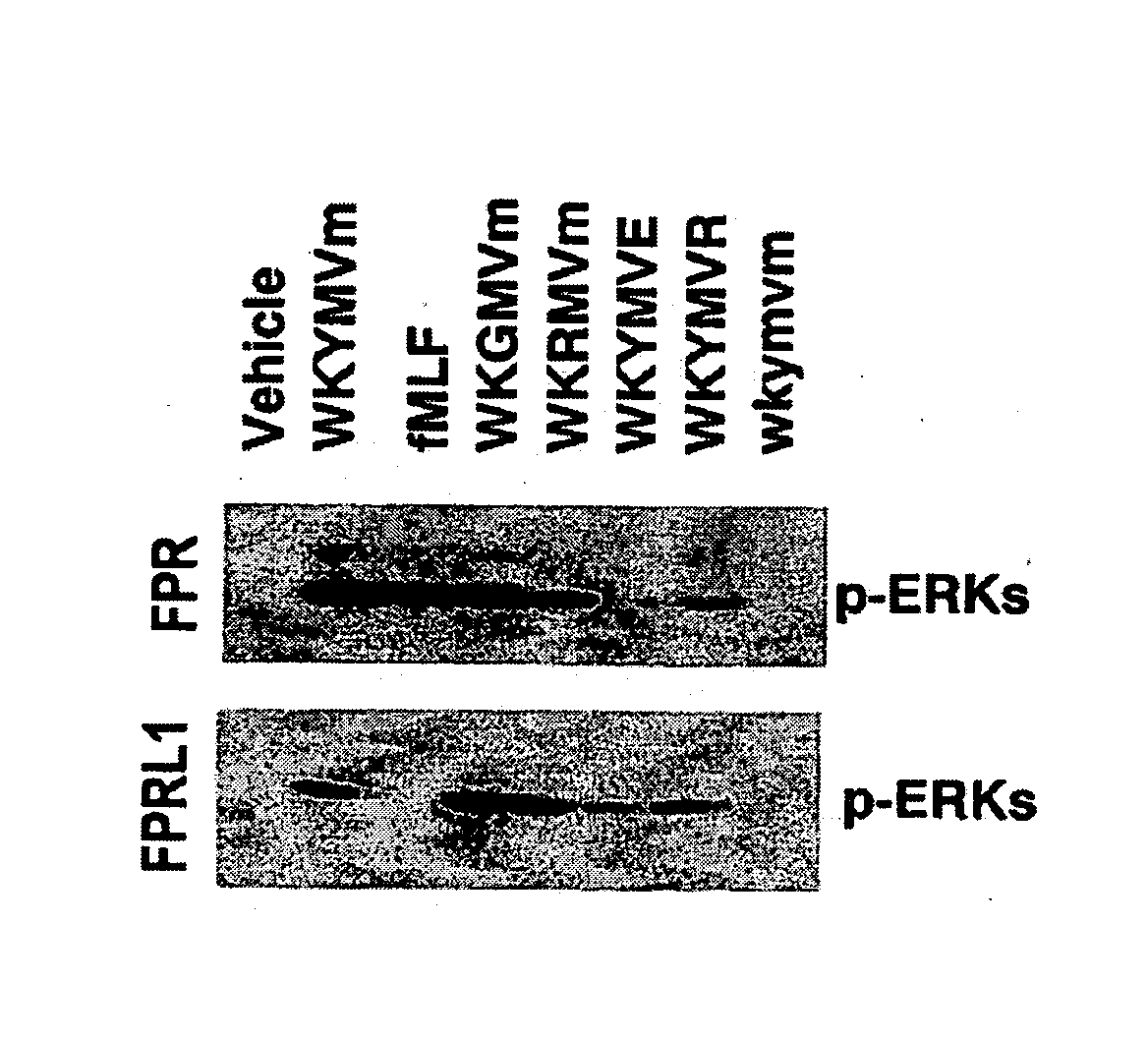
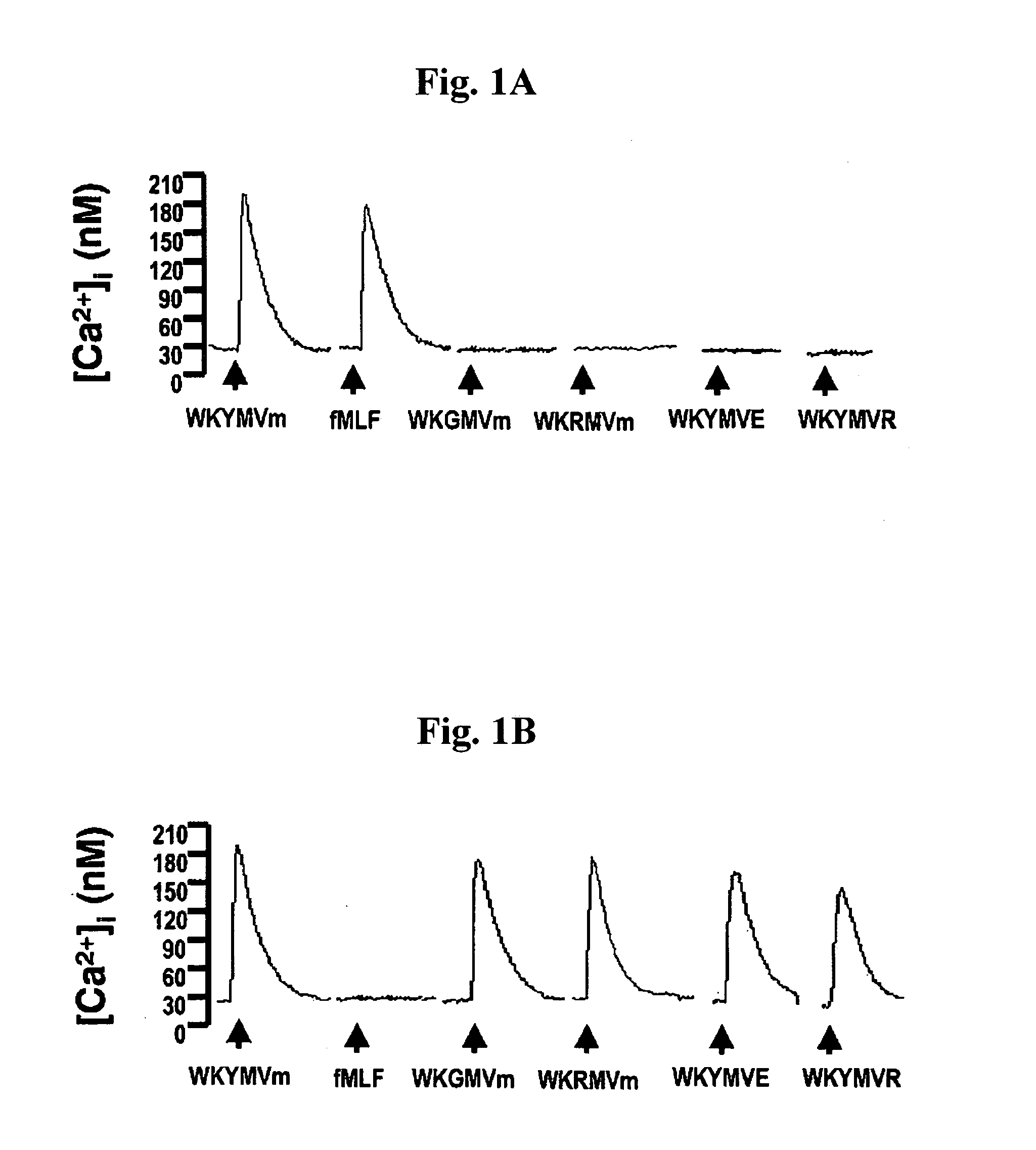
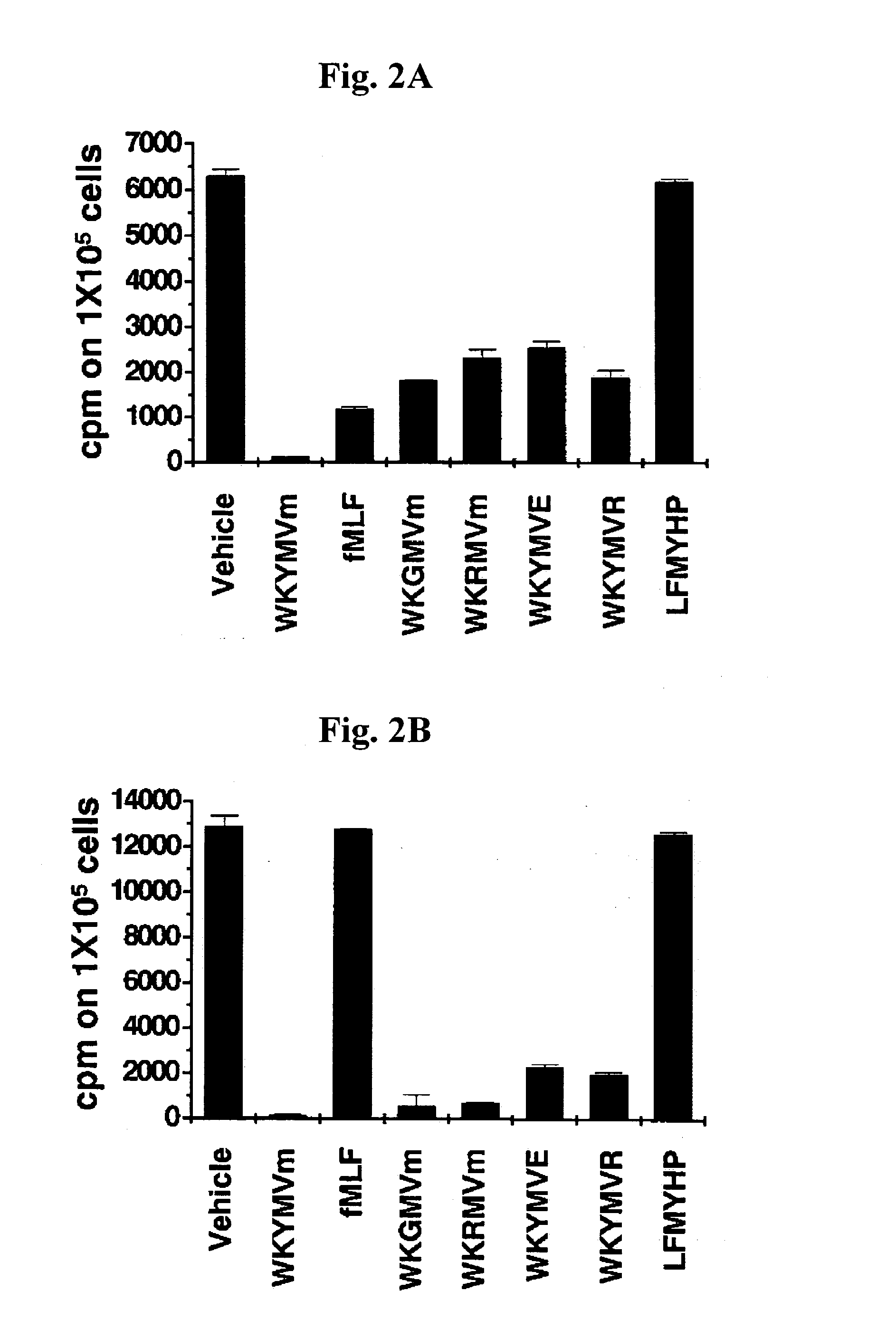
Immune-modulating peptide
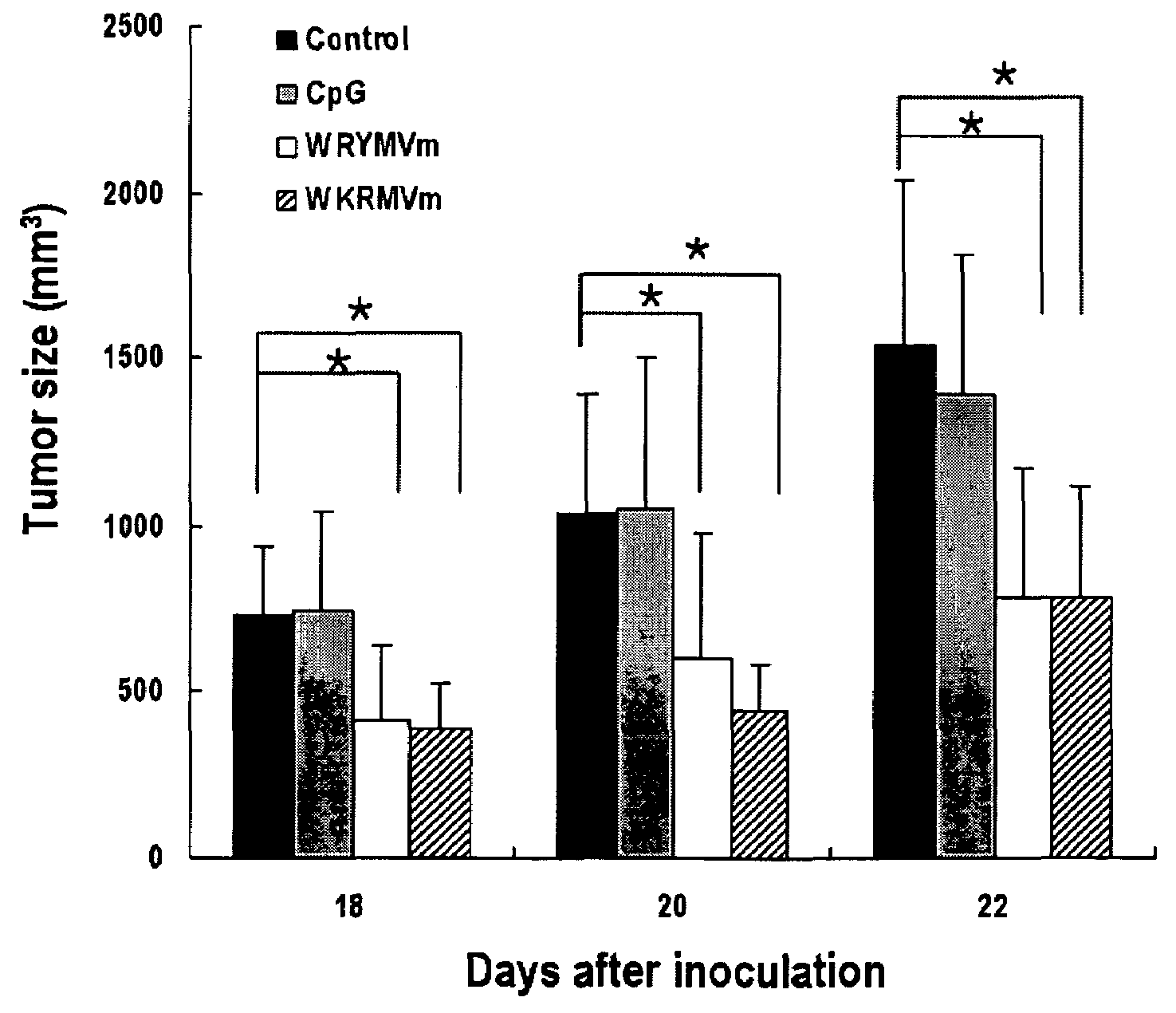
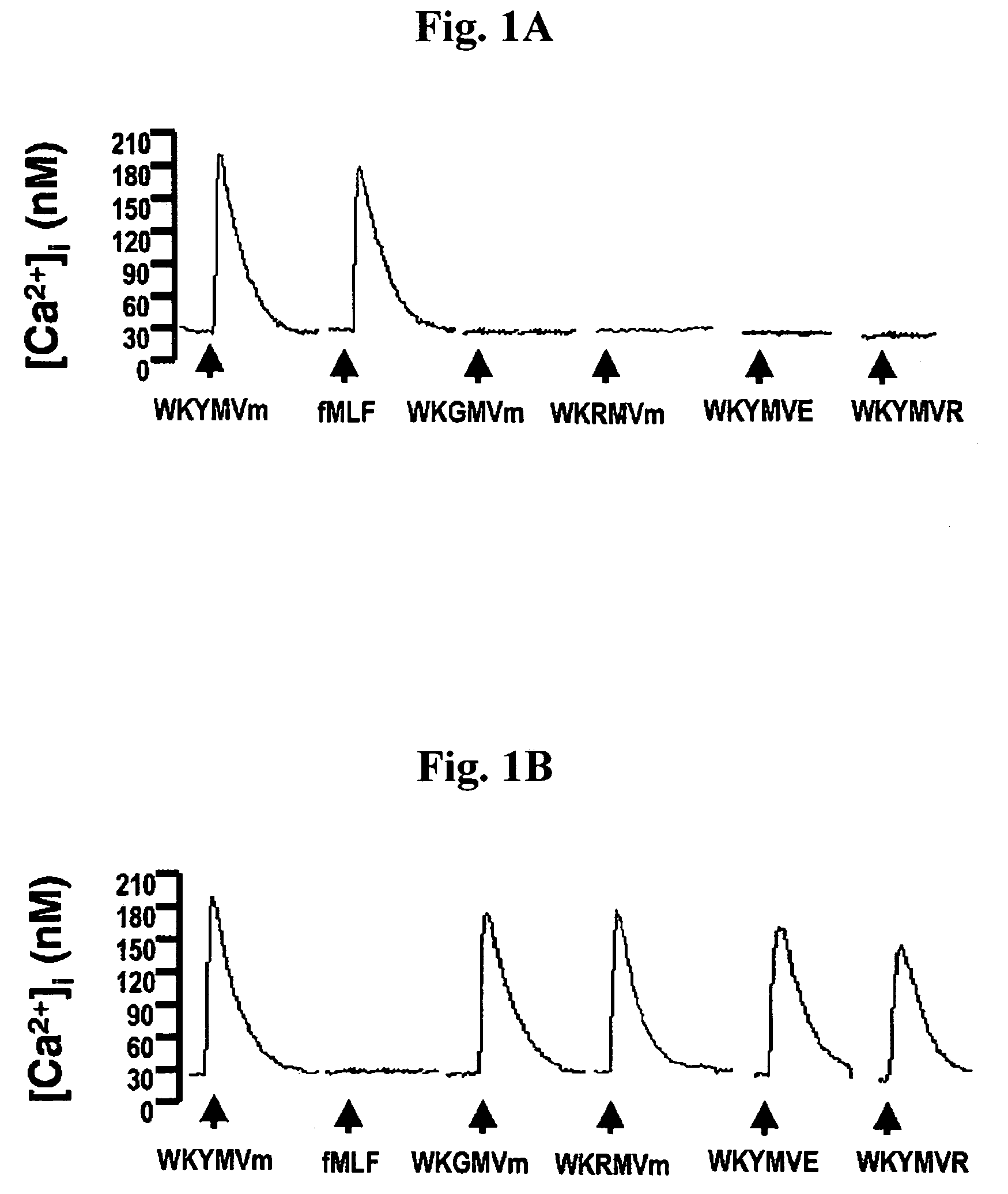
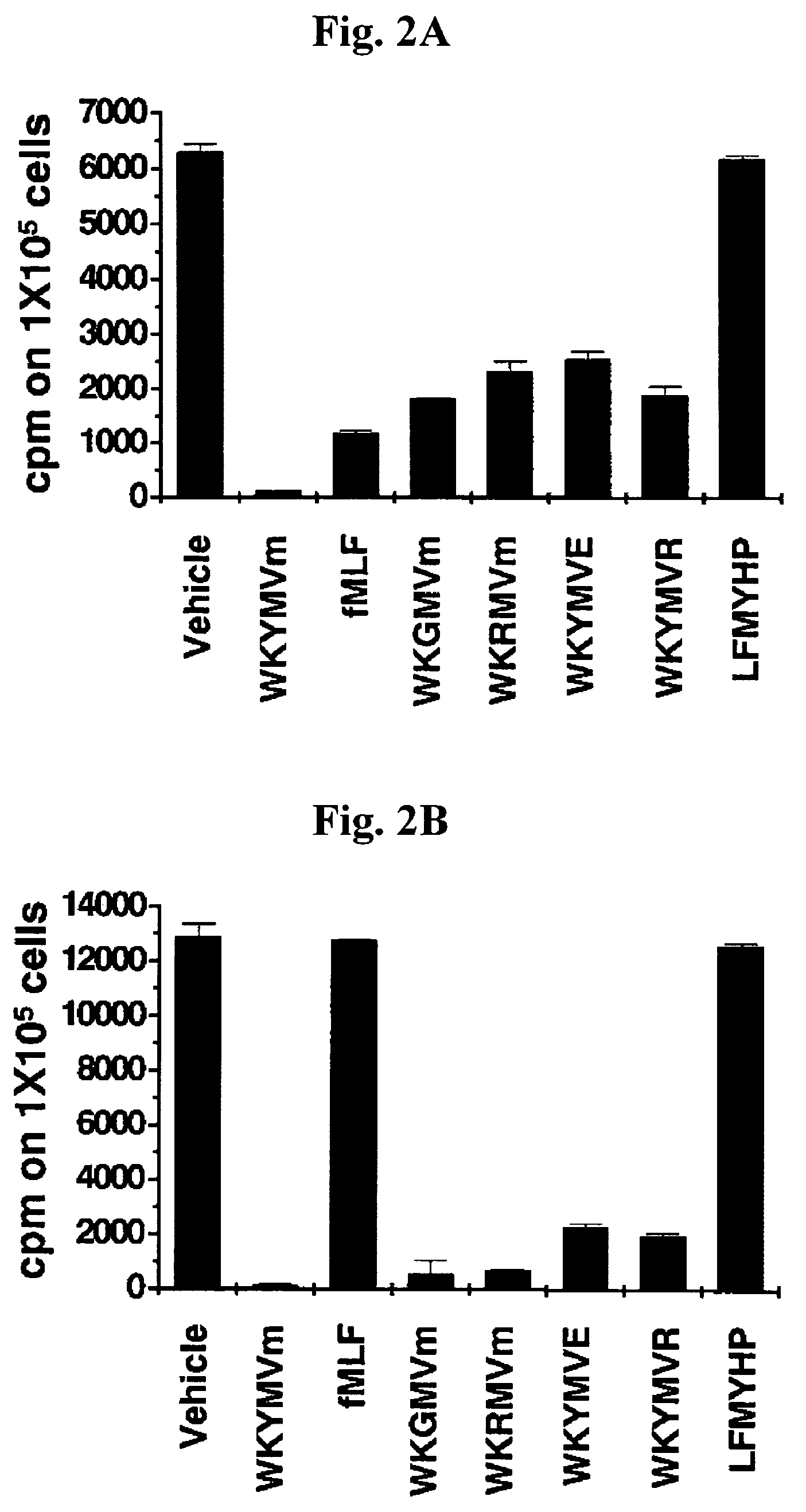
InactiveUS20070219139A1Inhibition of secretionPromote secretionBiocideNervous disorderExtracellular signalPhosphorylation
Disclosed are peptides having SEQ ID NOs: 1 to 24 that induce superoxide generation by human monocytes or neutrophils; that induce an intracellular calcium increase by human peripheral blood monocytes or neutrophils; binds to formyl peptide receptor or formyl peptide receptor-like 1; that induce chemotactic migration of human monocytes or neutrophils in vitro; that induce degranulation in formyl peptide receptor expressing cells or formyl peptide receptor-like 1 expressing cells; that stimulate extracellular signal regulated protein kinase phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1; or that stimulate Akt phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1.
