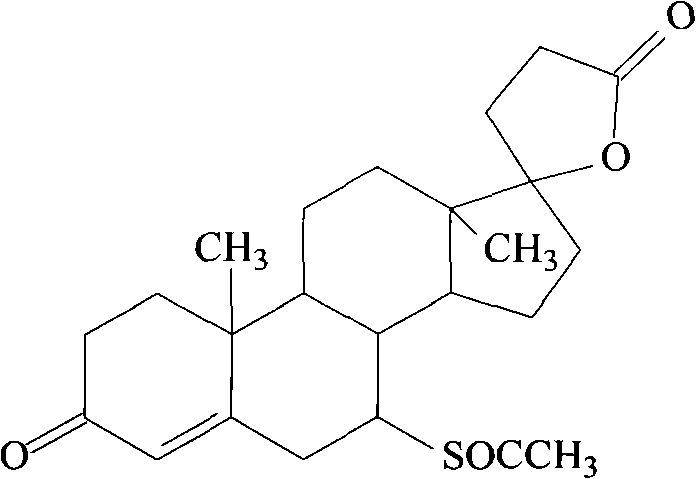
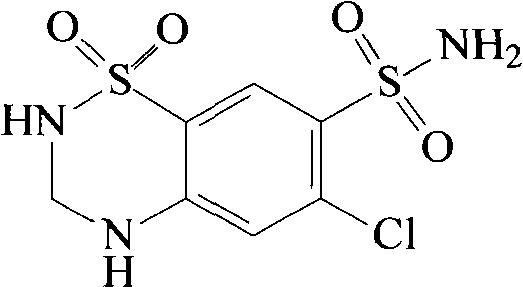
Spirolactone and hydrochlorothiazide pharmaceutical composition solid preparation
A technology of ester hydrochlorothiazide lipid and ester hydrochlorothiazide lipid, which is applied in the direction of drug combination, pharmaceutical formulation, liposome delivery, etc., can solve the problems of liposome stability and poor encapsulation efficiency, and achieve improved bioavailability, Better results, longer cycle times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 spironolactone hydrochlorothiazide liposome capsule
[0053] Prescription (1000 capsules)
[0054]
[0055]
[0056] Preparation Process
[0057] (1) 150g egg yolk lecithin, 75g cholesterol and 25g sodium glycocholate are dissolved in the mixed solvent of 4:1 isopropanol and acetone in 5000ml volume ratio, obtain lipid solution;
[0058] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 45° C. to form a uniform lipid film;
[0059] (3) Disperse 25g of spironolactone and 25g of hydrochlorothiazide in 800ml of water, add it into a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution, to obtain a liposome suspension;
[0060] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal solution;
[0061] (5) ...
Embodiment 2
[0065] The preparation of embodiment 2 spironolactone hydrochlorothiazide liposome capsules
[0066] Prescription (1000 capsules)
[0067]
[0068] Preparation Process
[0069] (1) 250g egg yolk lecithin, 125g cholesterol and 75g sodium glycocholate are dissolved in the mixed solvent of 4:1 isopropanol and acetone in 9000ml volume ratio, obtain lipid solution;
[0070] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 55° C. to form a uniform lipid film;
[0071] (3) Disperse 25g of spironolactone and 25g of hydrochlorothiazide in 800ml of water, add it into a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution, to obtain a liposome suspension;
[0072] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal solution;
[0073] (5) Filter the...
Embodiment 3
[0077] The preparation of embodiment 3 spironolactone hydrochlorothiazide liposome capsules
[0078] Prescription (1000 capsules)
[0079]
[0080] Preparation Process
[0081] (1) 400g egg yolk lecithin, 200g cholesterol and 100g sodium glycocholate are dissolved in a mixed solvent of 4:1 isopropanol and acetone in a volume ratio of 20000ml to obtain a lipid solution;
[0082] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 50° C. to form a uniform lipid film;
[0083] (3) Disperse 50g of spironolactone and 50g of hydrochlorothiazide in 1500ml of water, add it into a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution, to obtain a liposome suspension;
[0084] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal solution;
[0085] (5) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com