Method for rapidly detecting mifepristone
A technology for mifepristone and medicine, which is applied in the field of rapid detection of mifepristone and authenticity identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, detect whether medicine contains the general operating condition of mifepristone
[0069] 1. Sampling volume
[0070]Sampling recommendations for different types of samples are as follows:
[0071] sample type
Sampling volume
5mg-20mg
10mg tablet
Take 2 pieces, crushed
25mg strength tablet
Take 1 piece, crushed
200mg tablet
Take half a slice, crushed
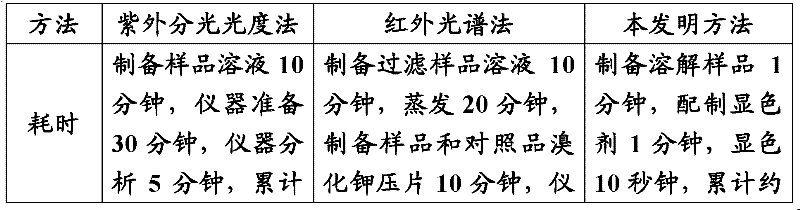
[0072] It can be seen that the amount of the sample to be inspected used by the method of the present invention is very small, so as to damage the interests of innocent operators as little as possible.
[0073] 2. Preparation of the test solution
[0074] After taking the medicine and grinding it, add 2ml of organic solvent (trichloromethane is used in the following test), and shake for 1 minute without filtering.
[0075] 3. Operation steps:
[0076] (1) Take a sample (5-100 mg containing the labeled mifepristone) and add 2 ml...
Embodiment 2
[0081] Embodiment 2, the test of whether mifepristone is contained in the detection medicine
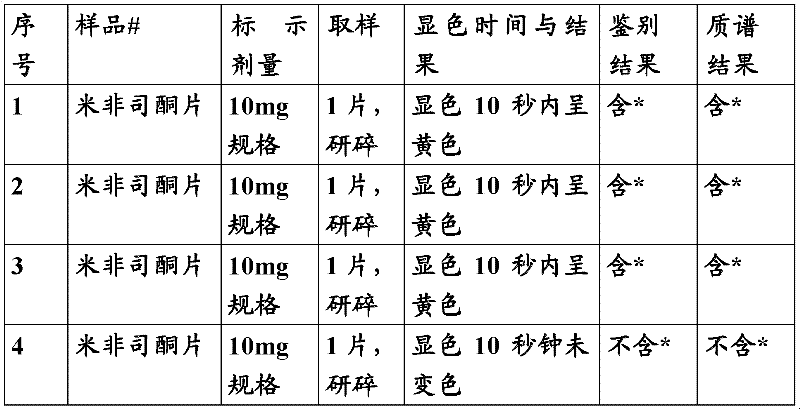
[0082] Adopt the method of embodiment 1, detect 20 batches of commercially available samples (comprising the unilateral preparation and the raw material that label contains mifepristone, omit manufacturer and production batch number, expressed with serial number), and with reference to " Chinese Pharmacopoeia 2005 edition two " The HPLC method chromatographic conditions under the related substance item of mifepristone raw material, use LCMS method to detect sample, specifically as follows:
[0083] Take the sample according to the table, add 2ml of chloroform, and shake for 1 minute. Then add 0.2ml of newly prepared sulfuric acid-acetic anhydride mixed developer into the sample solution, observe within 10 seconds, if the sample solution suddenly shows obvious yellow, orange yellow and / or orange red, then the tested sample can be judged may contain mifepristone; if there is no chan...
Embodiment 3
[0093] Embodiment 3, the performance of the inventive method
[0094] The present embodiment investigates the minimum detection limit of inventive method to detection mifepristone reference substance and sample, specifically as follows:
[0095] Take 10 mg of mifepristone reference substance provided by China National Institute of Pharmaceutical and Biological Products Identification, add chloroform to dissolve and dilute to a solution containing 0.25 mg of mifepristone in every 1 ml. Then add 0.2ml of newly prepared sulfuric acid-acetic anhydride mixed developer into the sample solution, and the sample solution turns yellow within 10 seconds.
[0096] Take 10 mg mifepristone tablets, grind finely, add chloroform to dissolve and dilute to a solution containing 0.25 mg mifepristone per 1 ml. Then add 0.2ml of newly prepared sulfuric acid-acetic anhydride mixed developer into the sample solution, and the sample solution turns yellow within 10 seconds.
[0097] According to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com