Owner:POSTECH ACAD IND FOUND
Application of engineered T cell with immune receptor for treatment of cancer
InactiveCN109734814AMild release responseEnhance killing activityMammal material medical ingredientsGenetic engineeringAntigenExtracellular signal
The invention discloses application of an engineered T cell with an immune receptor for treatment of cancer. A chimeric antigen receptor consists of an extracellular signal peptide, an antigen bindingstructural domain, a first intracellular conduction structural domain and a second intracellular conduction structural domain and comprises the first intracellular conduction structural domain in theform of the immune receptor. The invention provides a plurality of amino acid sequences of the chimeric antigen receptor and provides the chimeric antigen receptor specific for CD19 malignant hematological tumors and CAR-T cells. In a blood tumor killing test, the ability of the CAR-T cells to kill tumor cells is significantly improved, and high safety and high antitumor activity are shown in clinical application.
Owner:NANJING CART MEDICAL TECH LTD
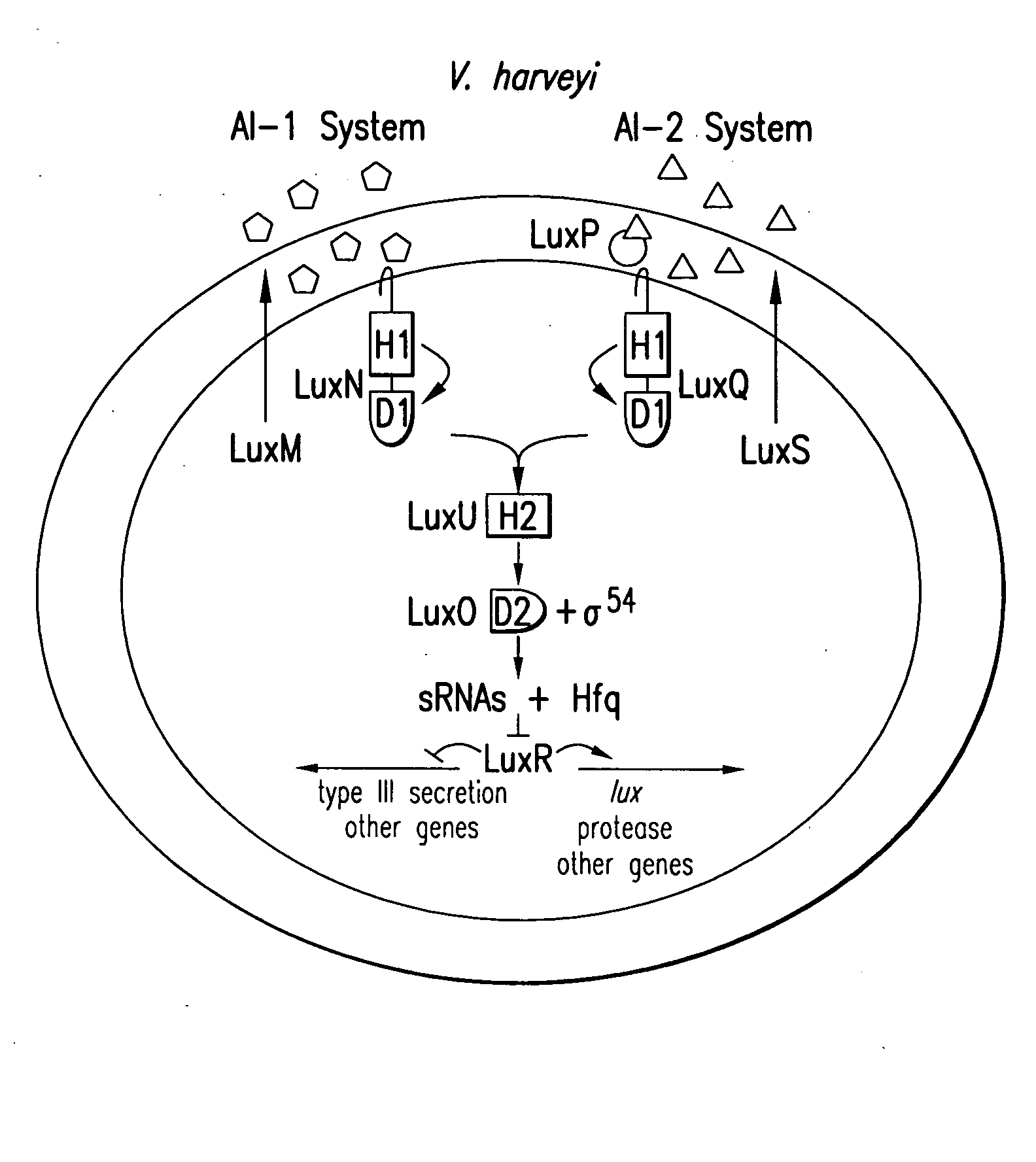
Small RNAs and bacterial strains involved in quorum sensing
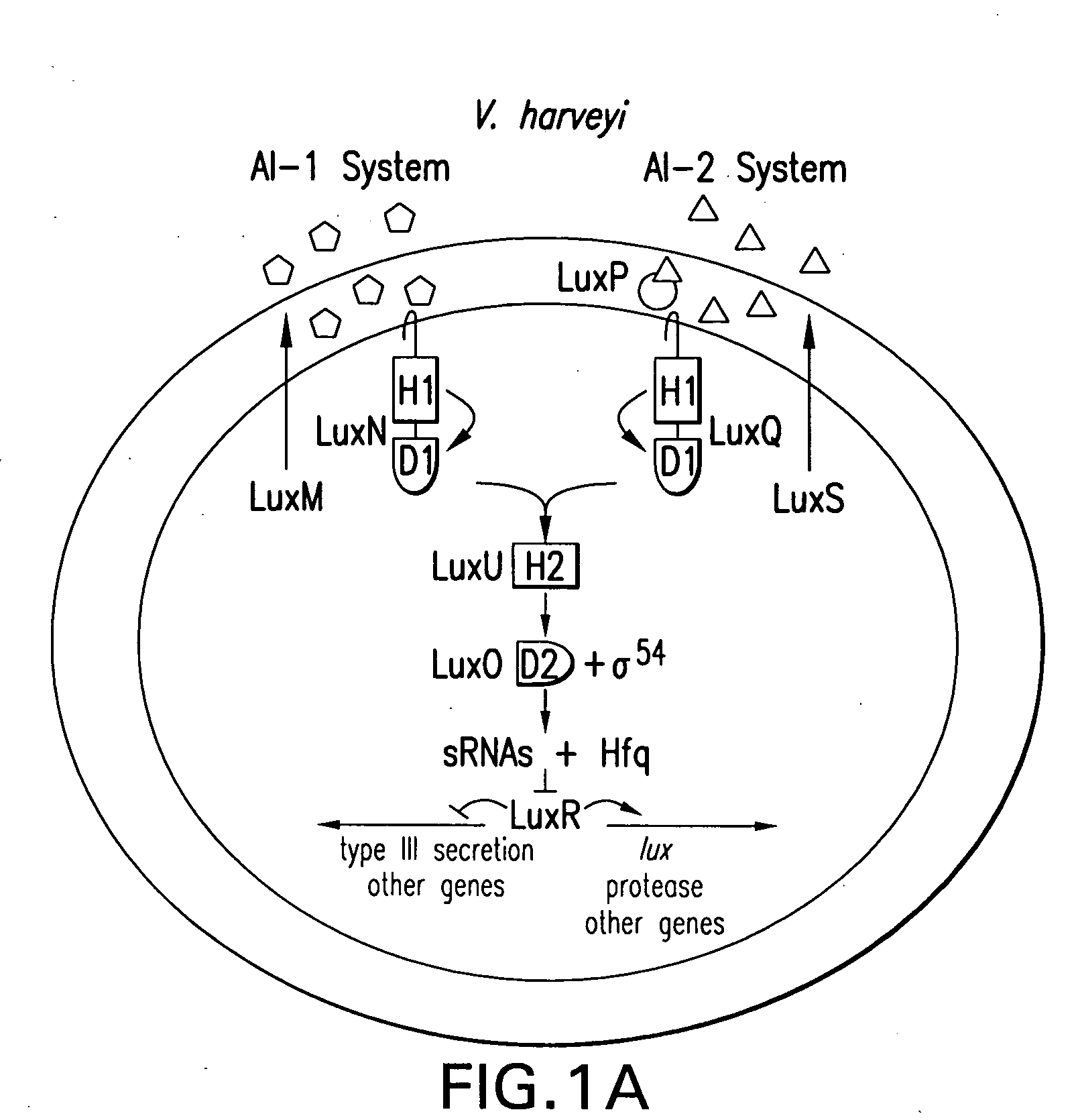
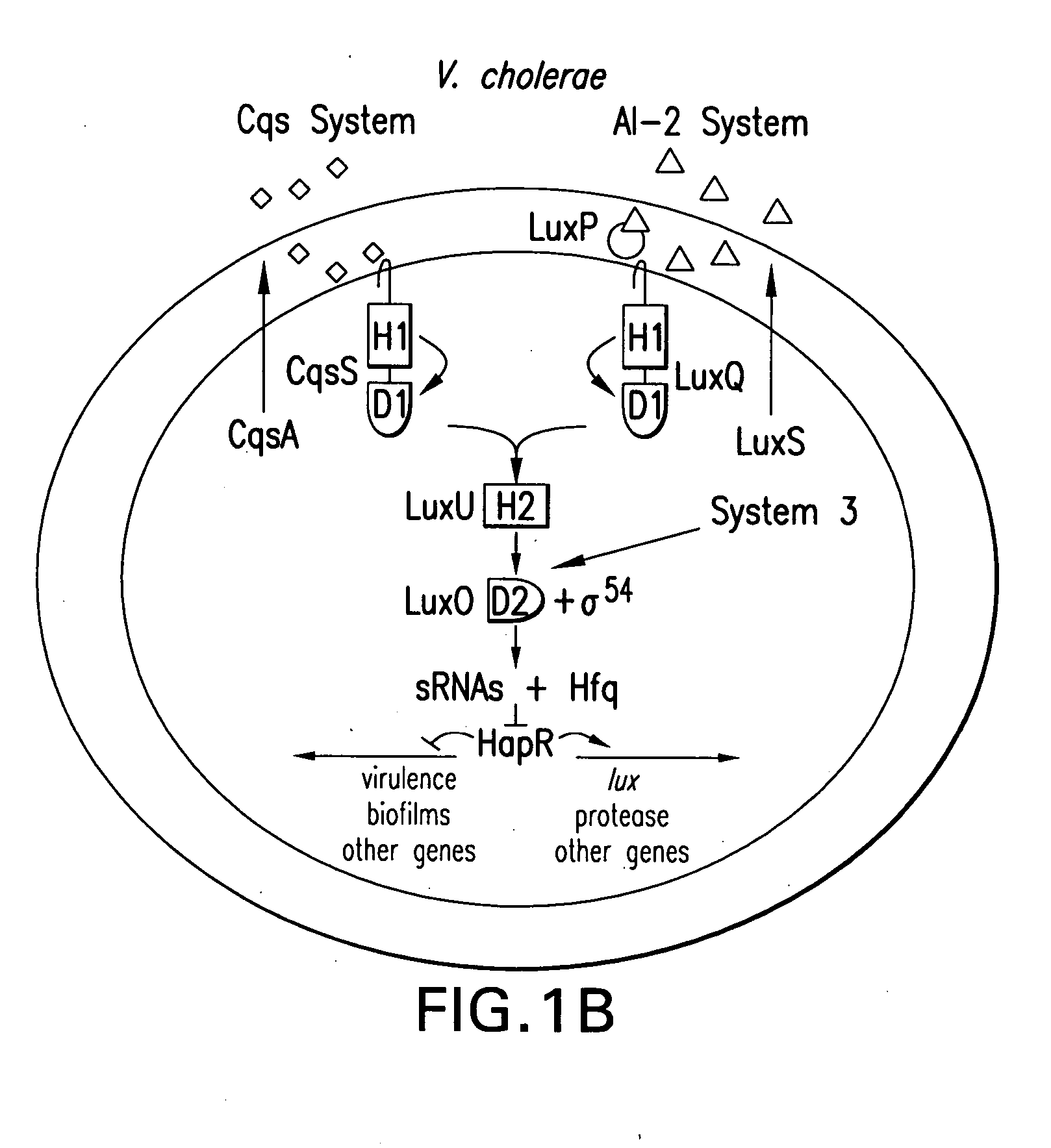
Quorum-sensing bacteria communicate with extracellular signal molecules called autoinducers to allow community-wide synchronization of gene expression. The present invention relates to the identification the Vibrio harveyi and Vibrio cholerae protein Hfq as mediating interactions between small, regulatory RNAs (sRNAs) and specific messenger RNA (mRNA) targets. Accordingly, the present invention provides nucleic acids encoding the Vibrio sRNAs, strains having various deletions and mutations of one or more qrr genes encoding these sRNA as well as methods of identifying quorum-sensing regulators. Additionally, the invention relates to an isolated V. harveyi Hfq protein and conservative amino acid substitutions thereof as well as nucleic acids encoding those proteins, recombinant methods of producing those proteins and antibodies against those proteins.
Owner:THE TRUSTEES FOR PRINCETON UNIV
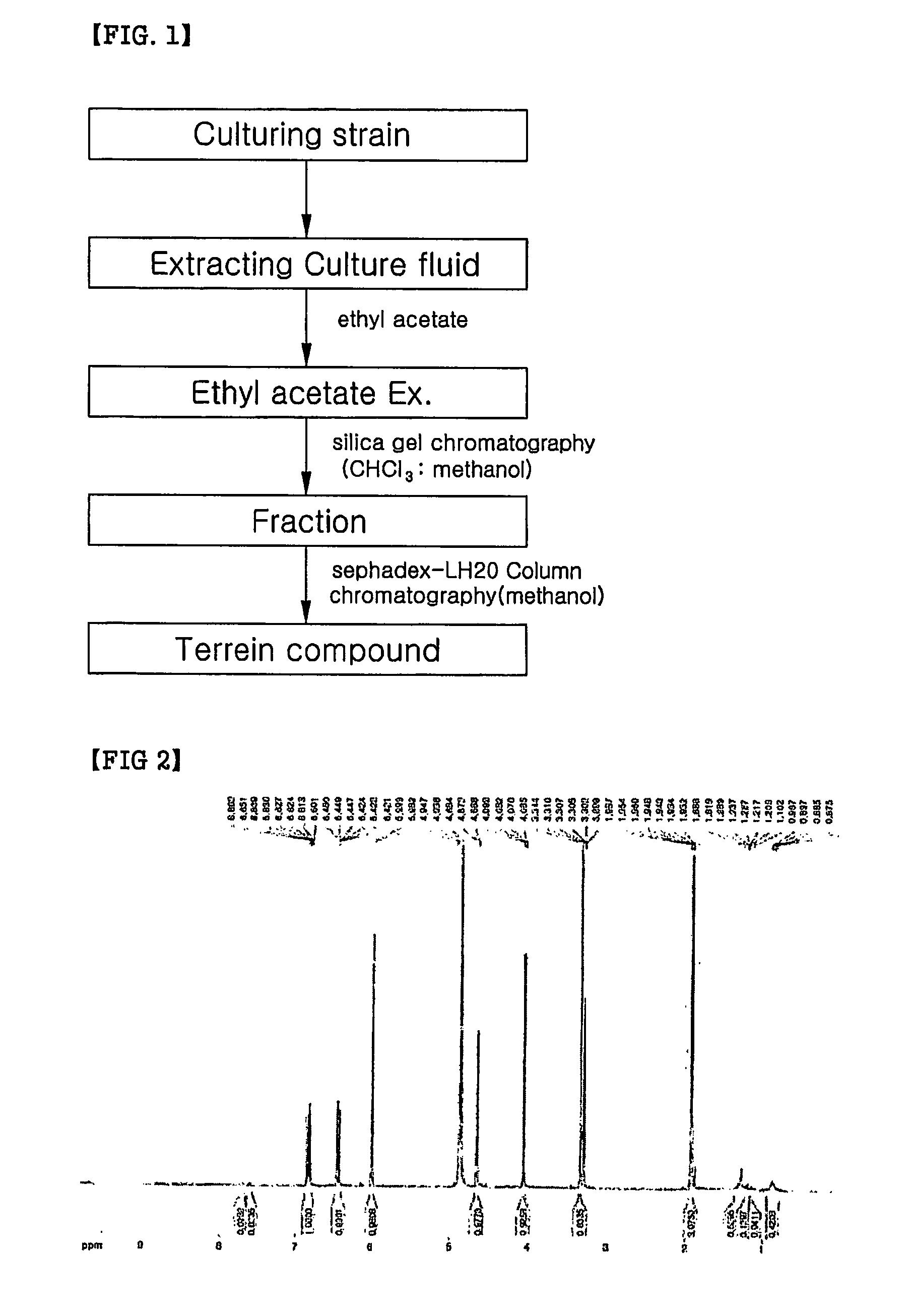
Terrein compound having melanin biosynthesis inhibitors and its preparation
InactiveCN1925848ANon-cytotoxicNo side effectsOrganic active ingredientsCosmetic preparationsExtracellular signalWhitening Agents
The present invention relates to a melanin biosynthesis inhibitor containing terrein compound as an effective ingredient. The terrein compound can be easily separated from Penicillium sp KCTC 26245, a fungal strain inhabited in domestic soil. It does not directly inhibit tyrosinase but inhibits the expression of MITF (microphthalmia-associated transcription factor) by activating ERK (extracellular signal-regulated kinase) in melanin chromatocytes to give whitening effect. So, the melanin biosynthesis inhibiting effect of the compound is much greater than that of any other conventional inhibitors, and further the effect can be raised when the compound is used together with other inhibitors, owing to their different mechanisms. Thus, the compound of the present invention can be effectively used as a skin trouble treating agent, a skin whitening agent and a browning inhibitor.
