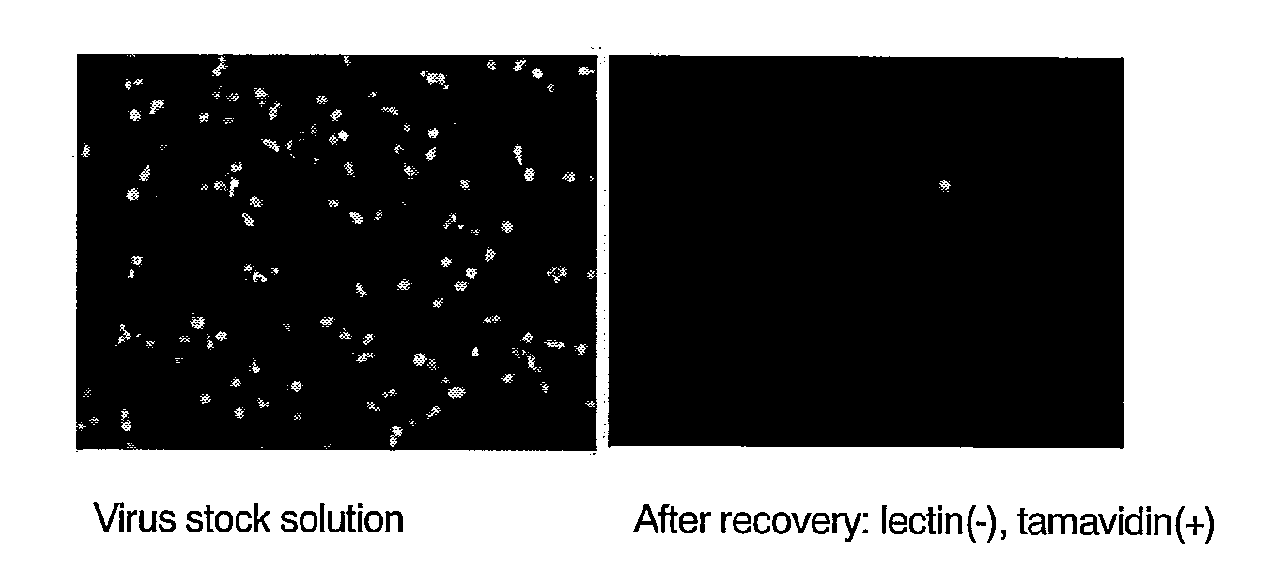
