New purpose of protein kinase Mnk2a
A technology of protein kinase and mnk2a, applied in peptide/protein components, medical preparations containing active ingredients, nervous system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
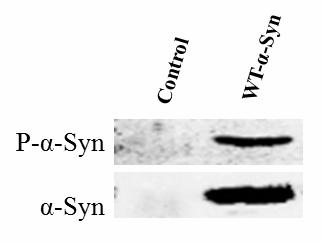
[0013] Example 1: Detection of the effect of Mnk2a on the phosphorylation of α-synuclein at position 129
[0014] 1. Construction of expression vector pcDNA3.1-α-synuclein, pcDNA3.1-α-synuclein-S129A (mutation of the serine at position 129 of α-synuclein to alanine) and pcDNA3.1-Mnk2a-myc using specific primers for PCR The α-synuclein and α-synuclein-S129A genes were cloned from fruit flies expressing human α-synuclein, and the Mnk2a gene was cloned from HEK293T cells, and then constructed into pcDNA3.1 and pcDNA3.1-myc empty loads. , Send it out for sequencing, and compare the sequencing result with the target gene sequence to confirm the success of the constructed vector.
[0015] The sequence of the upstream primer used to clone the Mnk2a target gene is: 5'-cccaagcttaccatgcccgccagccagcccattg -3'
[0016] The downstream primer sequence is: 5'-ccgctcgagtcaggcgtggtctcccaccag-3'
[0017] The full-length sequence of the Mnk2a target gene is:
[0018] atgcccgccagccagcccattgacatcccggacgc...
Embodiment 2
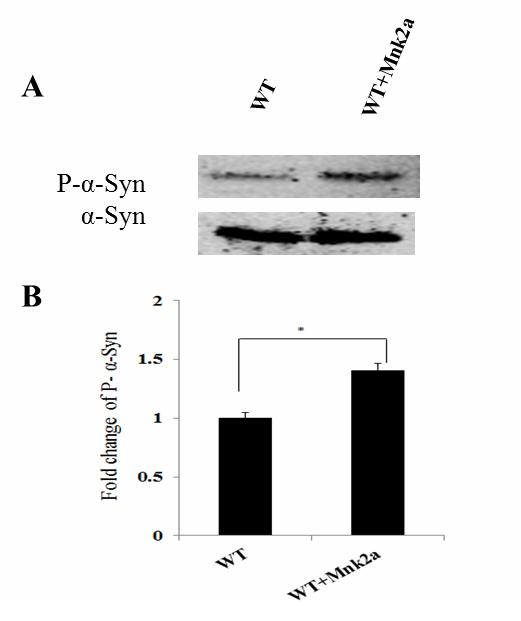
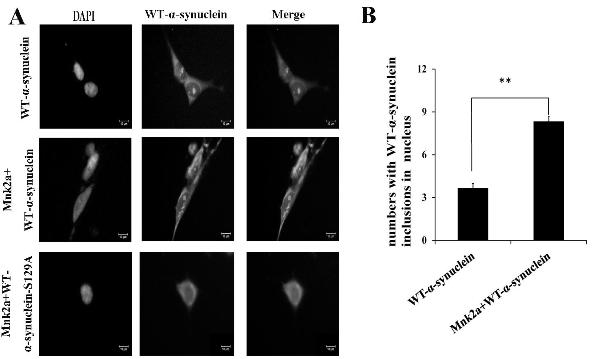
[0024] Example 2: Detection of the involvement of ERK signaling pathway in regulating the phosphorylation of Mnk2a on α-synuclein 129
[0025] The ERK signaling pathway activator PMA and inhibitor PD98059 were used to process cells co-transfected with the expression vectors α-synuclein and Mnk2a, and then the cell proteins were extracted for western analysis. The level of phosphorylation at serine 129 of α-synuclein in cells treated with PMA increased by 1.65 times, and the level of phosphorylation at serine 129 of α-synuclein in cells treated with PD98059 decreased by 0.80 times, and the activation of PMA could After being treated with PD98059, it decreased significantly. Experimental results indicate that Mnk2a may regulate the phosphorylation of the 129th serine of α-synuclein through the ERK signaling pathway. See results Figure 4 .
[0026] Shanghai University
[0027] New uses of protein kinase Mnk2a
[0028] 1
[0029]
[0030] 1
[0031] 1250
[0032] DNA
[0033...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com