Owner:KOREA RES INST OF BIOSCI & BIOTECH +1
Extracellular-signal-regulated-kinase (ERK) heteropolyligand polypeptide
Owner:PRECIGEN INC
Sorafenib tosylate-hydroxypropyl-beta-cyclodextrin clathrate compound and preparation method thereof
InactiveCN102145175APharmaceutical non-active ingredientsAntineoplastic agentsExtracellular signalCancer cell
The invention relates to a sorafenib tosylate-hydroxypropyl-beta-cyclodextrin clathrate compound and a preparation method thereof. Sorafenib plays the role of anticancer by inhibiting RAS / RAF / MEK (methyl ethyl ketone) / ERK (extracellular signal-regulated kinase) signal conducting channels and inhibiting tumor angiogenesis, and is directed to cancer cells instead of normal cells. The sorafenib is almost insoluble in water, and the average relative bioavailability of oral administration is 38% to 49%. According to the preparation method of the invention, the sorafenib tosylate-hydroxypropyl-beta-cyclodextrin clathrate compound is prepared based on the clathrate compound-forming property of hydroxypropyl-beta-cyclodextrin, thereby increasing medicine dissolvability, improving medicine dissolution and raising medicine bioavailability.
Owner:CHINA PHARM UNIV
Immune-modulating peptide
Disclosed are peptides having SEQ ID NOs: 1 to 24 that induce superoxide generation by human monocytes or neutrophils; that induce an intracellular calcium increase by human peripheral blood monocytes or neutrophils; binds to formyl peptide receptor or formyl peptide receptor-like 1; that induce chemotactic migration of human monocytes or neutrophils in vitro; that induce degranulation in formyl peptide receptor expressing cells or formyl peptide receptor-like 1 expressing cells; that stimulate extracellular signal regulated protein kinase phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1; or that stimulate Akt phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1.
Owner:POSTECH ACAD IND FOUND
Terrein compound having melanin biosynthesis inhibitors and its preparation
InactiveUS20070128136A1Inhibit expressionInhibits the synthesis of melaninCosmetic preparationsBiocideExtracellular signalAdditive ingredient
The present invention relates to a melanin biosynthesis inhibitor containing terrein compound as an effective ingredient. The terrein compound can be easily separated from Penicillium sp KCTC 26245, a fungal strain inhabited in domestic soil. It does not directly inhibit tyrosinase but inhibits the expression of MITF (microphthalmia-associated transcription factor) by activating ERK (extracellular signal-regulated kinase) in melanin chromatocytes to give whitening effect. So, the melanin biosynthesis inhibiting effect of the compound is much greater than that of any other conventional inhibitors, and further the effect can be raised when the compound is used together with other inhibitors, owing to their different mechanisms. Thus, the compound of the present invention can be effectively used as a skin trouble treating agent, a skin whitening agent and a browning inhibitor.
Owner:KOREA RES INST OF BIOSCI & BIOTECH +1
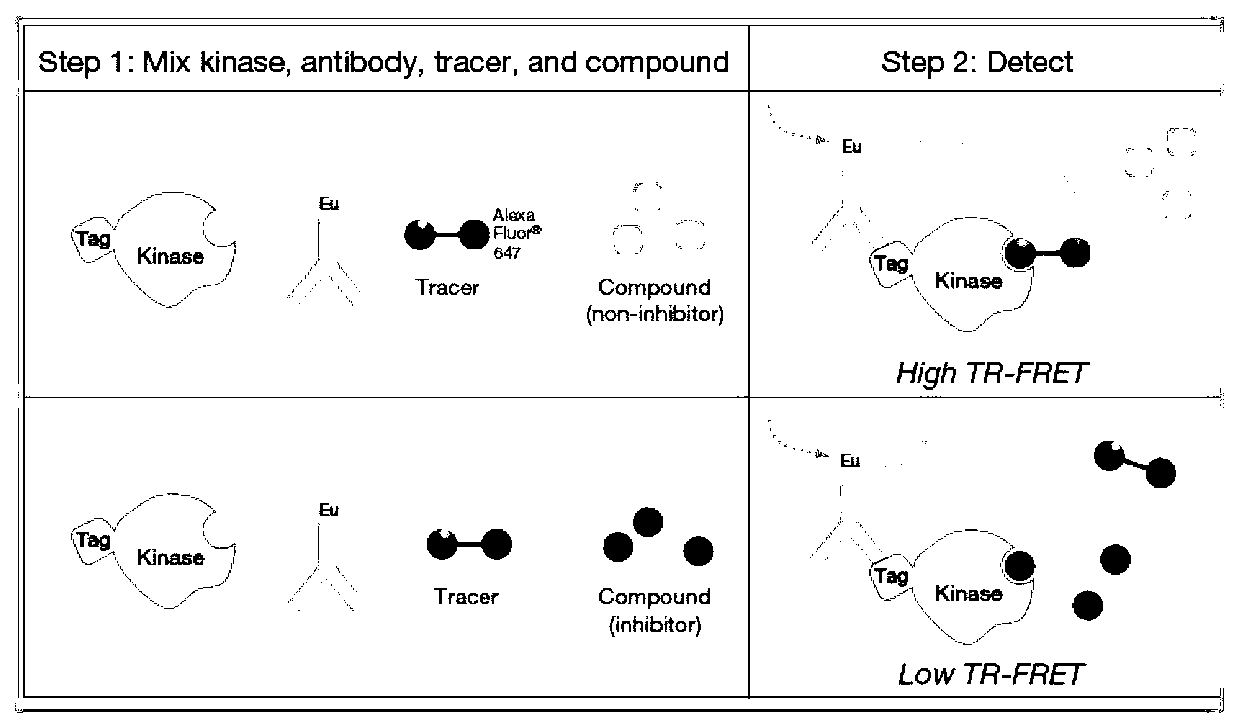
Non-atp dependent inhibitors of extracellular signal-regulated kinase (ERK)
Owner:UNIV OF MARYLAND
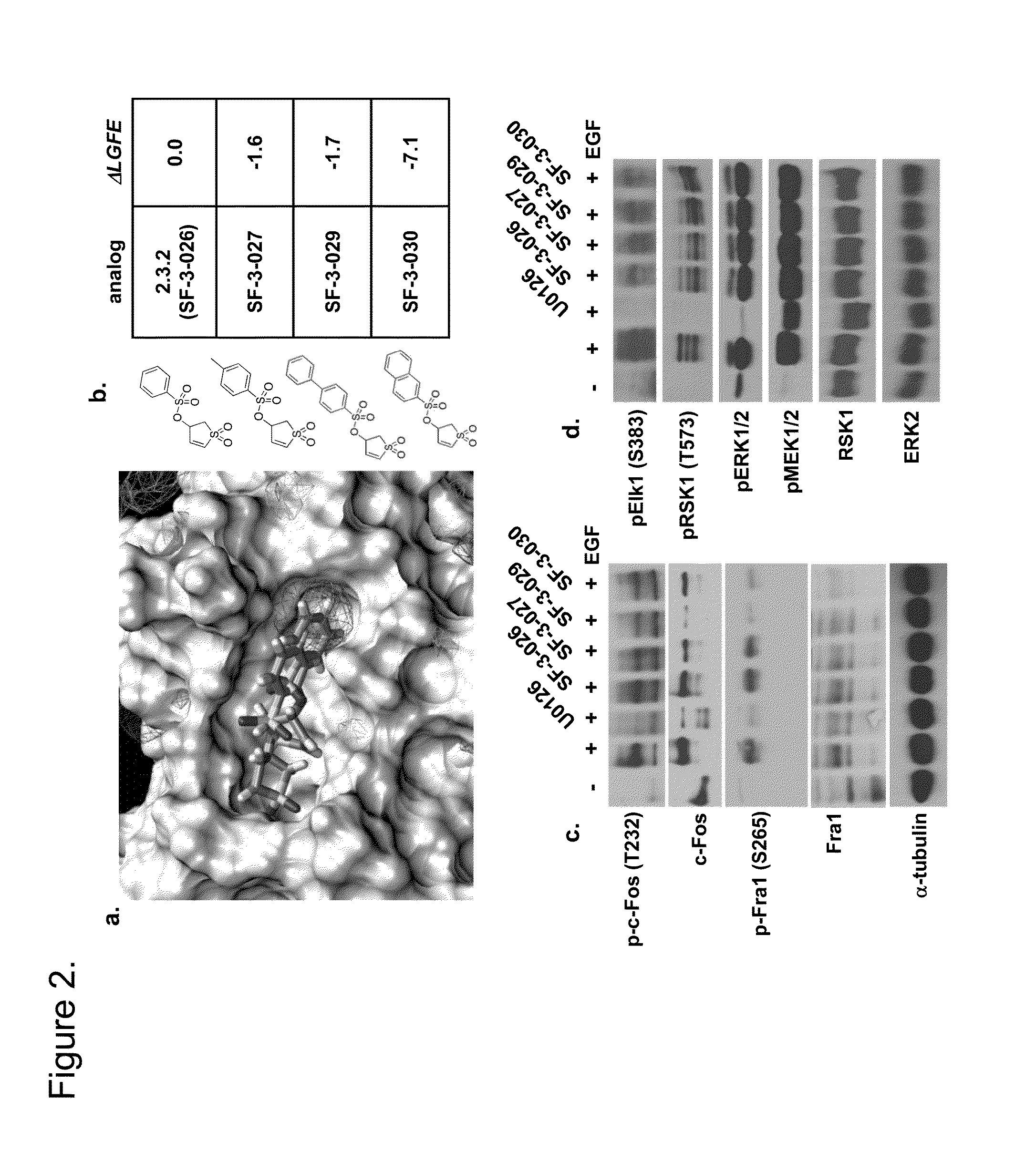
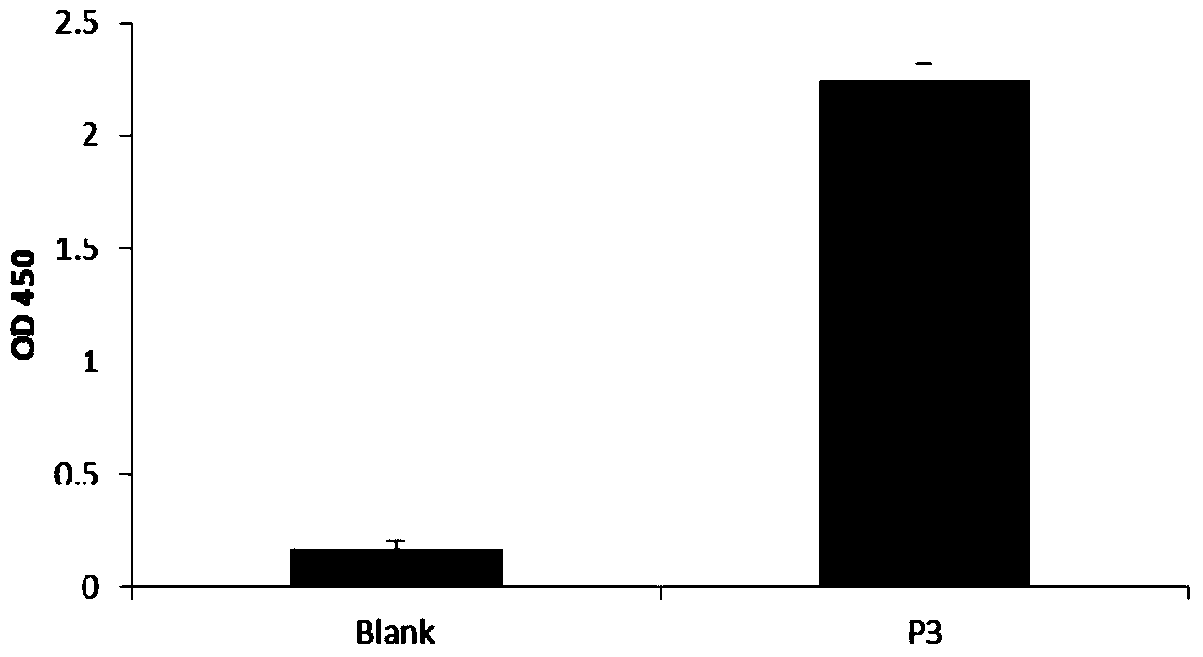
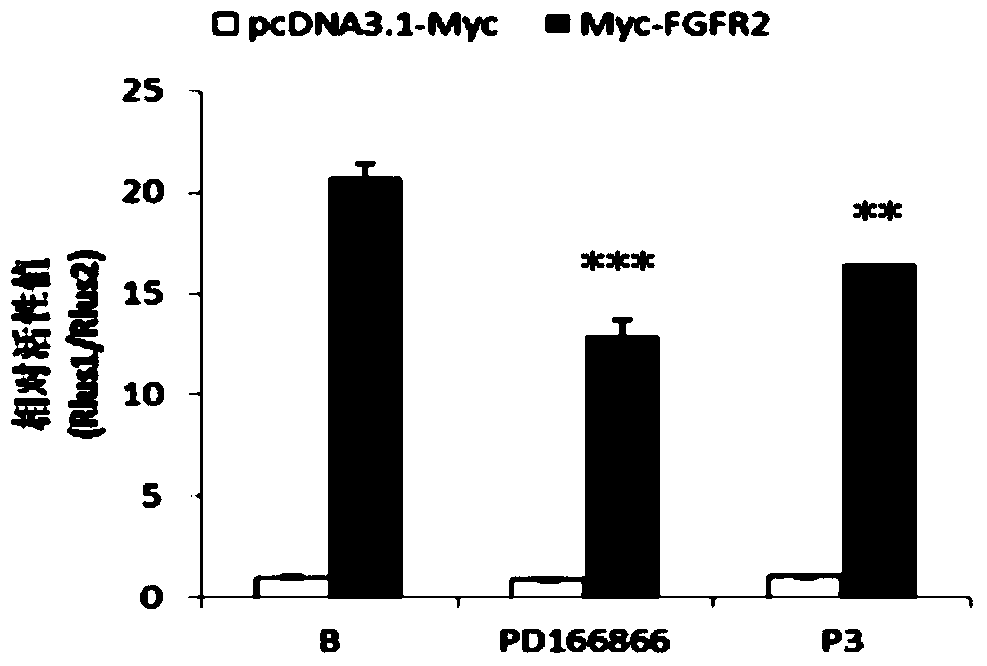
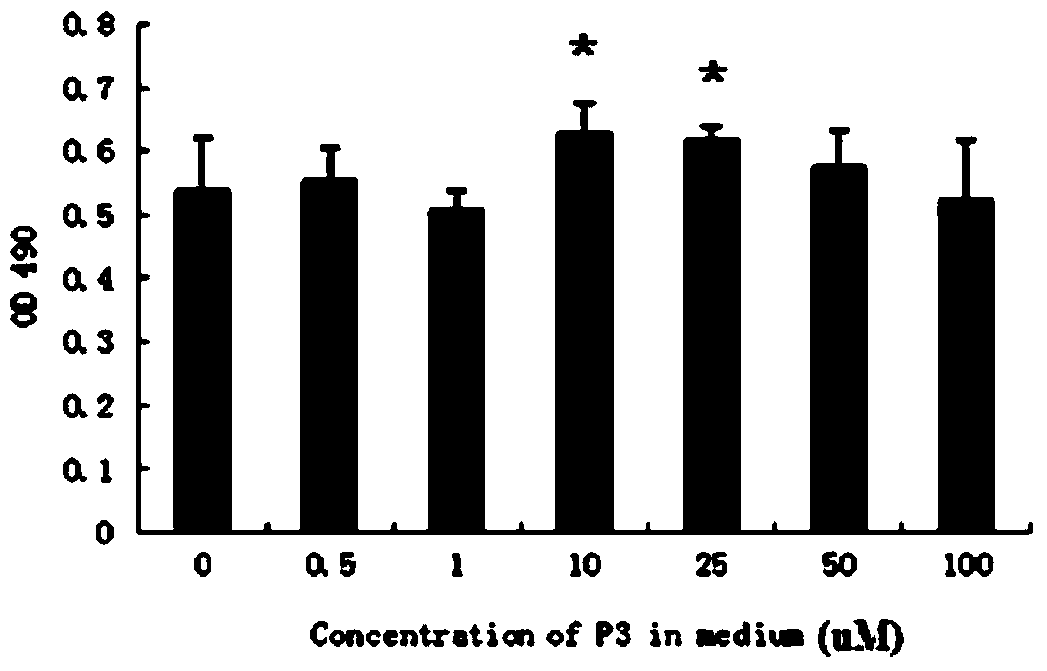
Polypeptide capable of regulating activity of FGFR2 (Fibroblast Growth Factor Receptor 2)
ActiveCN103980349AInhibitory activityEasy to adjustPeptidesExtracellular signalFibroblast growth factor receptor 2