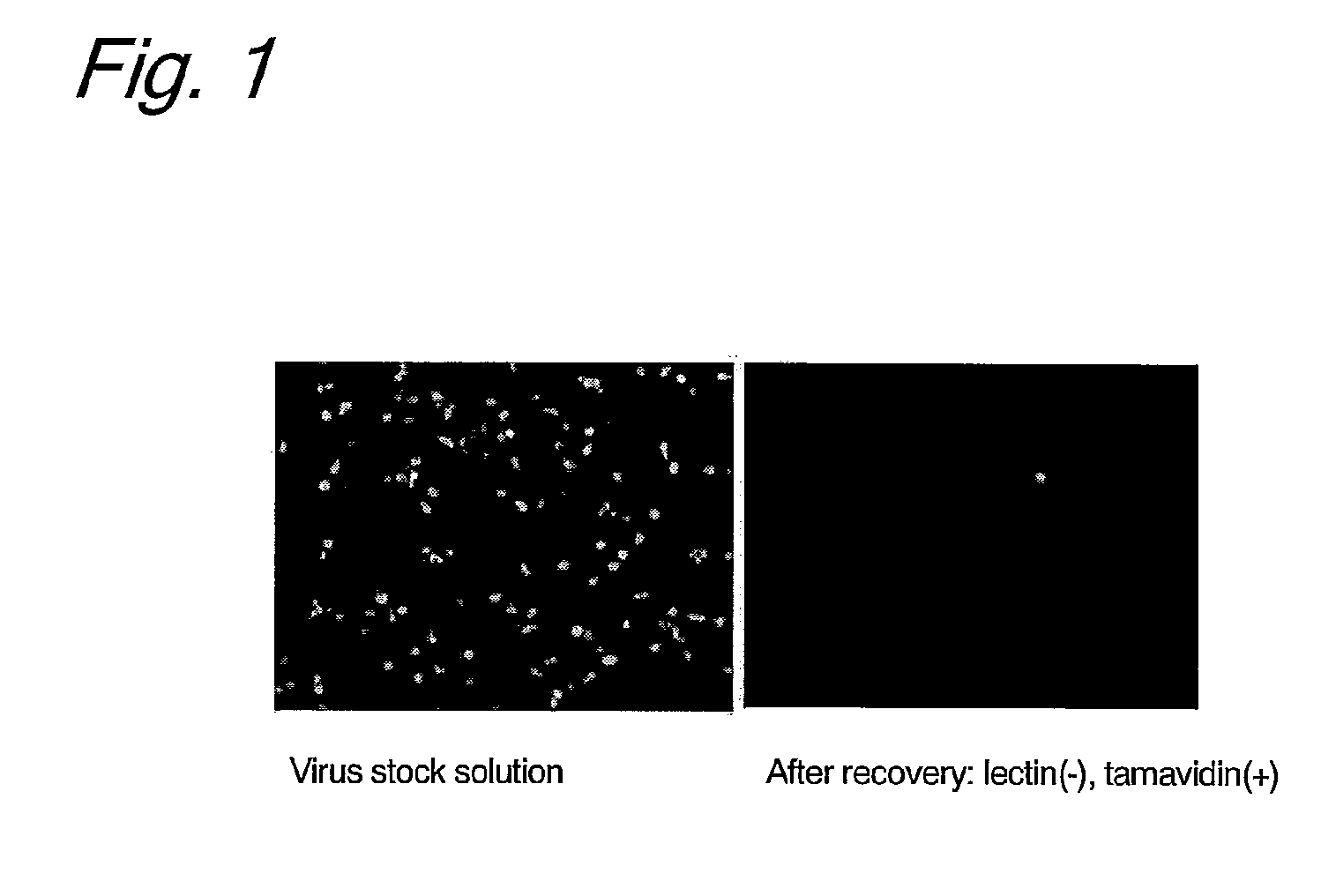
