Composition and tablet of mifepristone and anorethidrane dipropionate
A technology of anordrin and mifepristone, applied in pill delivery, drug combination, pharmaceutical formulation, etc., can solve the problem of solubilized products of mifepristone and anordrin compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The tablet prescription of this method is (1000 tablets)
[0034] Recipe 1:
[0035] Mifepristone 30g
[0036] Anordrin 5g
[0037] Macrogol 4000 50g
[0038] Lactose 20g
[0039] Starch 400g
[0040] Microcrystalline Cellulose 65g
[0041] Sodium Carboxymethyl Starch 20g
[0043] Hypromellose (HPMC) 0.2g
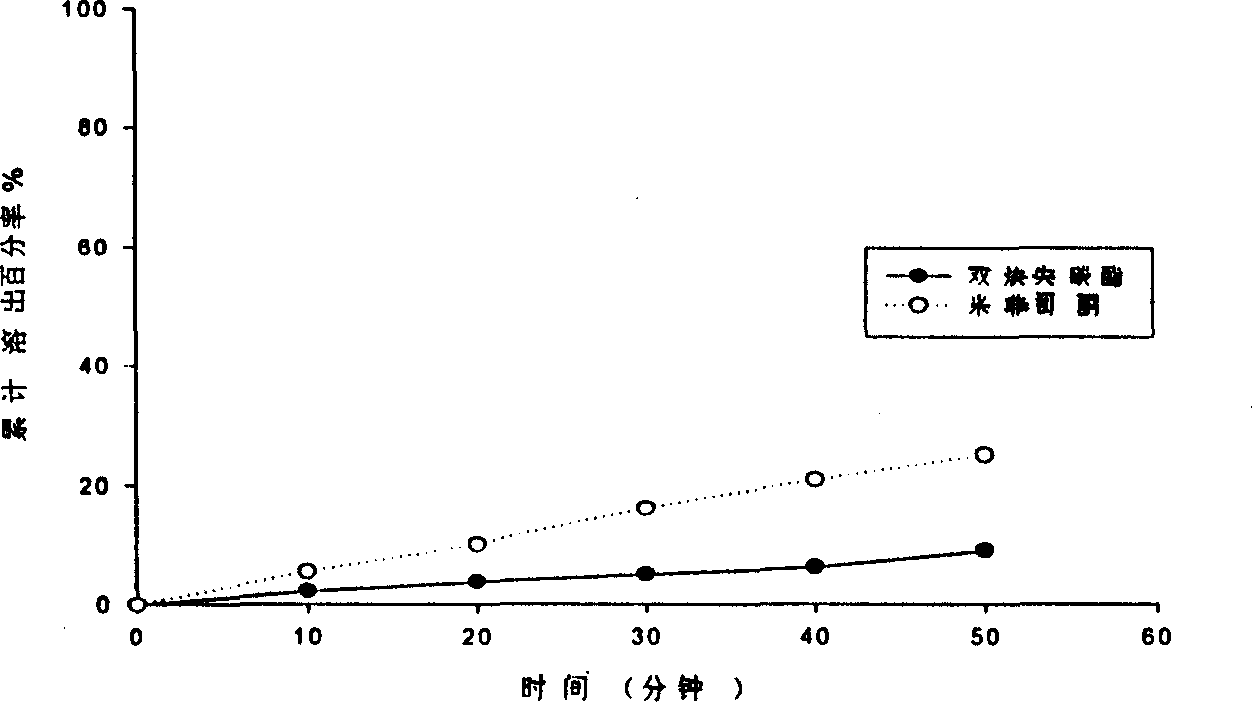
[0044] After melting polyethylene glycol 4000, add mifepristone and anordrin, pour it evenly into an appropriate amount of dry ice, solidify, and then grind it into a fine powder with a mill, and evaporate the CO 2 Then add lactose and starch, use HPMC slurry as the binder to granulate, add other ingredients after drying, mix and then compress into tablets to obtain. The dissolution rate is shown in Table 1.
Embodiment 2
[0050] Prescription 2: (1000 tablets)
[0051] Mifepristone 30g
[0052] Anordrin 5g
[0053] β-cyclodextrin 350g
[0054] Starch 10g
[0055] Microcrystalline Cellulose 5g
[0057] Hypromellose (HPMC) 0.2g
[0058]Process: Dissolve anordrin in 300ml of ethanol, add different amounts of β-cyclodextrin, put it in a grinder and grind it to a paste (note: the ventilation device is placed above), add mifepristone, and take it out after drying , put in a vacuum desiccator to remove the residual solvent, add starch, granulate with HPMC pulp, add microcrystalline cellulose, talcum powder, and tablet. The dissolution rate of this tablet is shown in Table 2.