The invention discloses a polypeptide capable of regulating the activity of FGFR2 (Fibroblast Growth Factor Receptor 2). The amino acid sequence of the polypeptide is Asn-Leu-Gly-Gln-Glu-Leu-Ala-Phe-Arg-Val-Pro-Asn. The polypeptide disclosed by the invention can be specifically combined with the FGFR2, plays an obviously regulating role on the activity of the FGFR2, can outstanding inhibit the activity of p-ERK (Phospho-Extracellular Signal-Regulated Kinase) contained in the main downstream signal channel MAPK (Mitogen Activated Protein Kinase) of the FGFR2, has the advantages of low molecular weight, easiness for preparation, controllability in quality, low immunogenicity and the like and has good potential application value in the field of medicines.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Non-ATP dependent inhibitors of extracellular signal-regulated kinase (ERK)
ActiveUS9115122B2Organic chemistryHeterocyclic compound active ingredientsExtracellular signalKinase
Owner:UNIV OF MARYLAND
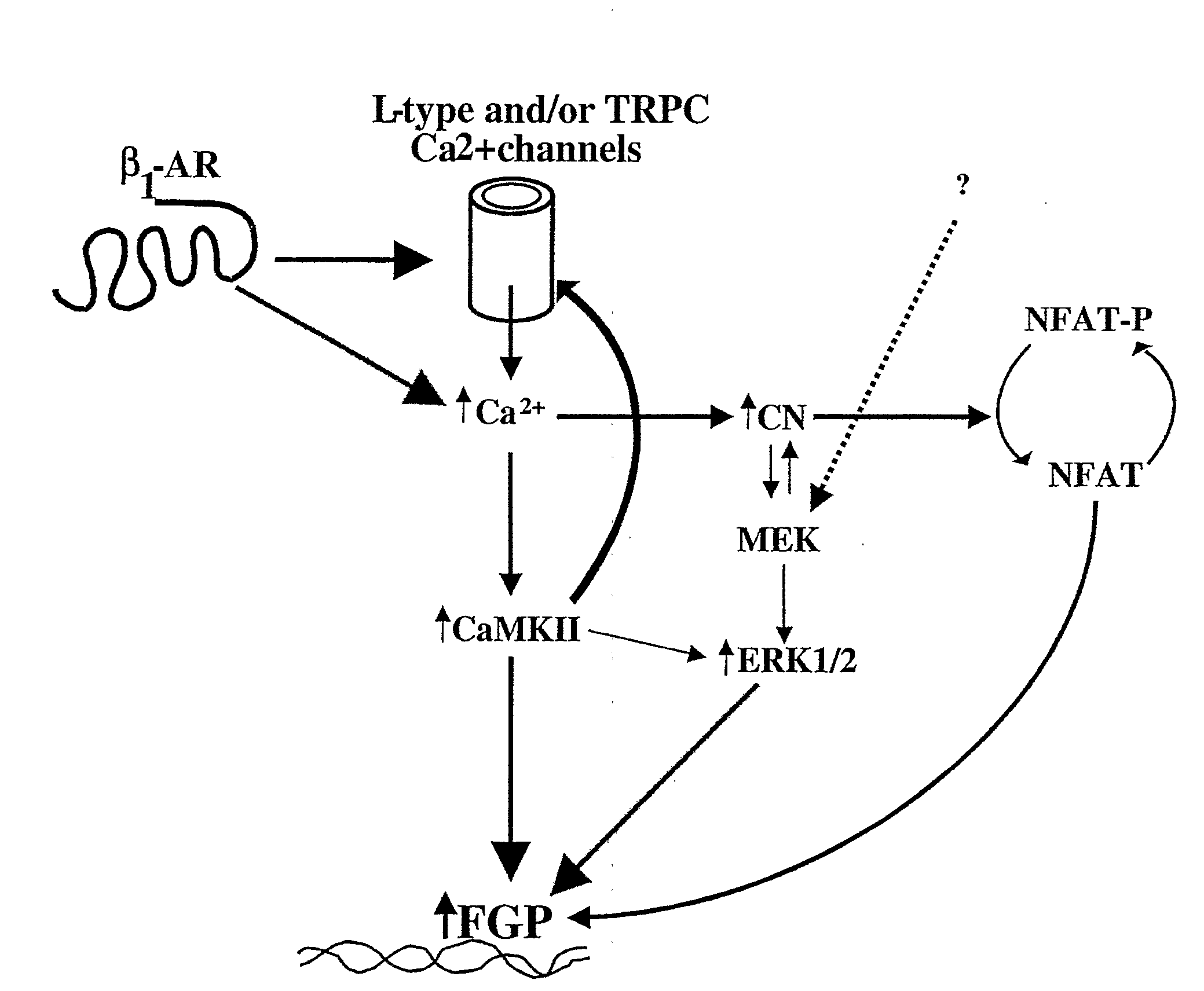
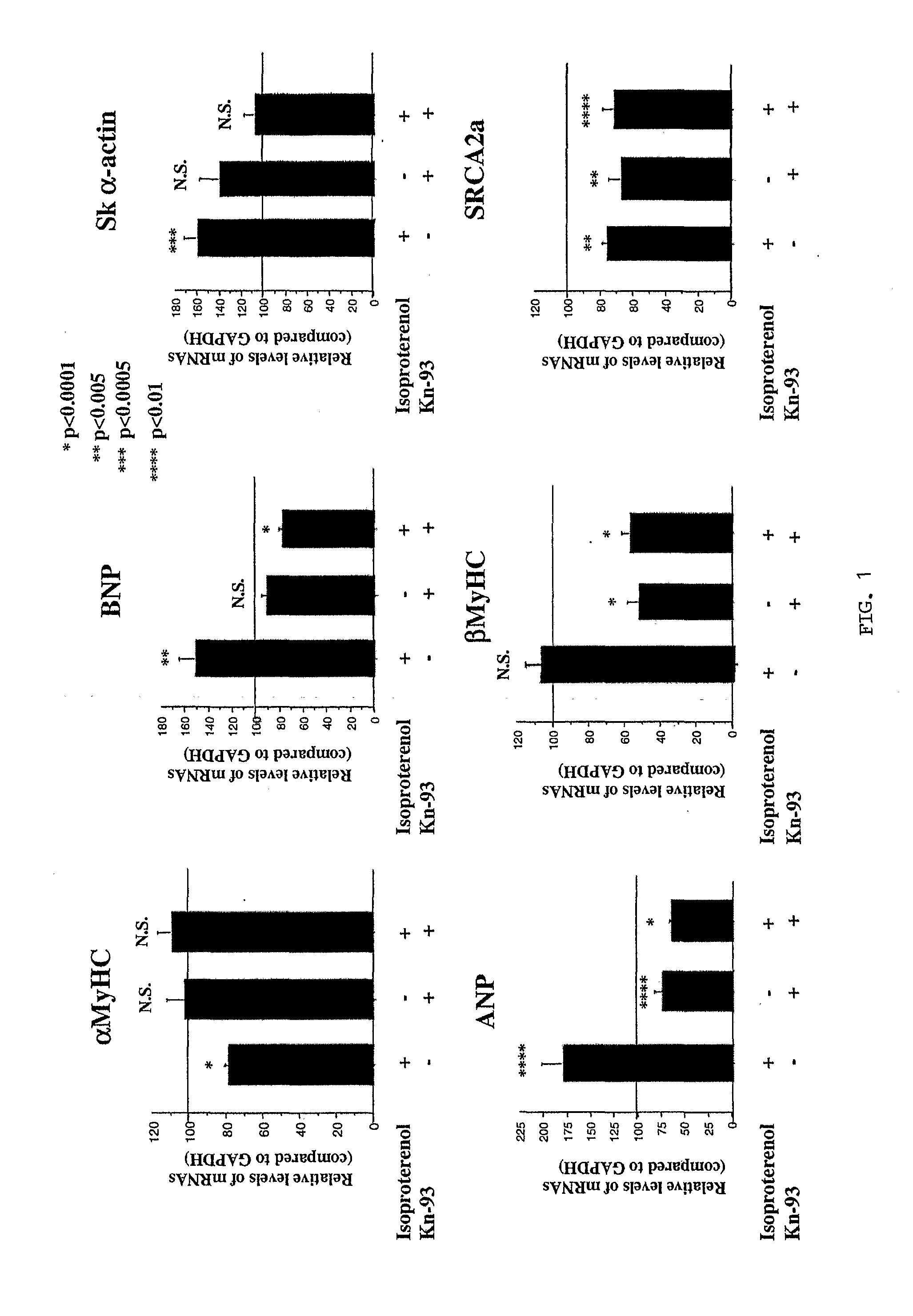
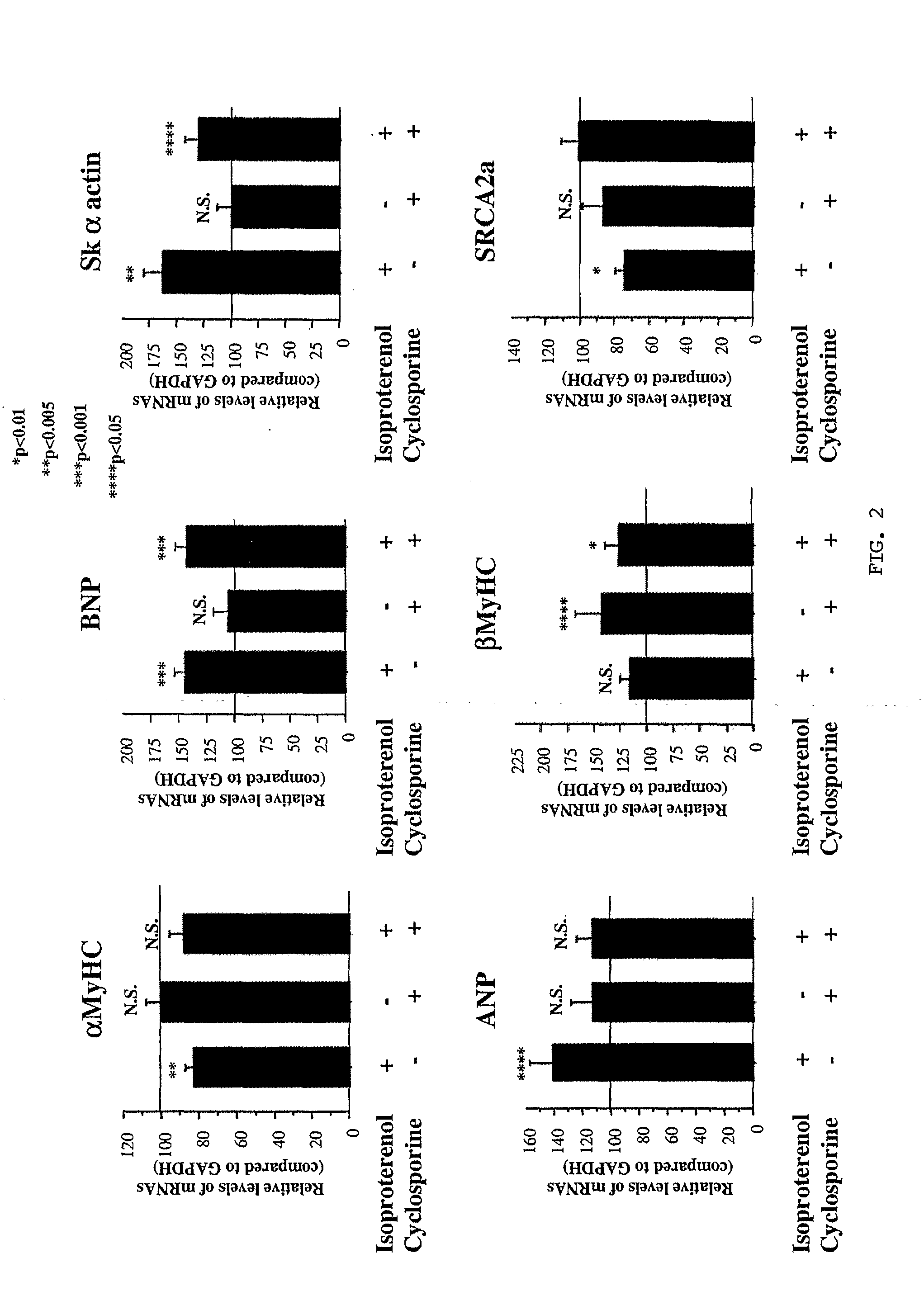
Inhibition of extracellular signal-regulated kinase 1/2 as a treatment for cardiac hypertrophy and heart failure
InactiveUS20090220507A1Improve athletic abilityIncrease injection volumeCompound screeningApoptosis detectionExtracellular signalManagement of heart failure
The present invention provides for methods of treating and preventing cardiac hypertrophy and heart failure. The present invention provides the link between ERK1 / 2 and calcineurin and CamKII. The present invention further demonstrates that inhibitors of ERK1 / 2 inhibit cardiac hypertrophy and heart disease by inhibiting, in part, the fetal cardiac gene expression that occurs when Ca2+-dependent signalling occurs in the heart.
Owner:UNIV OF COLORADO THE REGENTS OF
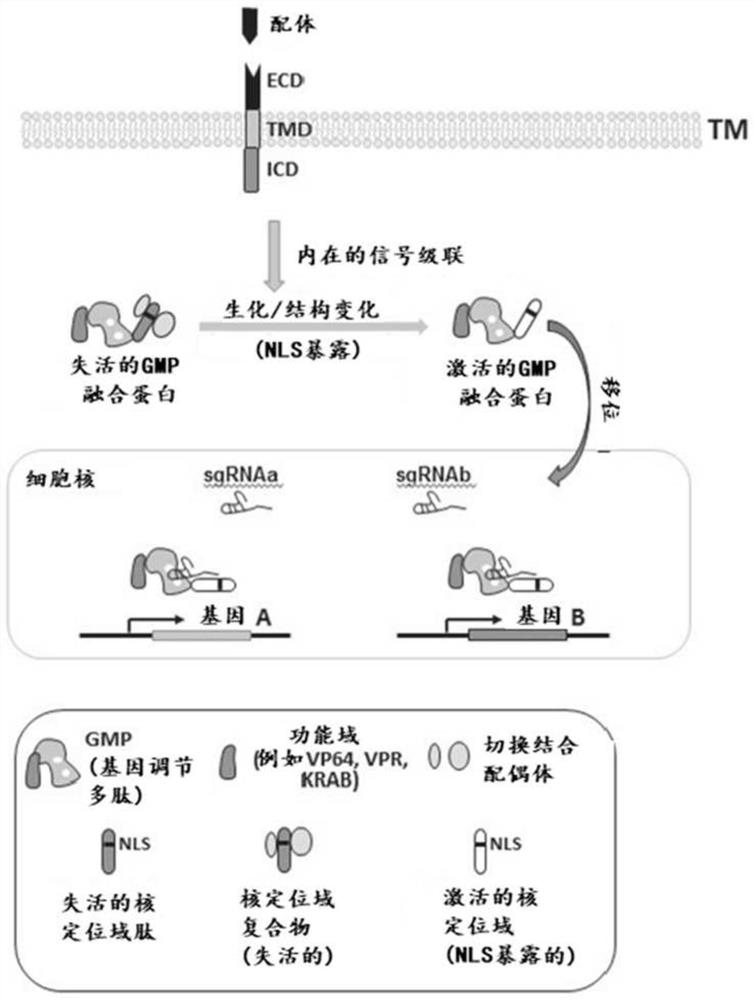
Gene regulation via conditional nuclear localization of gene modulating polypeptides
PendingCN112512594APolypeptide with localisation/targeting motifImmunoglobulin superfamilyHeterologousExtracellular signal
The present disclosure provides a system for regulating expression of a target polynucleotide in a cell. The system may comprise a chimeric polypeptide comprising a gene modulating polypeptide fused in-frame with a heterologous nuclear localization domain. The heterologous nuclear localization domain may be operable to translocate the chimeric polypeptide to a cell nucleus upon activation by an active cellular signaling pathway. The cellular signaling pathway may be inducible in response to an extracellular signal. In response to the extracellular signal, the chimeric polypeptide may localizeto the cell nucleus and the gene modulating polypeptide may regulate expression of a target polynucleotide in the cell nucleus.
Owner:REFUGE BIOTECH INC
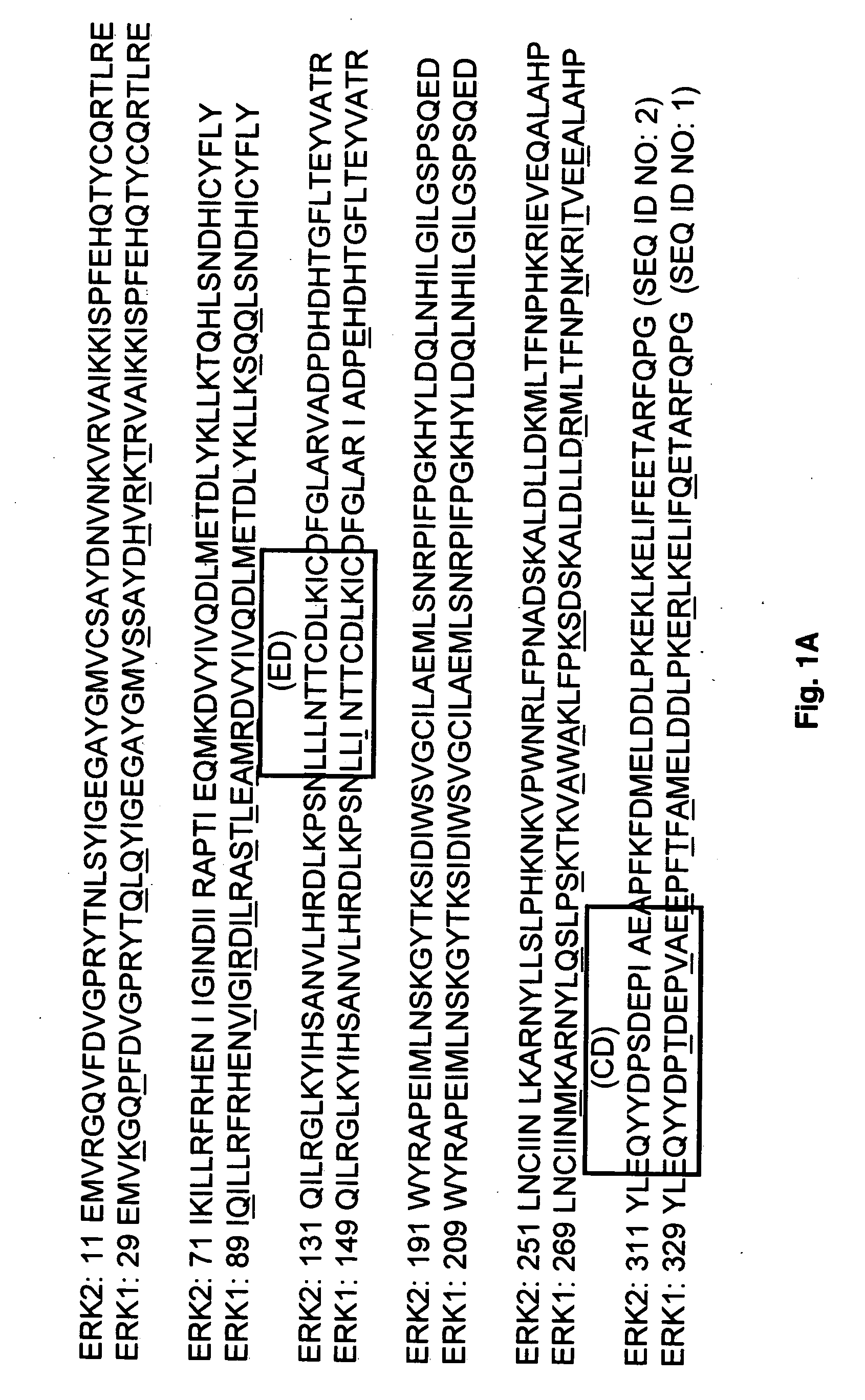
Antibodies directed toward extracellular signal-related kinases
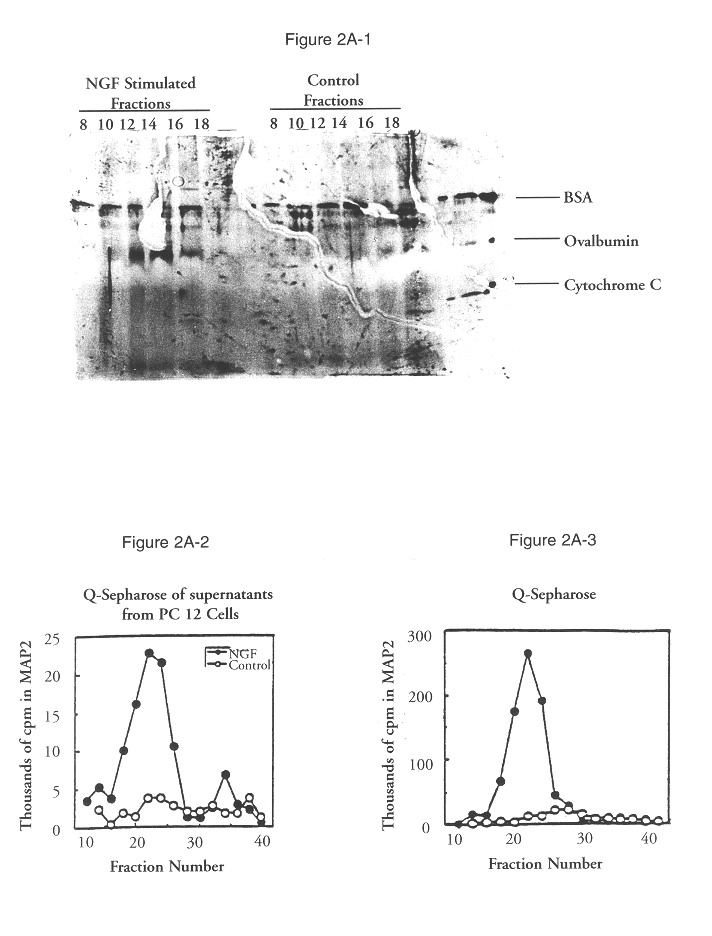
The present invention relates to a newly identified family of protein serine / threonine kinases which phosphorylate microtubule-associated protein 2 (MAP2). It is based, in part, on the cloning and characterization of novel MAP2 kinases designated extracellular signal-regulated kinase 1, 2, and 3 (ERK1, ERK2, ERK3) which are expressed in the central nervous system, and on the identification of another ERK family member, ERK4, with antisera. The present invention provides for recombinant nucleic acid molecules and proteins representing members of the MAP2 kinase family, and also for microorganisms, transgenic animals, and cell lines comprising recombinant MAP2 kinase molecules. In additional embodiments of the invention, the present invention provides for methods for assaying cellular factor activity, including, but not limited to, nerve growth factor activity, in which the activation of MAP2 kinase serves as an indicator of cellular factor activity. These methods may be extremely useful in screening compounds for the presence of a desired cellular factor activity. In specific embodiments, compounds which may be useful in the treatment of Alzheimer's disease, peripheral neuropathies, and diabetes may be identified using the methods of the invention.