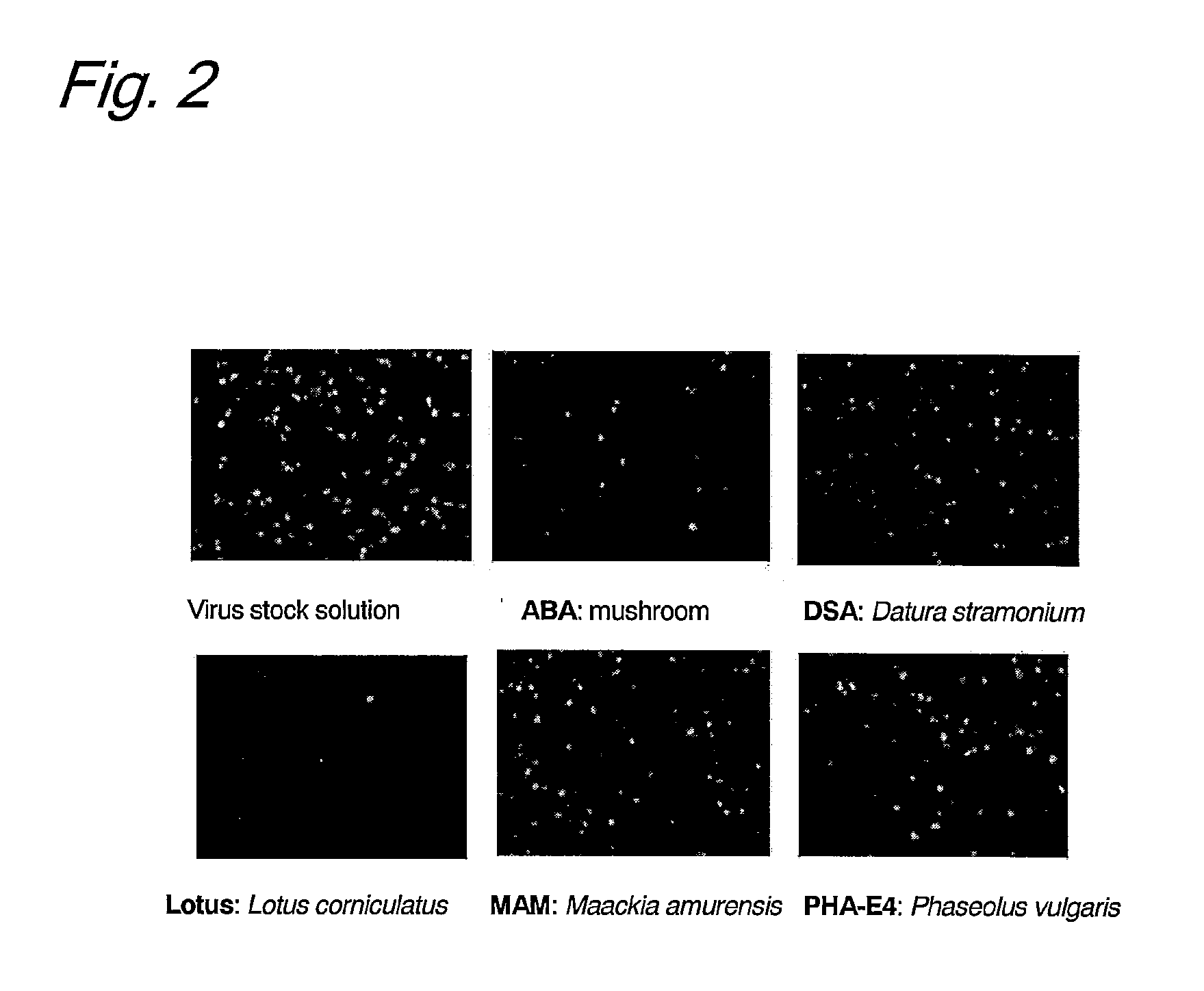