Embodiment 3
[0064] Prescription 3: (1000 tablets)
[0065] Mifepristone 30g
[0066] Anordrin 5g
[0067] Polyvinylpyrrolidone 50g
[0068] Starch 40g
[0069] Microcrystalline Cellulose 65g
[0070] Sodium carboxymethyl starch 20g
[0071] Talc powder 7g
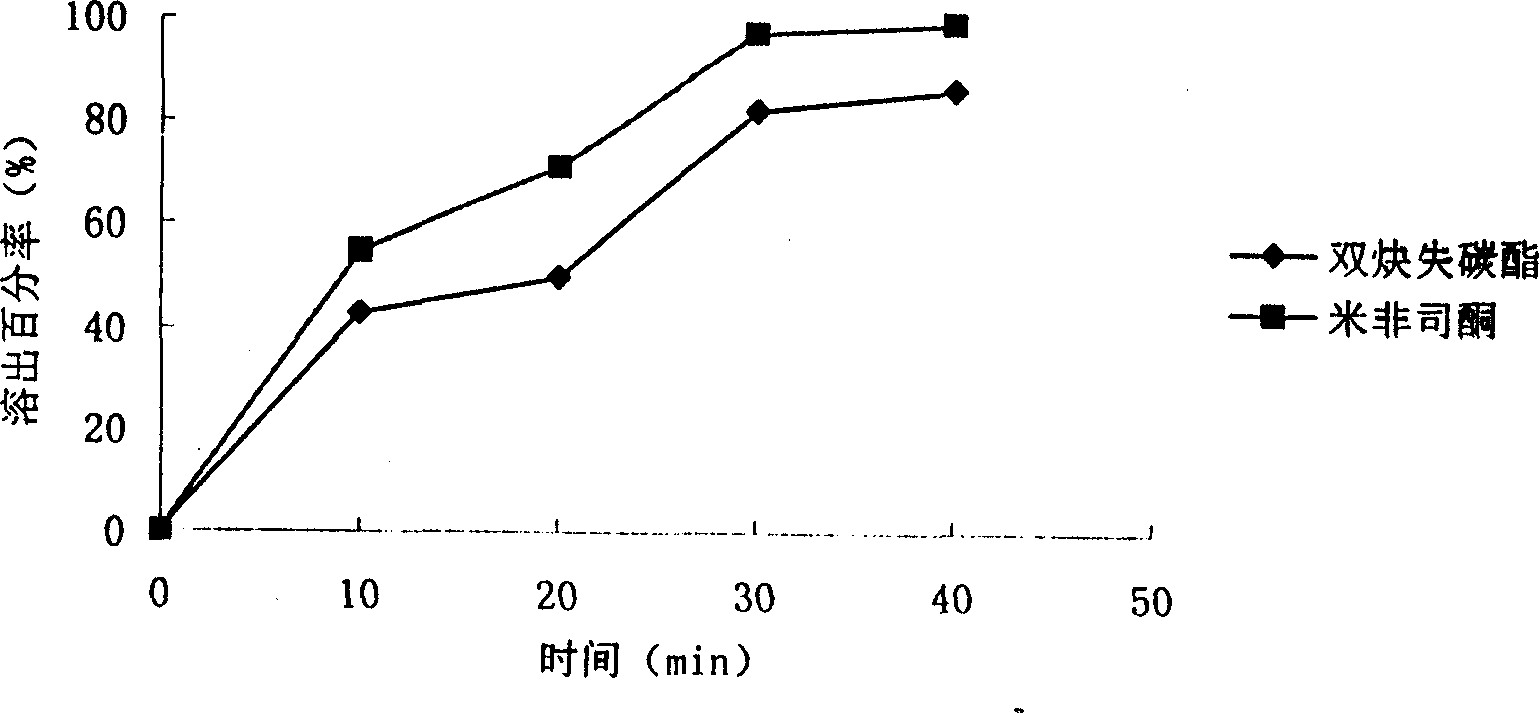
[0072] Preparation process: dissolve polyvinylpyrrolidone in 100ml ethanol, add anordrin and stir to dissolve, then continue stirring in the fume hood while adding mifepristone, then add microcrystalline cellulose, stir evenly, take out, and dry After being pulverized, it is made into granules, and sodium carboxymethyl starch is added, and the bone stone powder is compressed into tablets and wrapped in double-layer aluminum foil. The in vitro dissolution profile of the tablet is shown in figure 2 .
[0073] from figure 2 Visible 30min dissolution rate anordrin is more than 80%, and mifepristone is about 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com