Owner:REGENERON PHARM INC +1
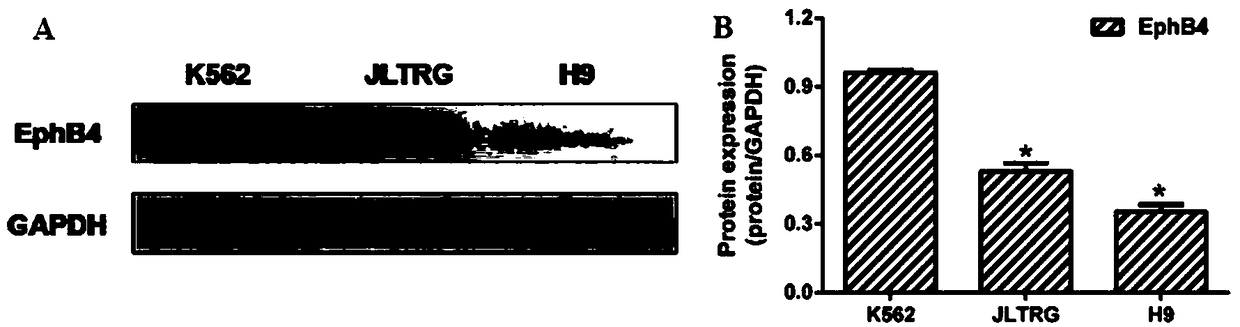
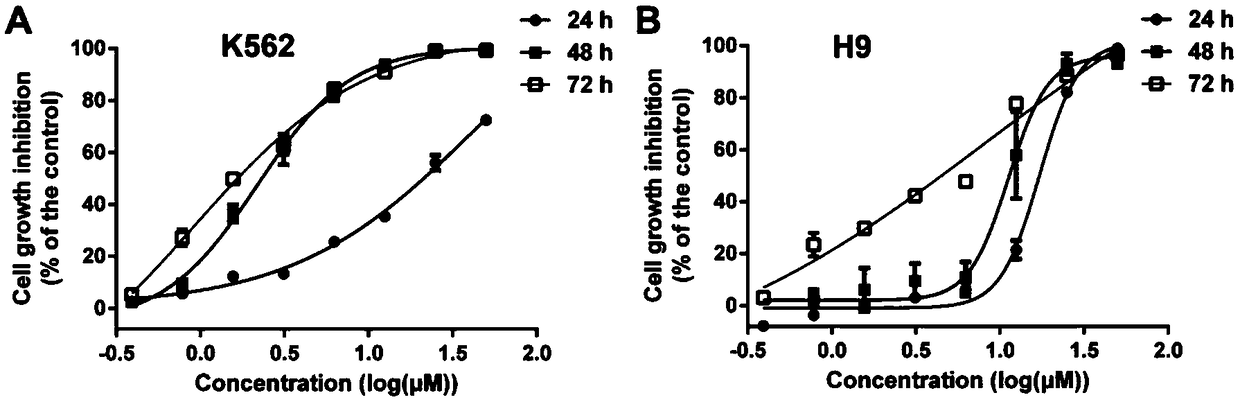
Application of Vandetanib to preparation of targeted EphB4 anti-tumor drug
InactiveCN108785310AInhibits kinase activityPrevent proliferationOrganic active ingredientsAntineoplastic agentsAntileukemic agentExtracellular signal
The invention discloses an application of Vandetanib to preparation of a targeted EphB4 anti-tumor drug, and belongs to the technical field of medicines. Experiments verify the application of the Vandetanib, and a molecular docking method, an SPR (surface plasmon resonance) method and MST (modulated scatterer technique) experiment results indicate that the Vandetanib can interact with EphB4. EphB4kinase experiments display that the Vandetanib can obviously inhibit EphB4 kinase activity. Proliferation of leukemic cells K562, JLTRG and H9 is remarkably inhibited. Besides, the Vandetanib remarkably inhibits expression of signal molecules p-PI3K p85 / p55 and PI3K p85 at the downstream of the EphB4, and the phosphorylation level of MEK (methyl ethyl ketone) and ERK (extracellular signal-regulated kinase) is reduced. Therefore, the experiment results sufficiently support the application of the Vandetanib to an anti-leukemia drug and the targeted EphB4 anti-tumor drug.
Owner:XI AN JIAOTONG UNIV
Methods of treating inflammatory bowel disease
InactiveUS20060088533A1Increase gene expressionInhibition of activationOrganic active ingredientsPeptide/protein ingredientsExtracellular signalClostridium difficile
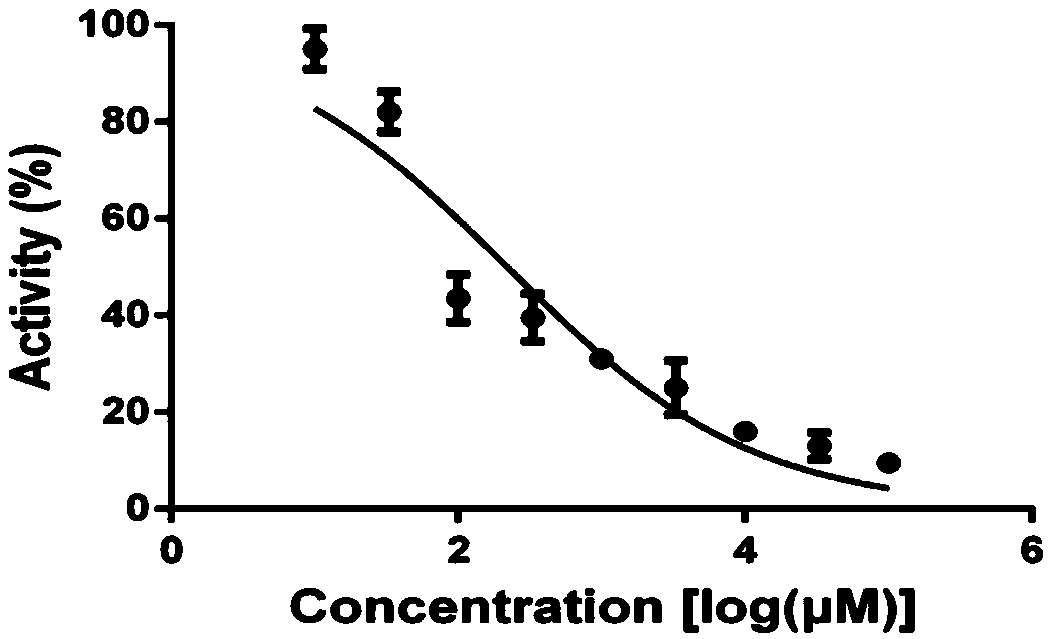
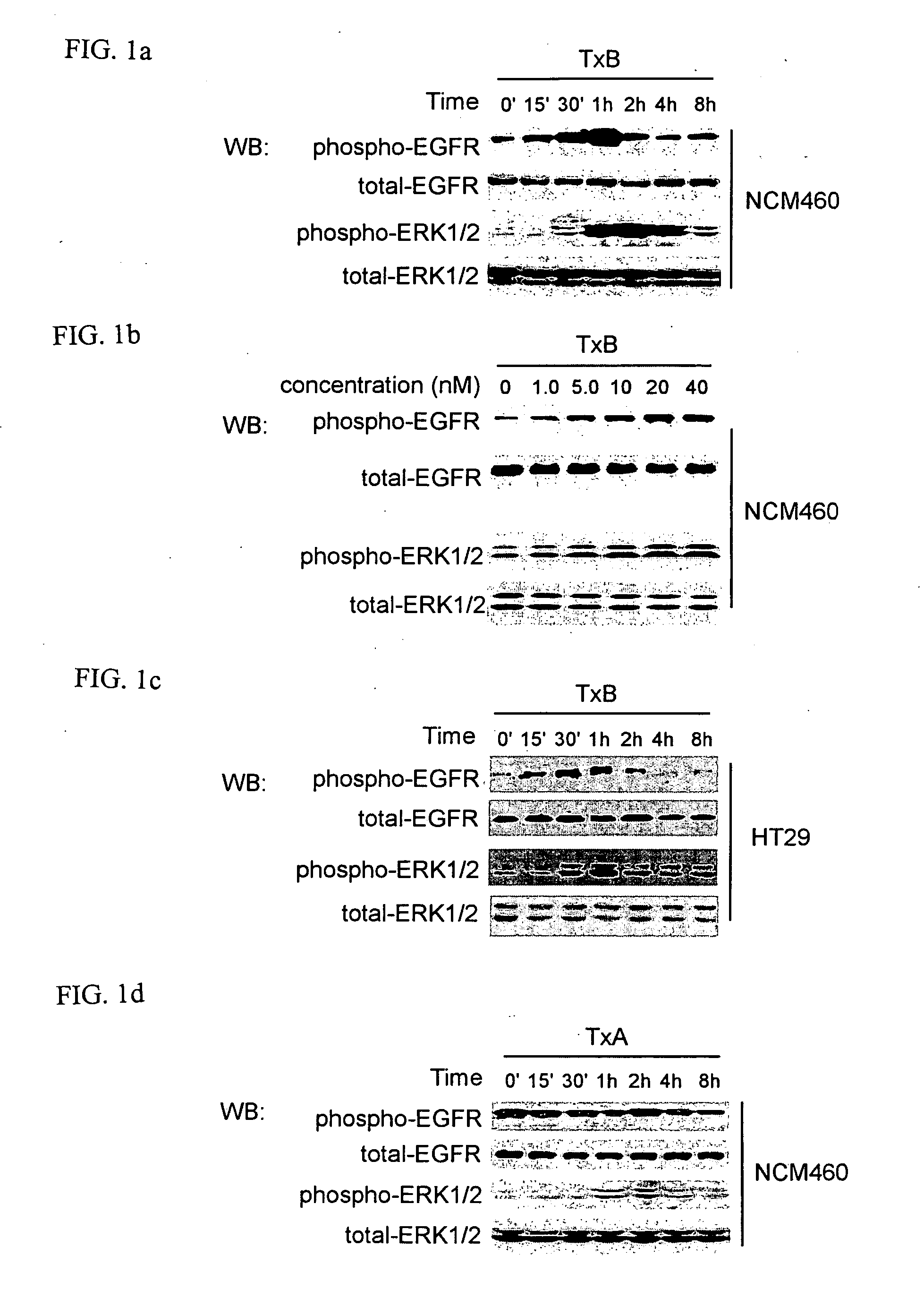
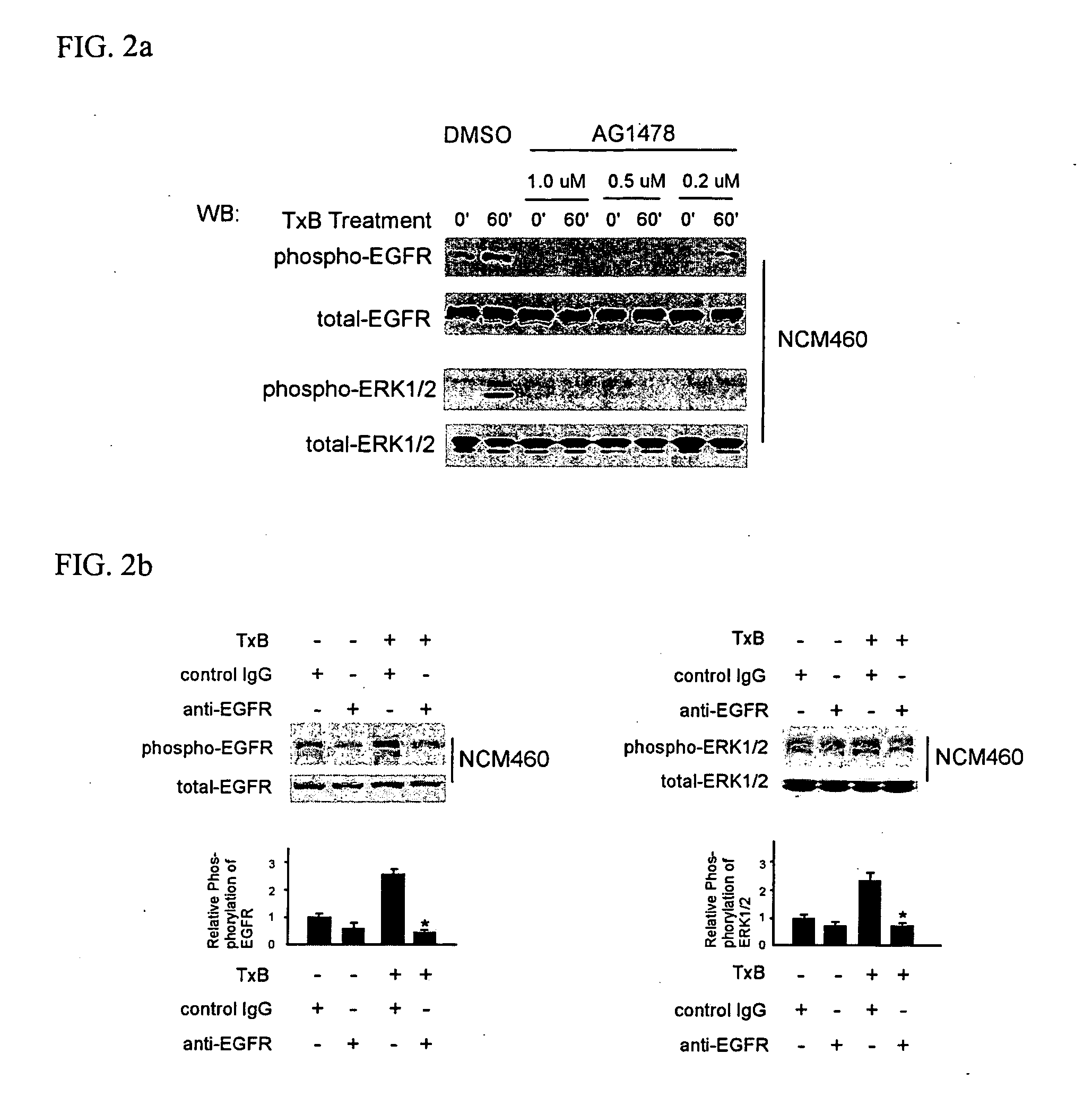
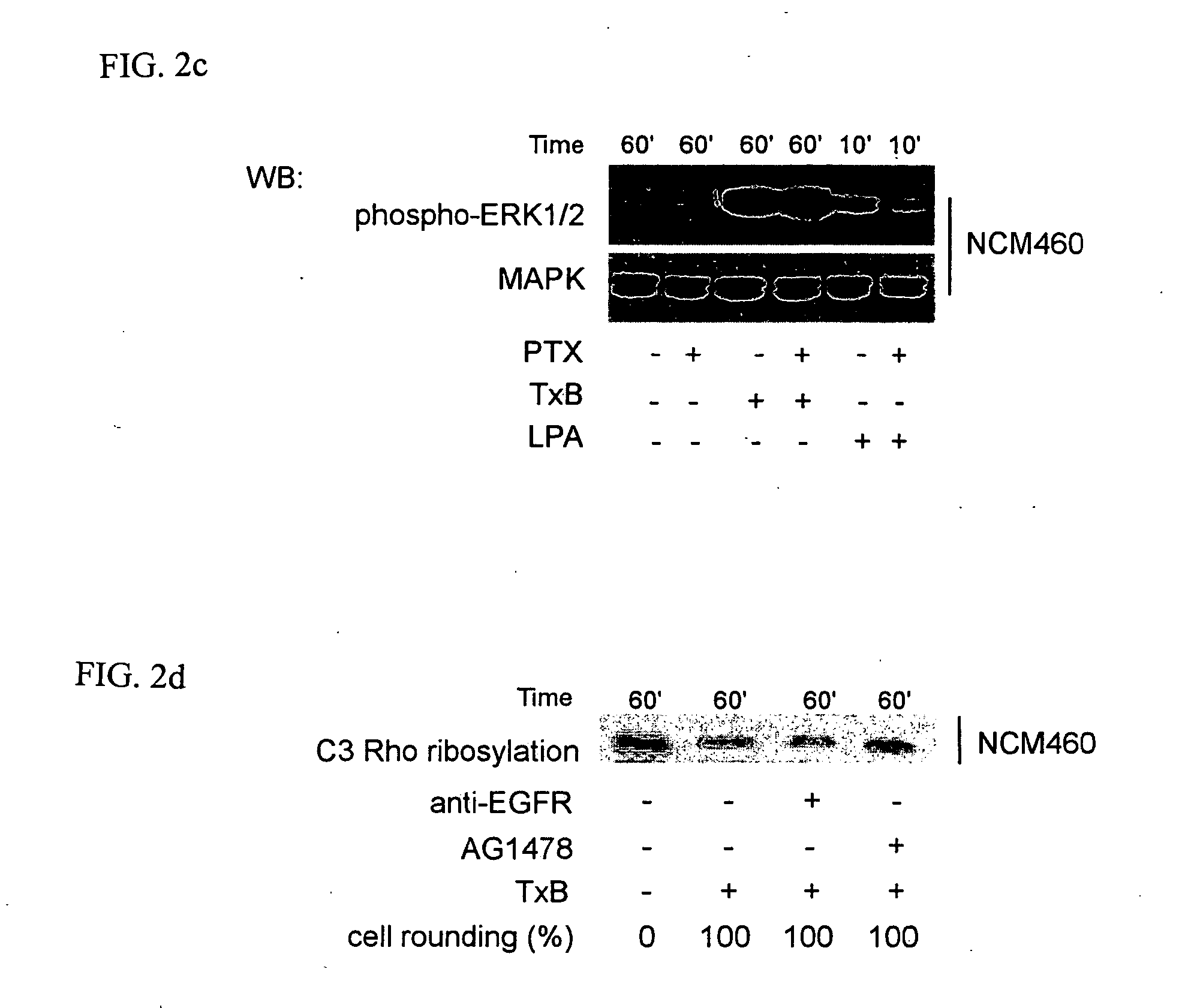
Methods for treating intestinal inflammation by inhibiting Clostridium difficile toxin B-mediated activation of the epidermal growth factor receptor or by inhibiting Clostridium difficile toxin B-mediated activation of the extracellular signal-regulated kinase 1 / 2 are described.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
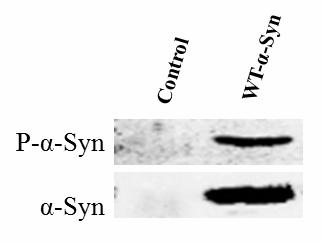
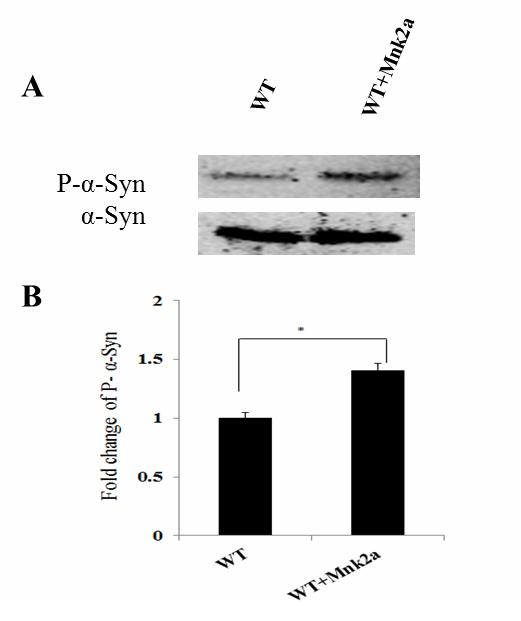
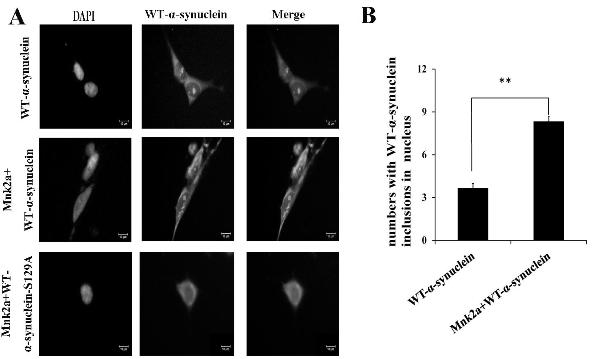
New purpose of protein kinase Mnk2a
Owner:SHANGHAI UNIV
ERK1 MAP2 protein kinase
The present invention relates to a newly identified family of protein serine / threonine kinases which phosphorylate microtubule-associated protein 2 (MAP2). It is based, in part, on the cloning and characterization of novel MAP2 kinases designated extracellular signal-regulated kinase 1, 2, and 3 (ERK1, ERK2, ERK3) which are expressed in the central nervous system, and on the identification of another ERK family member, ERK4, with antisera. The present invention provides for recombinant nucleic acid molecules and proteins representing members of the MAP2 kinase family, and also for microorganisms, transgenic animals, and cell lines comprising recombinant MAP2 kinase molecules. In additional embodiments of the invention, the present invention provides for methods for assaying cellular factor activity, including, but not limited to, nerve growth factor activity, in which the activation of MAP2 kinase serves as an indicator of cellular factor activity. These methods may be extremely useful in screening compounds for the presence of a desired cellular factor activity. In specific embodiments, compounds which may be useful in the treatment of Alzheimer's disease, peripheral neuropathies, and diabetes may be identified using the methods of the invention.