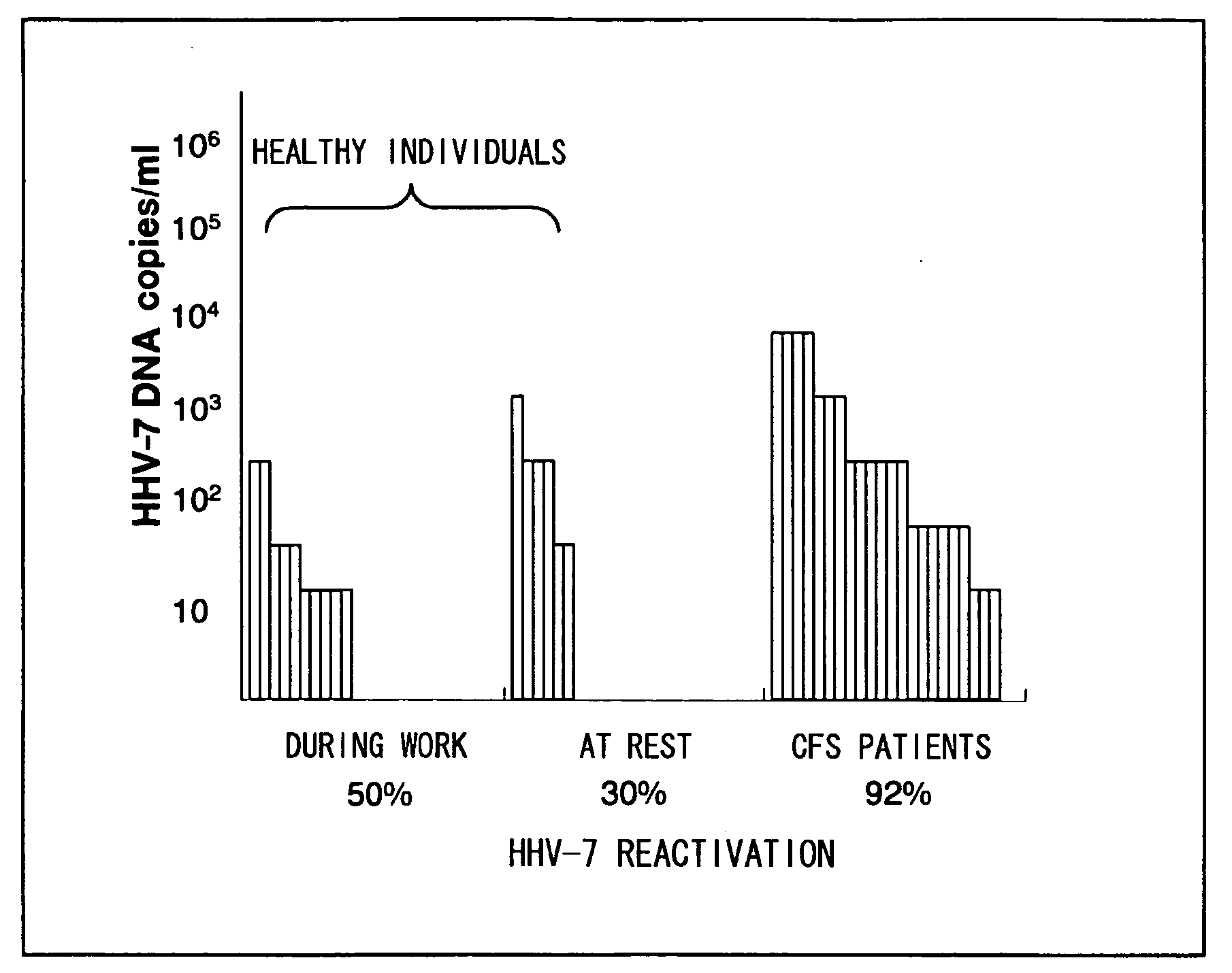
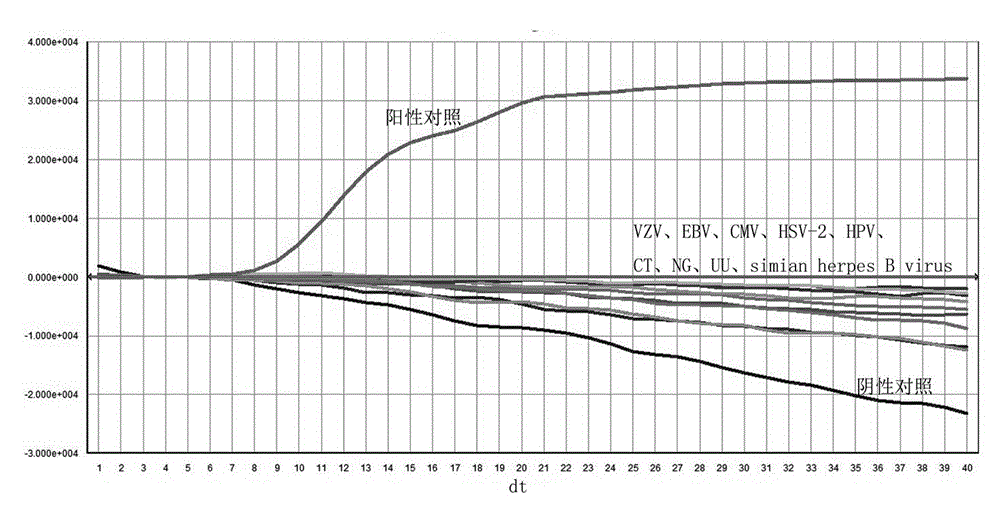
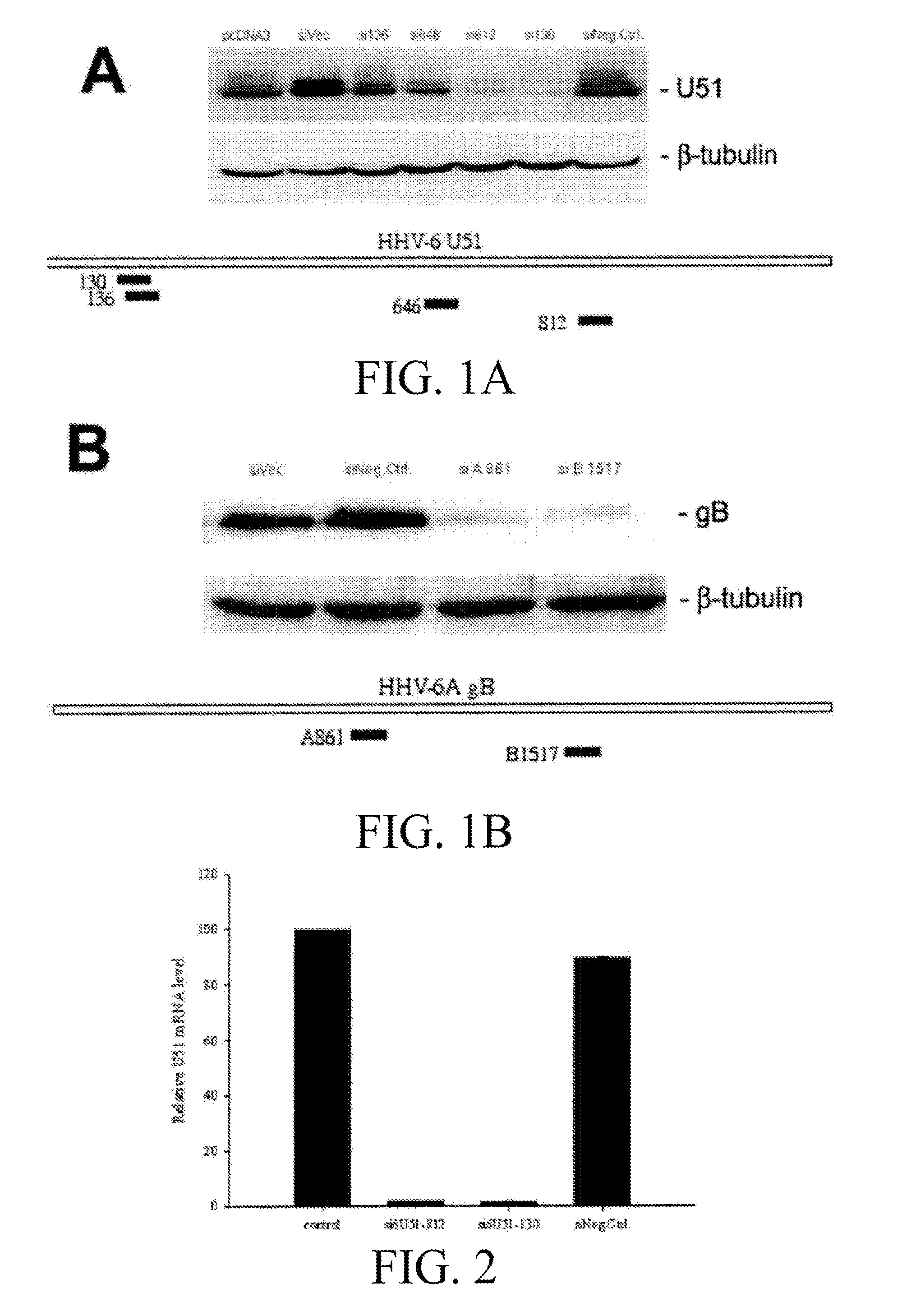