Owner:REGENERON PHARM INC +1
Attenuation of hyperoxia-induced cell death with mitochondrial aldehyde dehydrogenase
InactiveUS20080058278A1Reducing and eliminating generationPrevent and reduce incidenceGenetic material ingredientsGenetically modified cellsExtracellular signalPhosphorylation
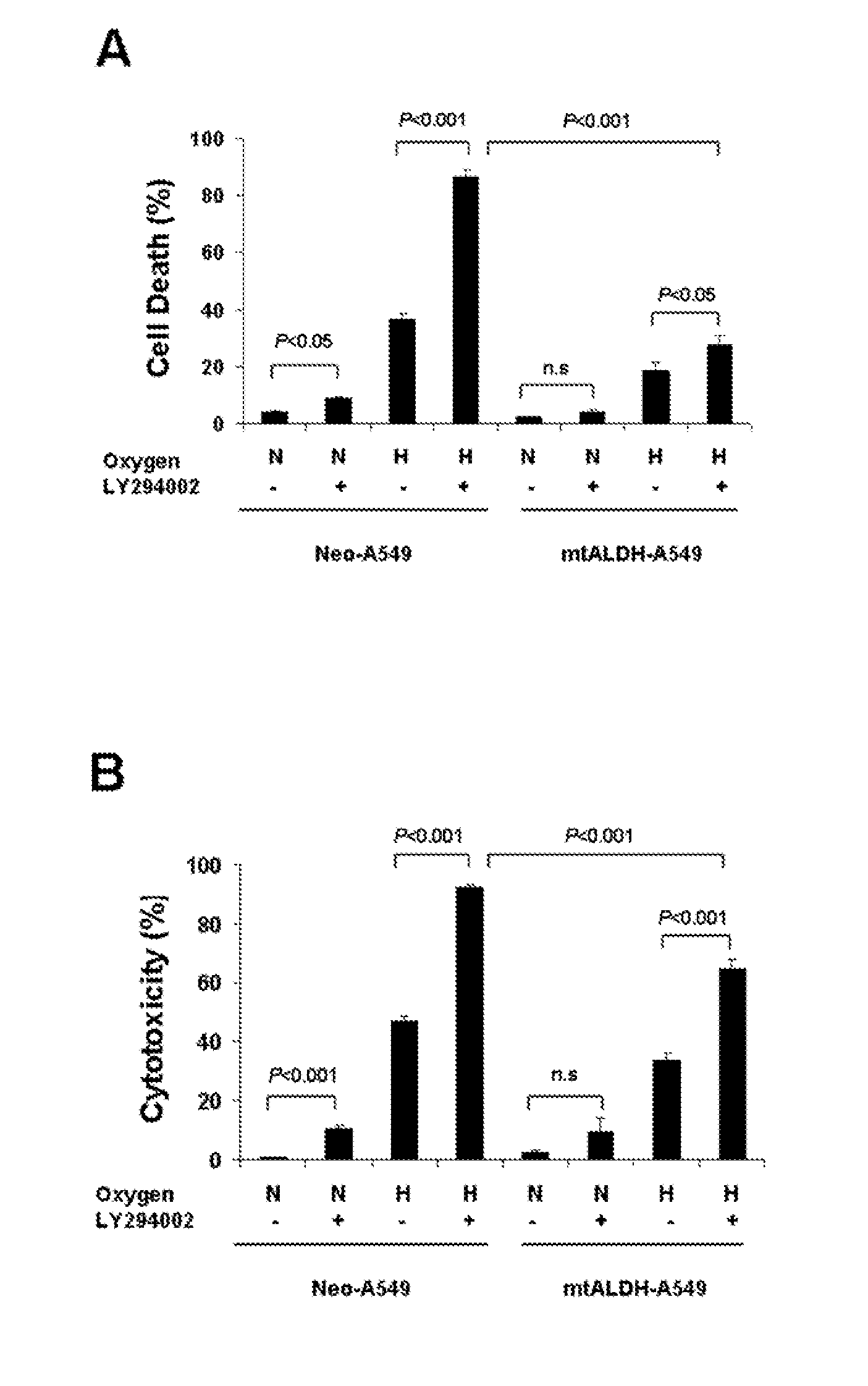
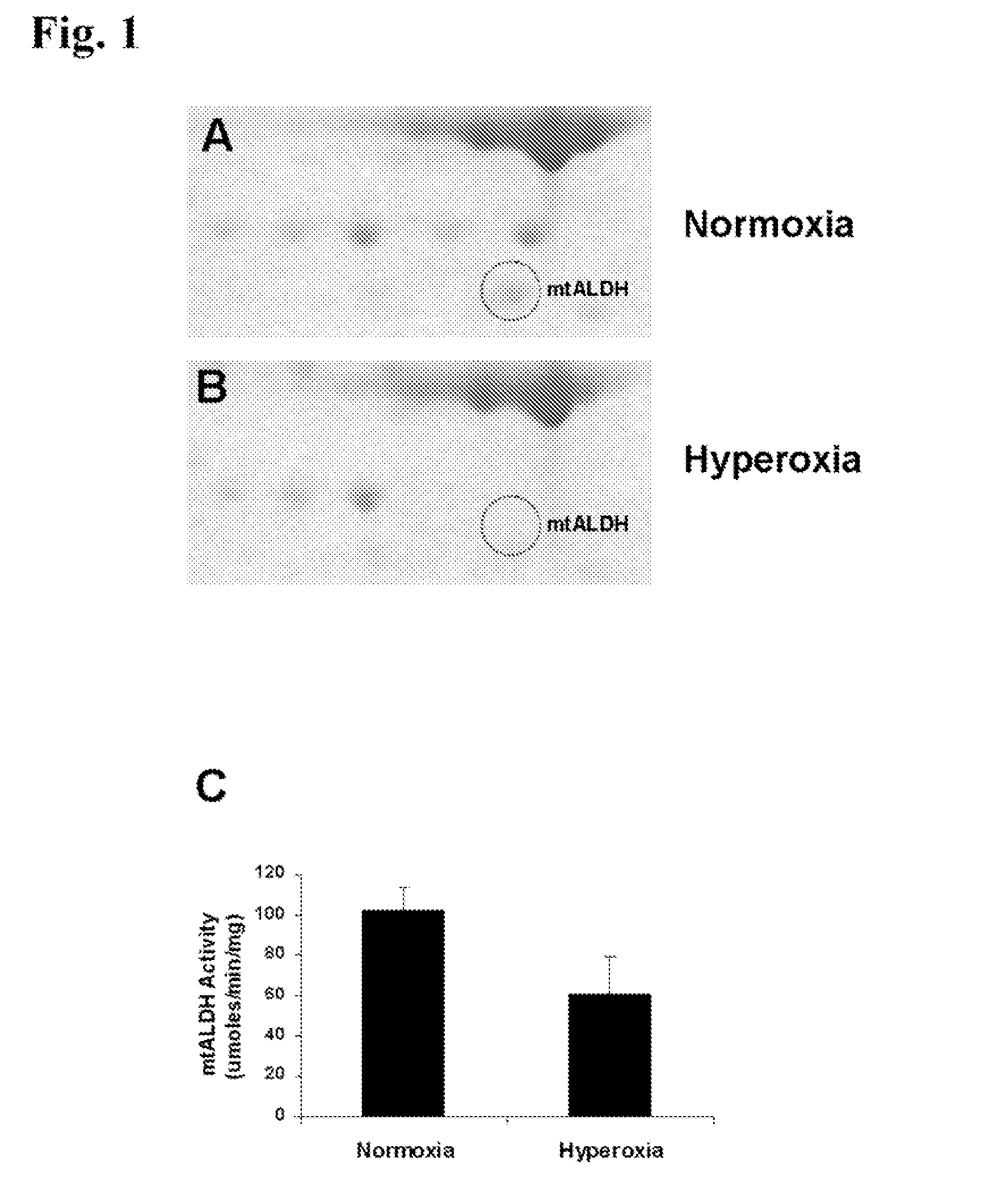
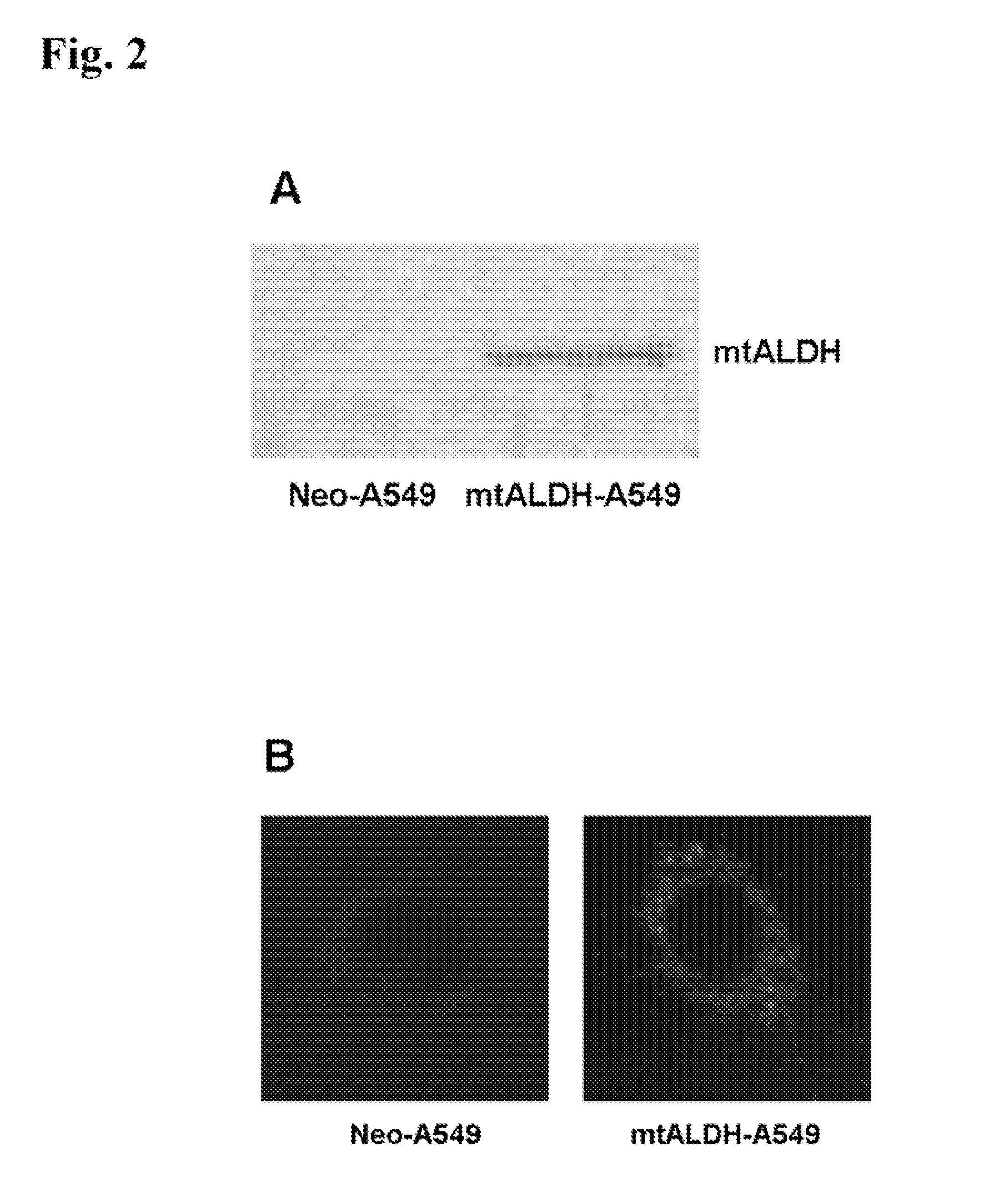
Oxygen toxicity is one of the major risk factors in the development of the chronic lung disease or bronchopulmonary dysplasia in premature infants. Using proteomic analysis, we discovered mitochondrial aldehyde dehydrogenase (mtALDH or ALDH2) was down-regulated in neonatal rat lung after hyperoxic exposure. To study the role of mtALDH in hyperoxic lung injury, we overexpressed mtALDH in human lung epithelial cells (A549) and found that mtALDH significantly reduced hyperoxia-induced cell death. Compared to control cells (Neo-A549), the necrotic cell death in mtALDH overexpressing cells (mtALDH-A549) decreased from 25.3% to 6.5%, 50.5% to 9.1% and 52.4% to 15.06% after 24-, 48- and 72-hour hyperoxic exposure, respectively. The levels of intracellular and mitochondria-derived reactive oxygen species (ROS) in mtALDH-A549 cells after hyperoxic exposure were significantly lowered compared to Neo-A549 cells. mtALDH overexpression significantly stimulated extracellular signal regulated kinase (ERK) phosphorylation under normoxic and hyperoxic conditions. Inhibition of ERK phosphorylation partially eliminated the protective effect of mtALDH in hyperoxia-induced cell death, suggesting ERK activation by mtALDH conferred cellular resistance to hyperoxia. mtALDH overexpression augmented Akt phosphorylation and maintained the total Akt level in mtALDH-A549 cells under normoxic and hyperoxic conditions. Inhibition of PI3K activation by LY294002 in mtALDH-A549 cells significantly increased necrotic cell death after hyperoxic exposure, indicating that PI3K / Akt activation by mtALDH played an important role in cell survival after hyperoxia. Taken together, these data demonstrate that mtALDH overexpression attenuates hyperoxia-induced cell death in lung epithelial cells through reduction of ROS, activation of ERK / MAPK and PI3K / Akt cell survival signaling pathways.
Owner:CHILDRENS MERCY HOSPITAL
Kit for quickly detecting expression quantities of related genes of sorafenib chemotherapeutic medicament
InactiveCN102618656AImprove accuracyStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceExtracellular signalTumor vessel
The invention relates to the technical field of biology. High expression of kinase insert domain receptors (KDR) and platelet-derived growth factor receptors alpha (PDGFRA) can be viewed in multiple tumor tissues; and sorafenib directly inhibits tumor growth by inhibiting the activities of the KDR and the PDGFR and inhibiting a recombinant activated factor / methyl ethyl ketone / extracellular signal-regulated kinase (RAF / MEK / ERK) signal transduction pathway, and blocks the formation of new tumor vessels by inhibiting vascular endothelial growth factors (VEGF) and platelet-derived growth factors (PDGF), so that the sorafenib achieves double inhibiting and multi-target blocking anti-hepatic cell carcinoma (HCC) effects. Detection of KDR and PDGFR mRNA levels can assist doctors in forecasting the curative effect of medicaments and the clinical outcome of patients, and has important clinical significance. The invention aims to provide a kit capable of quickly, conveniently, sensitively and specifically detecting expression quantities of related genes KDR and PDGFRA of a sorafenib chemotherapeutic medicament. According to the kit, the KDR and PDGFR mRNA levels are detected by adopting a fluorescent quantitative polymerase chain reaction (PCR) technology with high sensitivity and specificity, so that the sensitivity and the specificity are remarkably improved; and the kit is quick in detection and high in flux, and can finish the detection in 3 to 4 hours.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Inhibitors for Extracellular Signal-Regulated Kinase Docking Domains and Uses Therefor
InactiveUS20090299063A1Low toxicityInhibit cell proliferationOrganic active ingredientsOrganic chemistryExtracellular signalMedicine
Provided herein are compounds and methods of using compounds that selectively inhibit binding to one or more docking domain regions of an extracellular signal-recognition kinase to inhibit in a cell having an extracellular signal-regulated kinase activity. Such methods may be used to inhibit cell proliferation of a neoplastic cell, to treat a cancer and further may be used in conjunction with administration of an anticancer drug at a reduced dosage to treat a cancer with a concomitant reduction in toxicity to an individual receiving the treatment. Also provided is a method to design and screen for compounds to inhibit binding within the extracellular signal-regulated kinase docking domain region, using at least in part computer-aided drug design modeling.
Owner:SHAPIRO PAUL +1
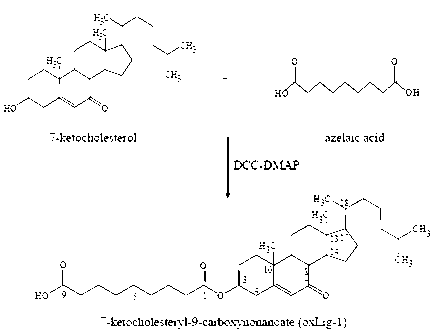
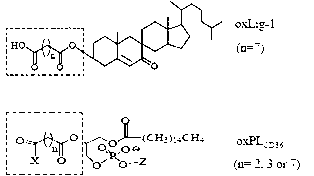
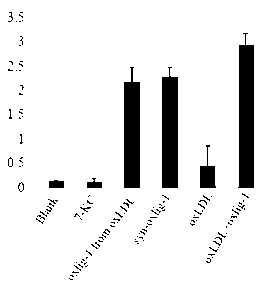
Chemical synthetic method of low affinity ligand of oxidized low density lipoprotein receptor CD36
The invention belongs to the field of biochemical engineering, and in particular relates to a chemical synthetic method of a low affinity ligand of an oxidized low density lipoprotein receptor CD36. The method comprises the following steps of: carrying out a chemical reaction on 4-Dimethylaminopyridine, N, N'-Dicyclohexylcarbodiimide, azelaic acid and 7-ketocholesterol; and obtaining oxLig-1 through extraction, sample mixing, packing, purification and collection. Experiments show that the chemically synthesized oxLig-1 has same physiological activity with that of oxLig-1 separated and purified from oxLDL (oxidized low density lipoprotein), and the oxLig-1 activates cell signaling mediated by CD36. According to the chemical synthetic method disclosed by the invention, oxLig-1 is synthesized by the chemical method, and oxLig-1 has a completely consistent chemical structure with that of the low affinity ligand of CD36 and can activate ERK (Extracellular Signal-Regulated Kinase) and JNK (Jun N-Terminal Kinase) and up-regulate expression of a cholesterol outflow gene, namely, a triphosadenine binding cassette transporter A1 (ABCA1).