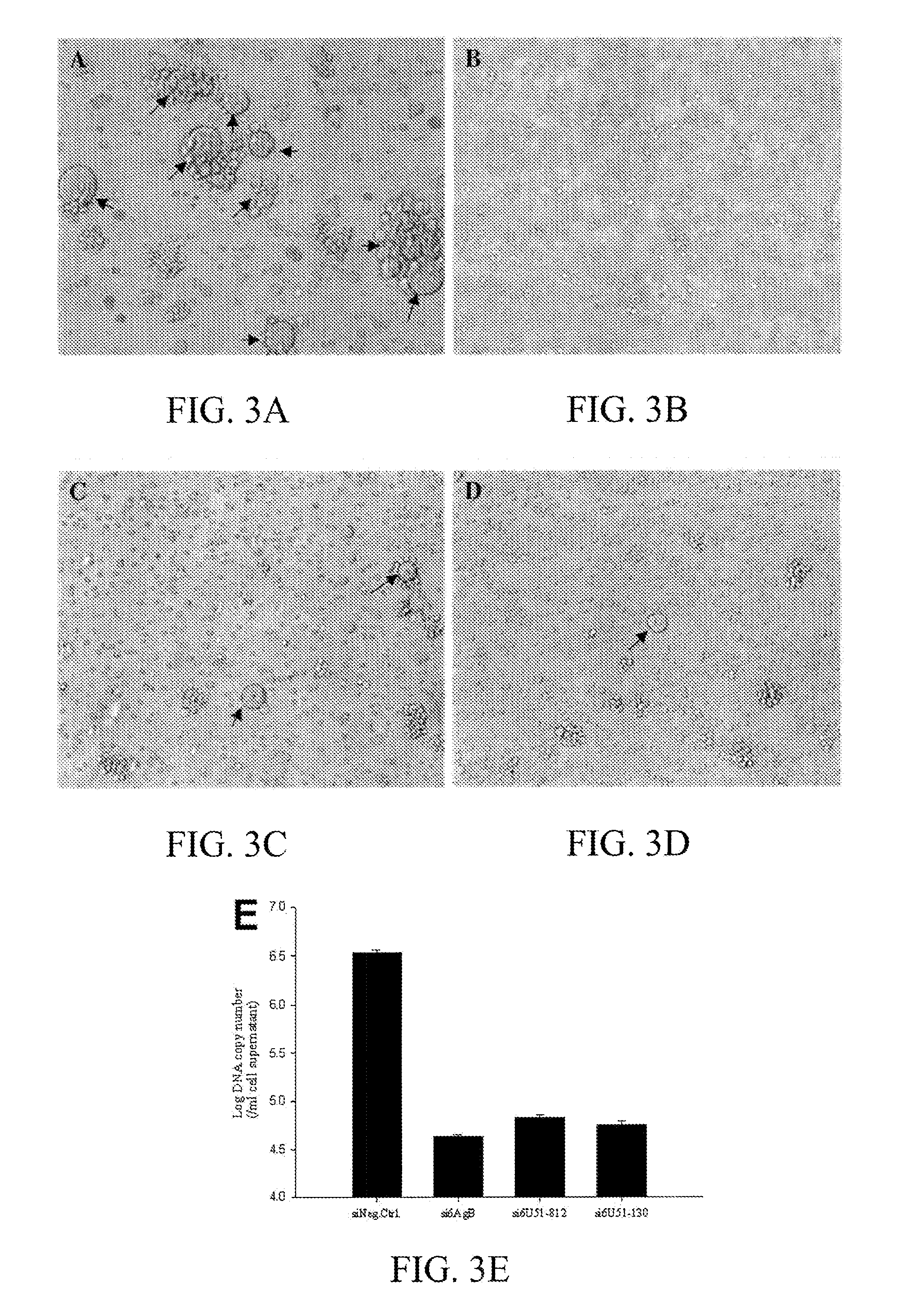