Owner:DALIAN UNIV
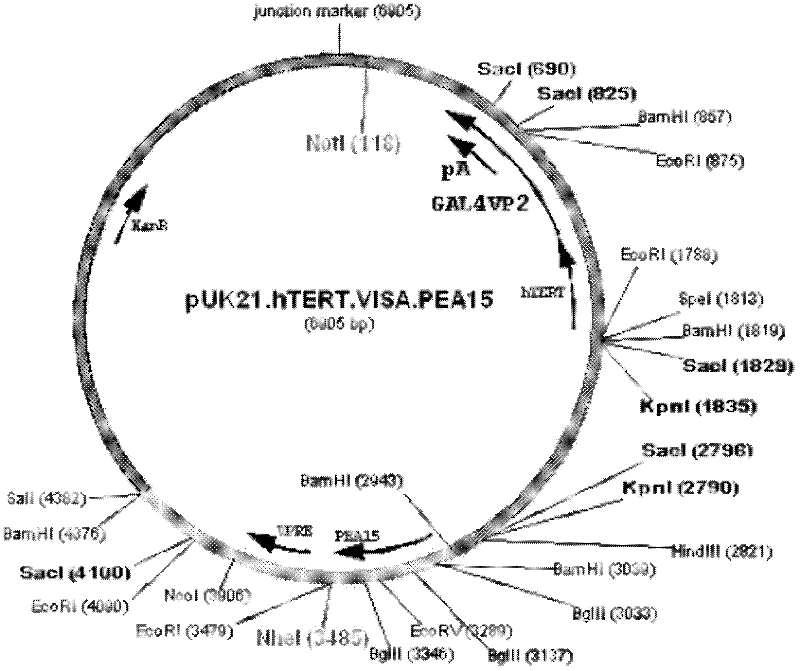
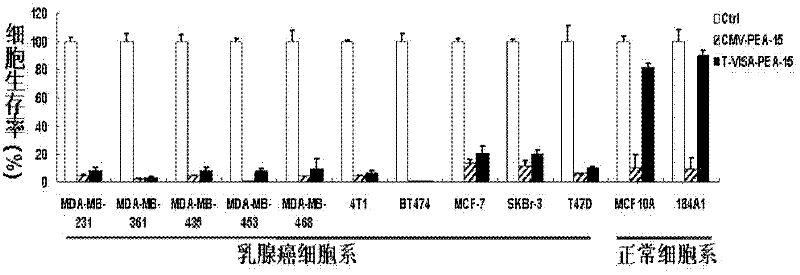
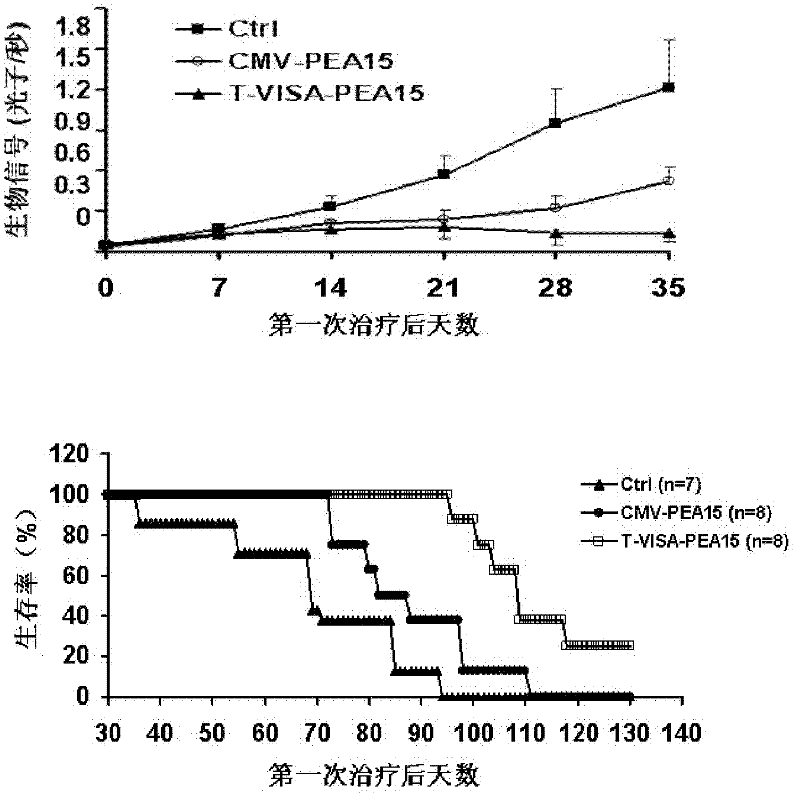
Medicine T-VISA-PEA15 capable of effectively and specifically killing breast cancer cells
ActiveCN102634531AGrowth inhibitionNo killing effectGenetic material ingredientsAntineoplastic agentsExtracellular signalSide effect
The invention discloses a medicine T-VISA-PEA15 capable of effectively and specifically killing breast cancer cells. The sequence of a T-VISA-PEA15 therapy vector capable of effectively and specifically killing the breast cancer cells is shown as SEQ ID NO.1. A liposome of the medicine T-VISA-PEA15 liposome capable of effectively and specifically killing the breast cancer cells comprises a liposome and the T-VISA-PEA15 therapy vector coated by the liposome, wherein the nucleotide sequence of the T-VISA-PEA15 therapy vector is shown as SEQ ID NO.1. After being coated by the liposome and transferred, the T-VISA-PEA15 therapy vector is enriched on the breast cancer part, so as to effectively target the breast cancer ERK (extracellular signal-regulated kinase) target point and inhibit the breast cancer ERK target point from entering the nucleus, thereby promoting the apoptosis of tumor cells, but not killing the normal cells. The medicine T-VISA-PEA15 can be applied to a whole body and has of the advantages of high gene expression amount, long gene expression time, high gene expression efficiency, definite efficacy, low toxicity and side effect, no toxicity to liver and kidneys and wide prospect in the treatment of the breast cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
Substituted pyrimidines, pharmaceutical compositions and therapeutic methods thereof
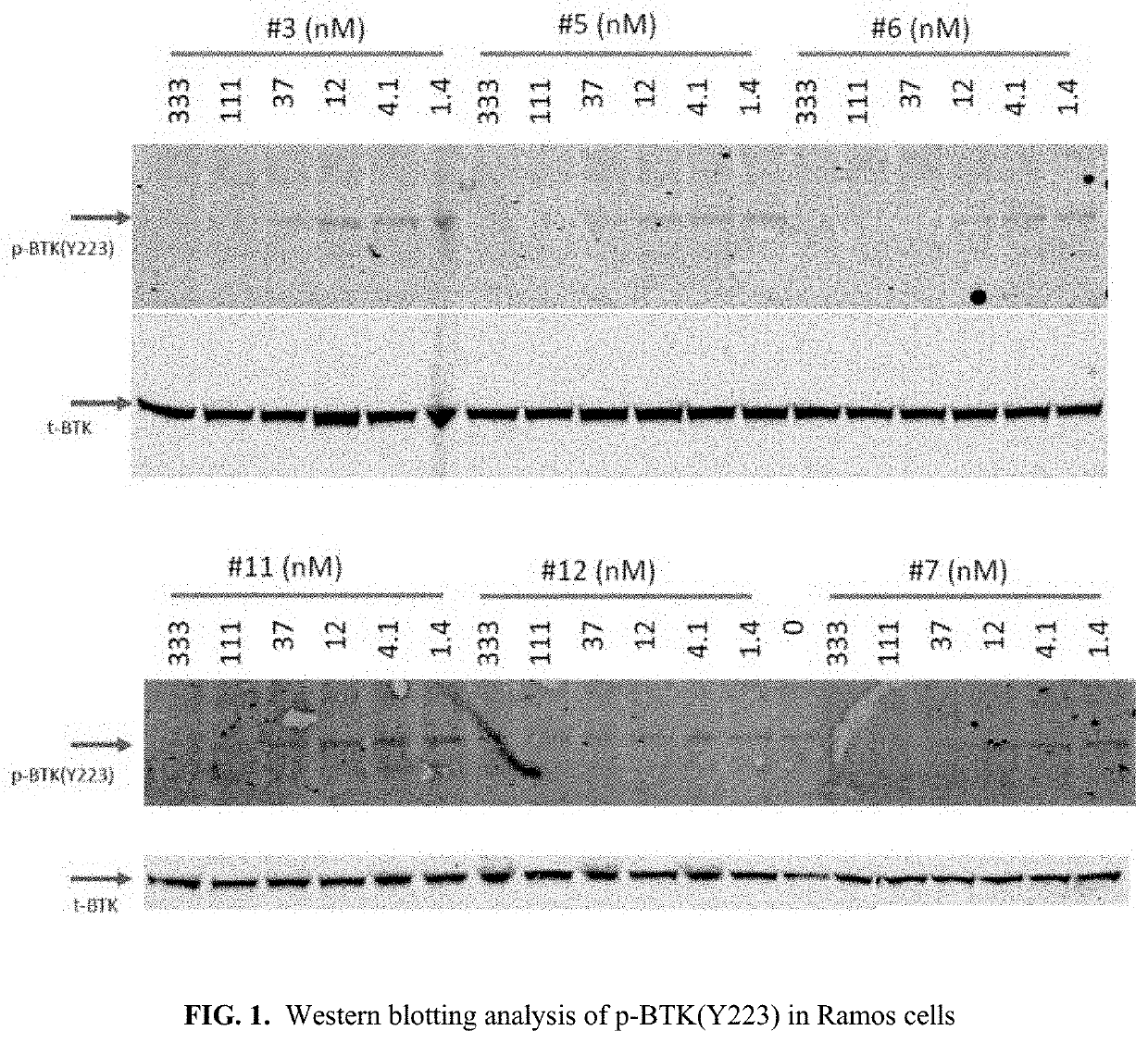
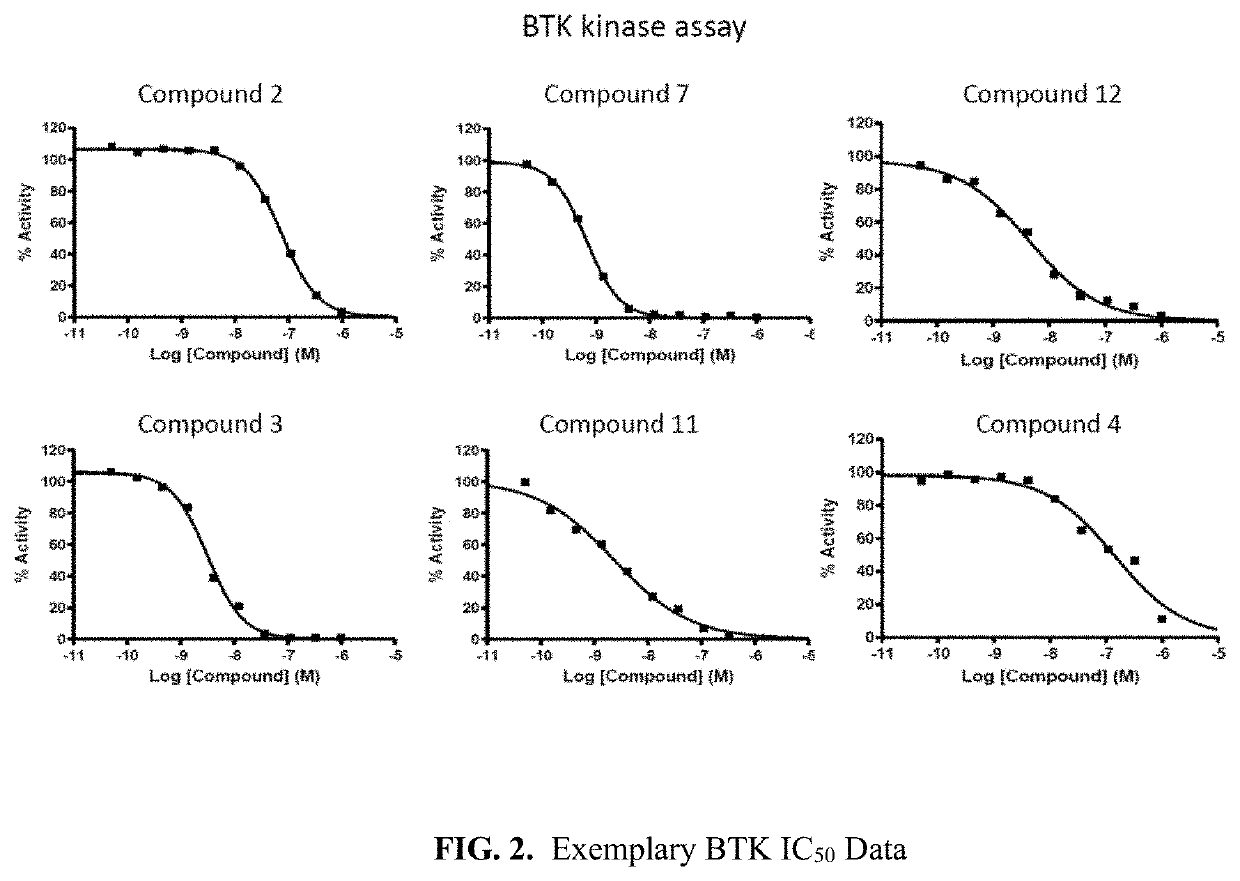
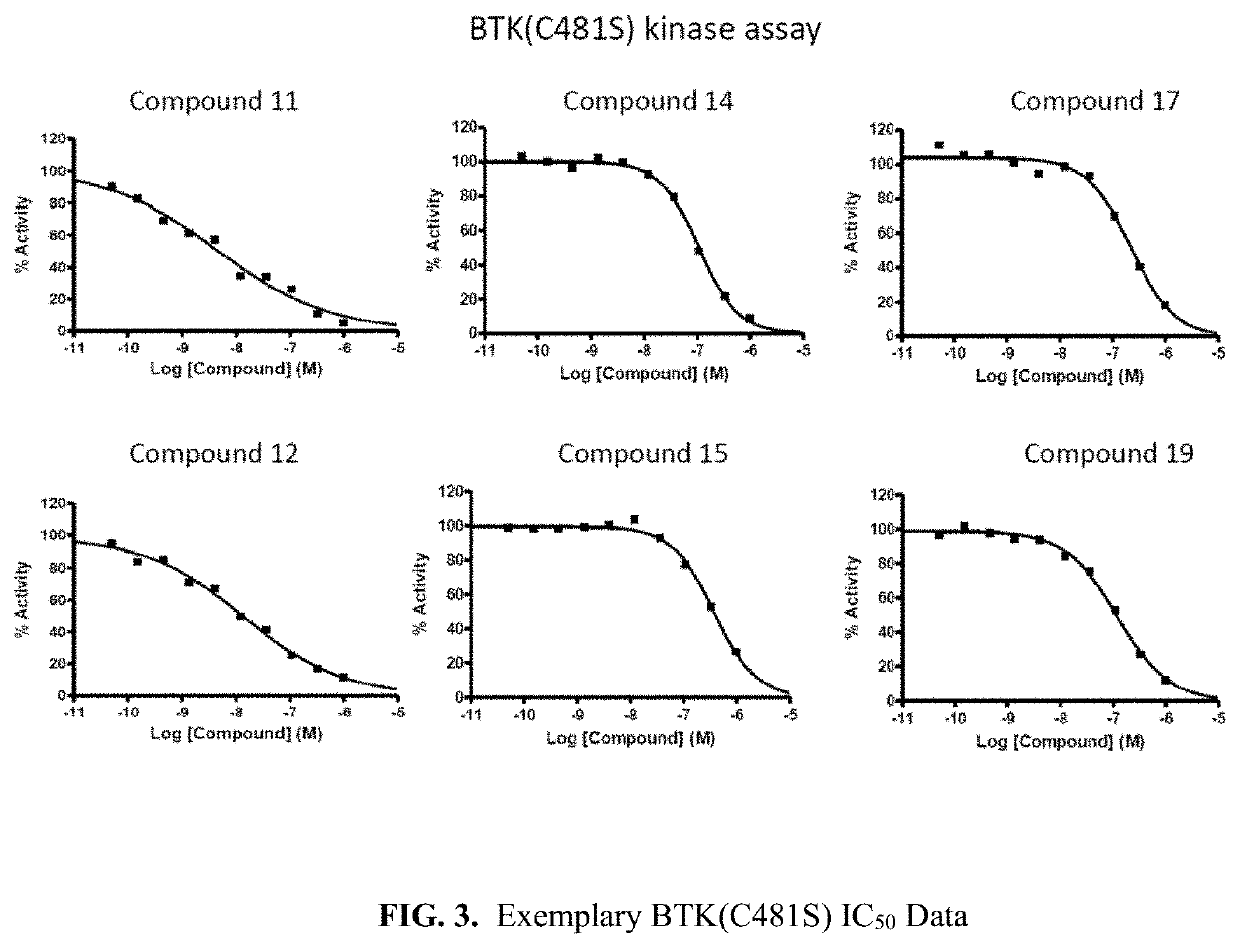
The invention provide novel pyrimidine derivatives and analogs having inhibitory activities towards certain tyrosine kinases, e.g., Bruton's tyrosine kinase (Btk) and / or Focal adhesion kinase (FAK), extracellular signal-regulated kinase (ERK), pharmaceutical compositions thereof, and methods of treatment, reduction or prevention of certain diseases or conditions mediated by such by tyrosine kinases, e.g., cancers, tumors, fibrosis, inflammatory diseases, autoimmune diseases, diabetes, or immunologically mediated diseases.
Owner:CHEN ZHIHONG
Fungus secretory expression vector as well as construction method and application thereof
ActiveCN110938648AEffective secretionRapid identificationPolypeptide with localisation/targeting motifFungiBiotechnologyExtracellular signal
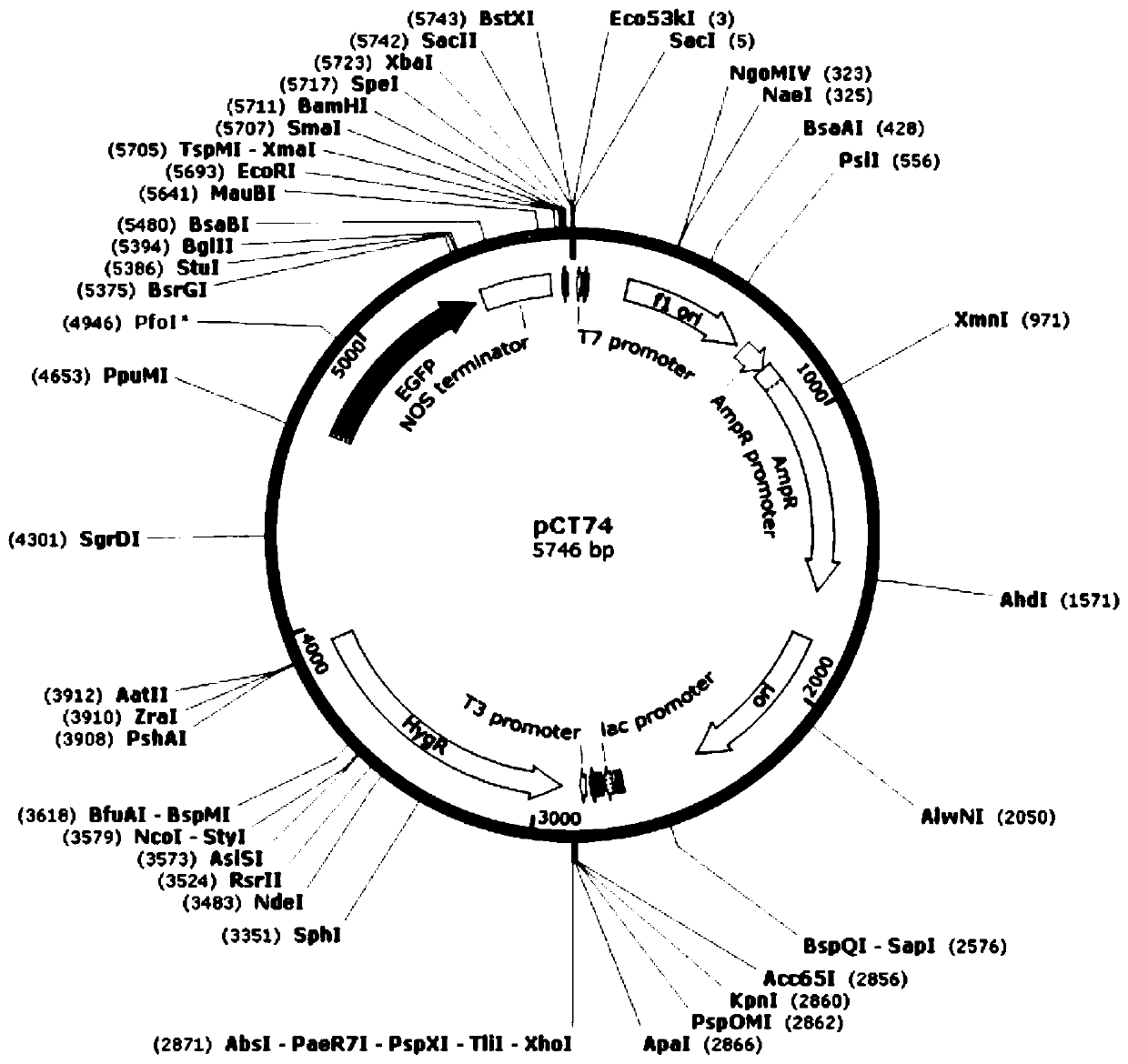
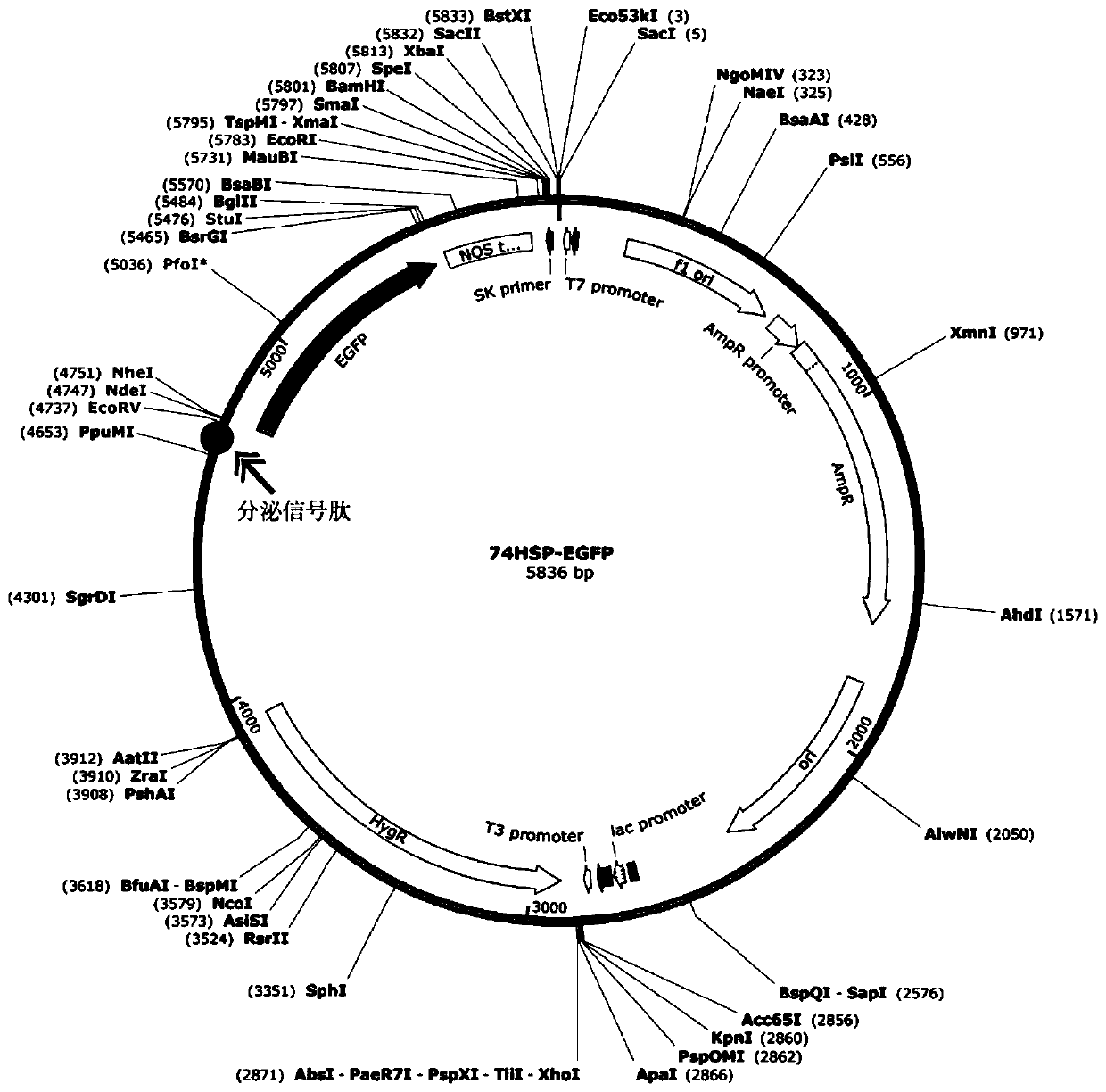
The invention provides a fungus secretory expression vector as well as a construction method and application thereof. The fungus secretory expression vector is a fungus secretory expression vector 74HSP formed by inserting extracellular signal peptide of a Six1d secretory protein N-terminal 23aa (MAPYSMVLLGALSILGFGAYAQE) into a 4665bp position of a pCT74 vector. According to the invention, a fungus secretory expression vector capable of effectively secreting plant protein to the outside of the pathogenic bacteria cell is successfully constructed and the target protein is effectively expressedin fungus and secreted into the plant in the infection process, so that the rapid identification of the plant gene function is realized.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
A kind of culture system and culture method of lacrimal gland stem cells and lacrimal gland stem cells
ActiveCN109486766BA large amountContinuous passageCulture processNervous system cellsExtracellular signalMatrigel
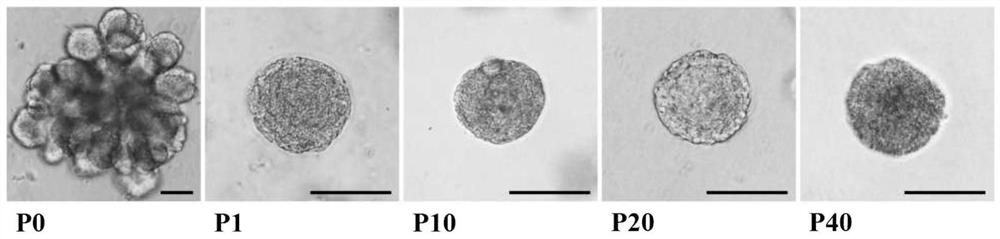
The invention relates to a lacrimal gland stem cell, a culture system and a culture method of the lacrimal gland stem cell, and relates to the field of cell engineering. The culture system of lacrimal gland stem cells of the present invention uses DMEM / F12 as the base medium, and adds the following components to form the culture medium: cell culture additives, non-essential amino acids, L-alanyl-L-glutamine, mitogen-activated protein Kinase / extracellular signal regulates the composition of kinase signaling pathway ligand, fibroblast growth factor, Wnt signaling pathway ligand and ROCK signaling pathway inhibitor, and uses matrigel as a three-dimensional culture support. The culture method of the present invention is as follows: the mouse lacrimal gland tissue is digested into single cells and inoculated into Matrigel, and after solidification, the lacrimal gland stem cell culture medium is added to carry out primary culture. The mouse lacrimal gland stem cells cultured by the method of the present invention have a relatively large number, are stable enough for continuous passage, and have the function of restoring lacrimal gland secretion function, and can effectively treat dry eye.
Owner:SUN YAT SEN UNIV
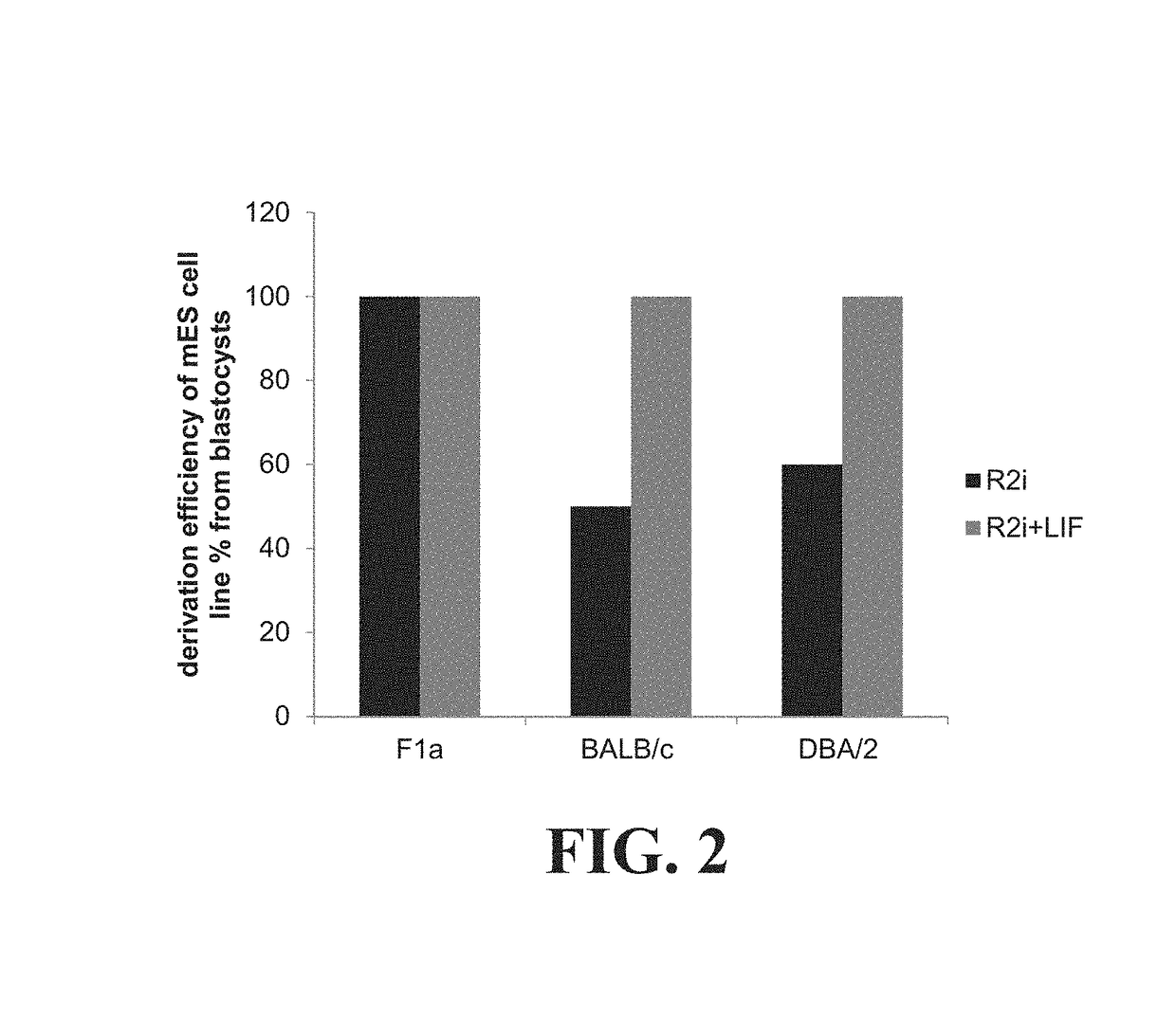
Establishing pluripotency in mouse embryonic stem cells
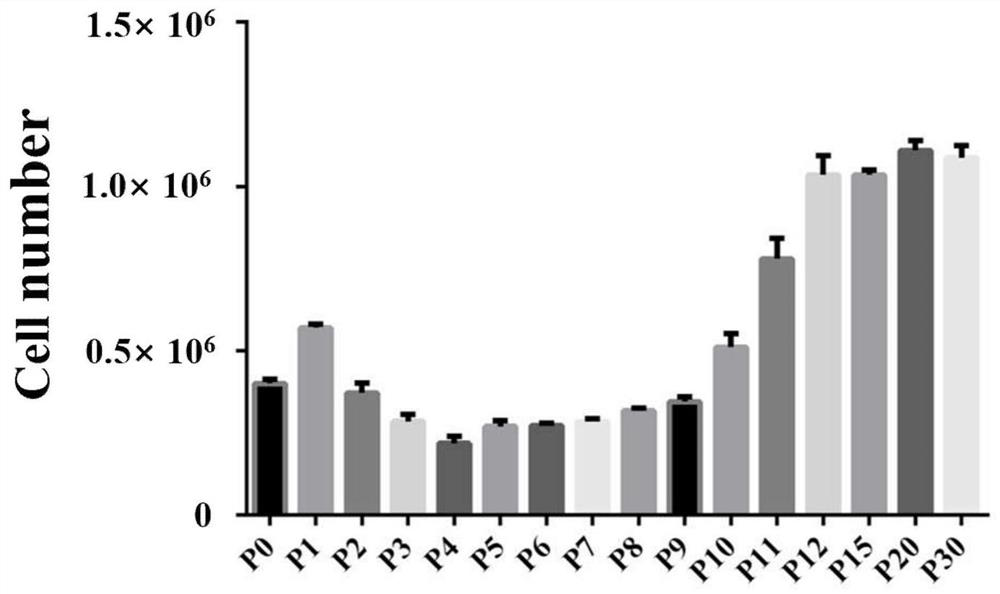
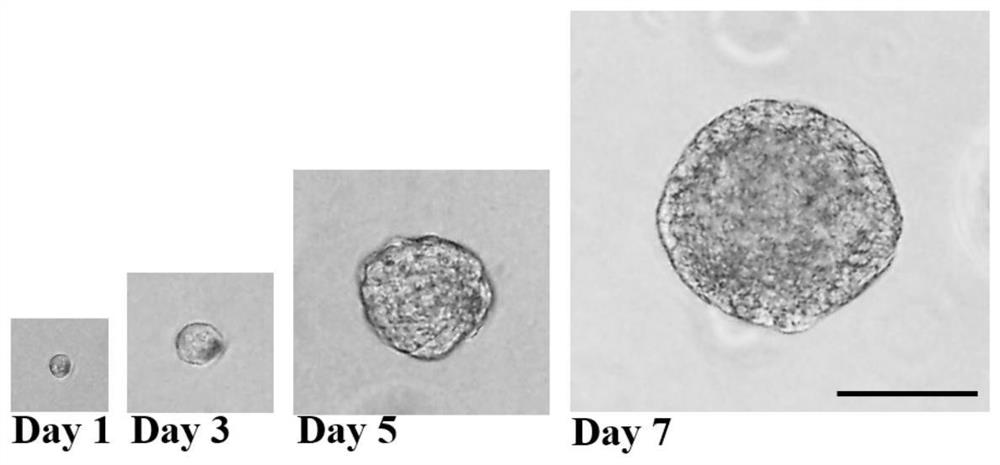
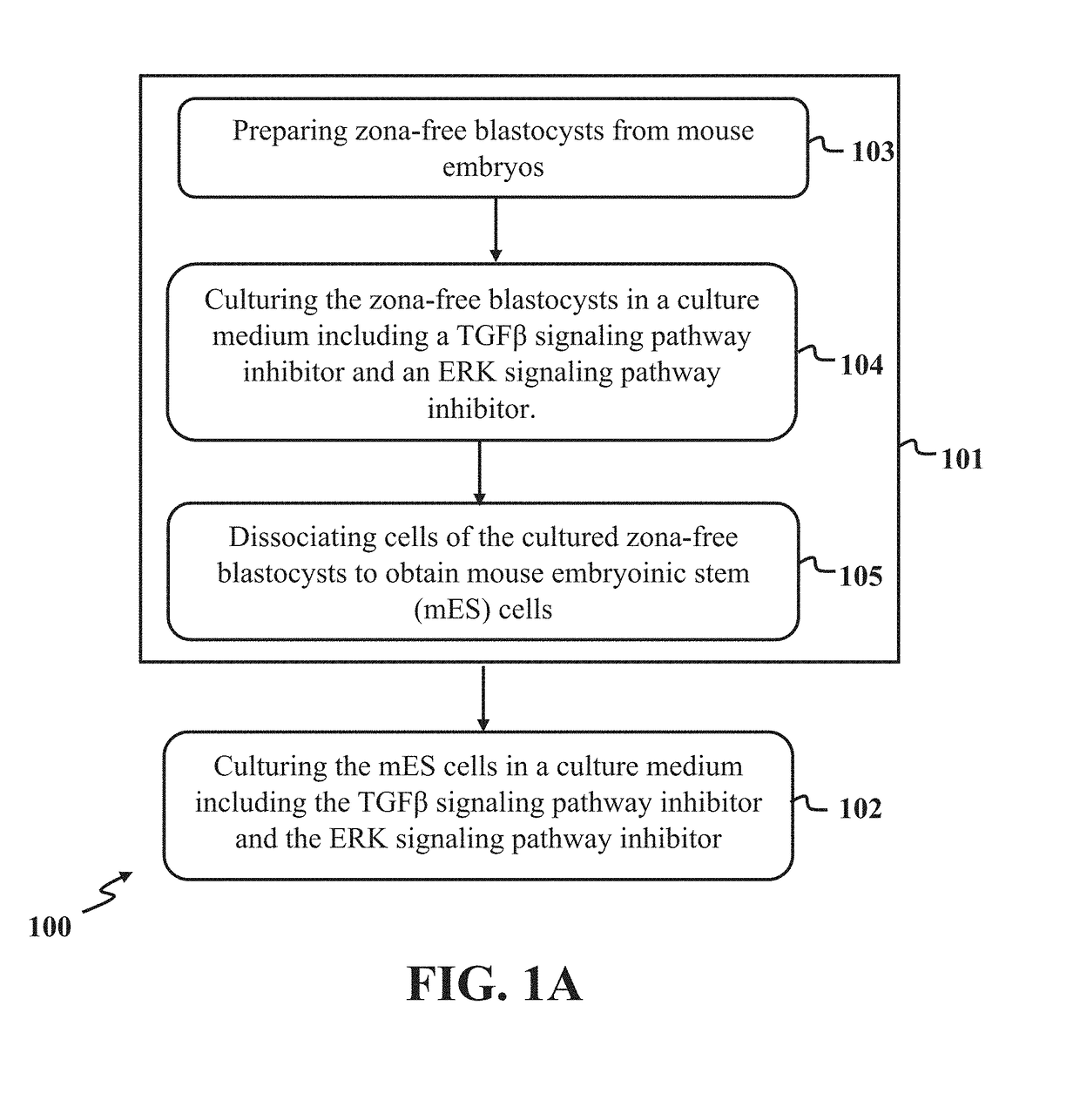
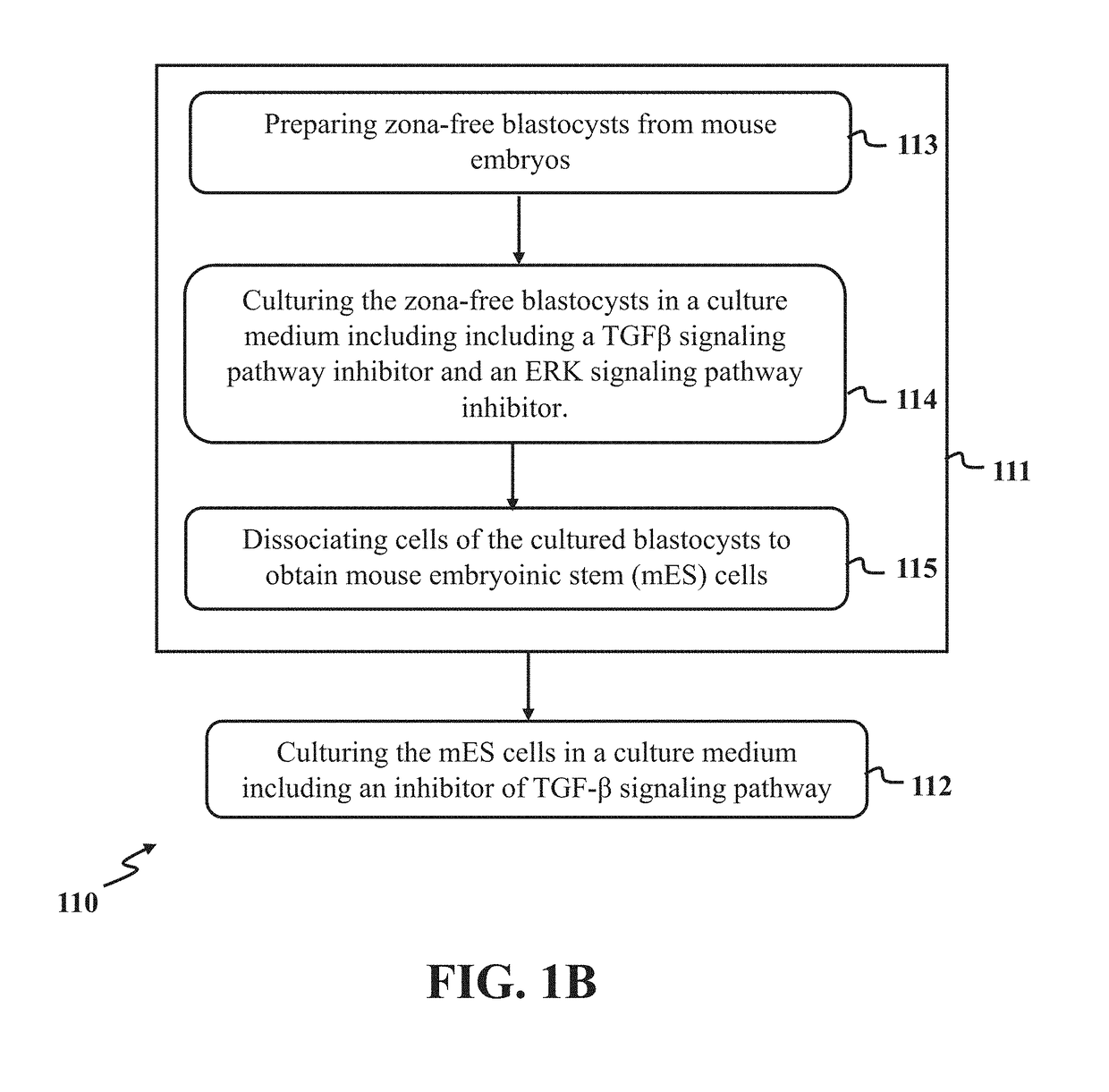
A method for derivation and maintenance of pluripotency in mouse embryonic stem (mES) cells is disclosed. The method includes isolating mES cells from mouse embryos in a culture medium including an R2i compound, and culturing the mES cells in a medium including the R2i compound or a transforming growth factor beta (TGF-β) signaling pathway inhibitor. The R2i compound includes combination of a transforming growth factor beta (TGF-β) signaling pathway inhibitor and an extracellular signal-regulated kinases (ERK) signaling pathway inhibitor. This method can facilitate the homogeneous expression of pluripotency factors in embryonic stem cells.
Owner:ROYAN INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com