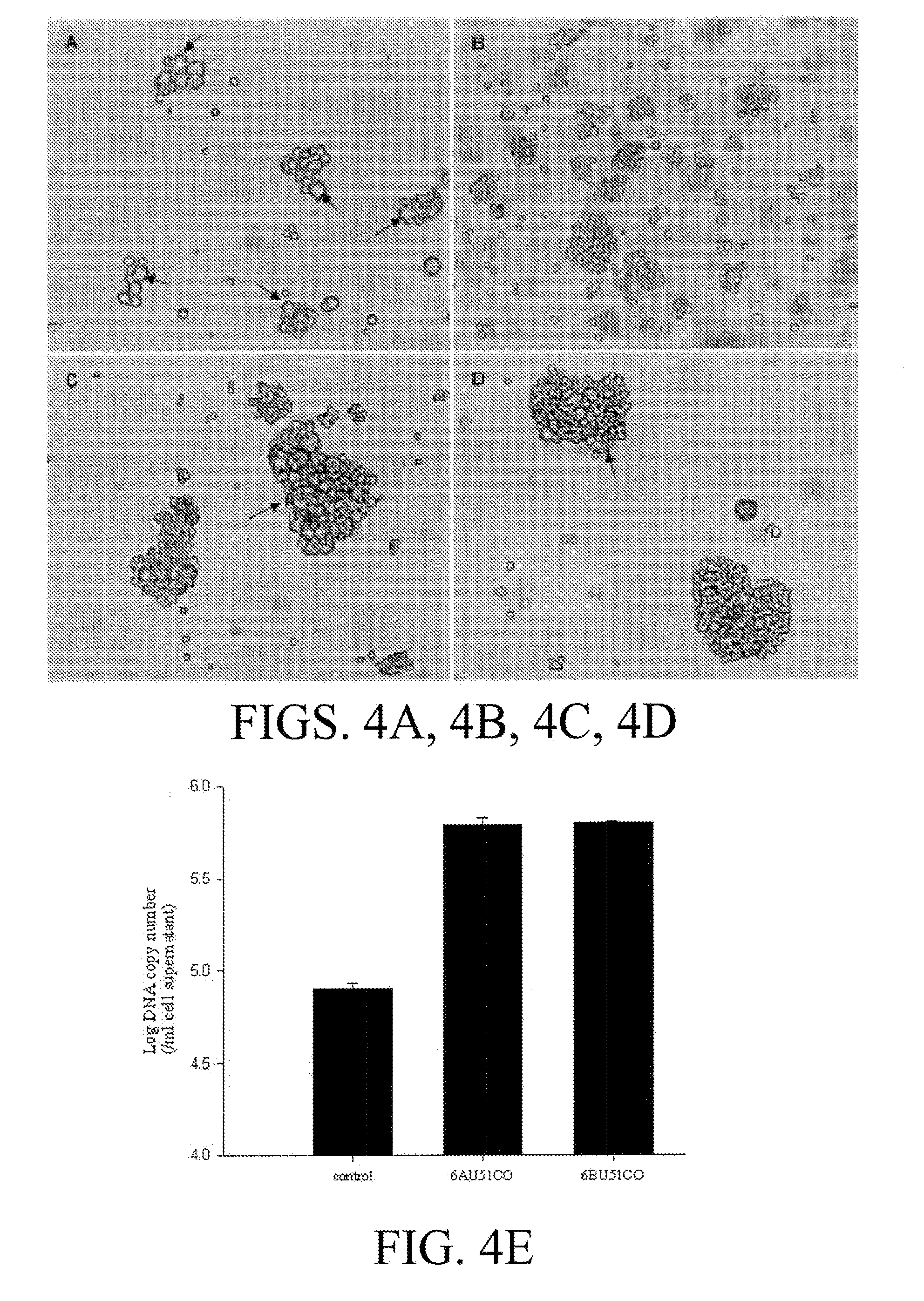
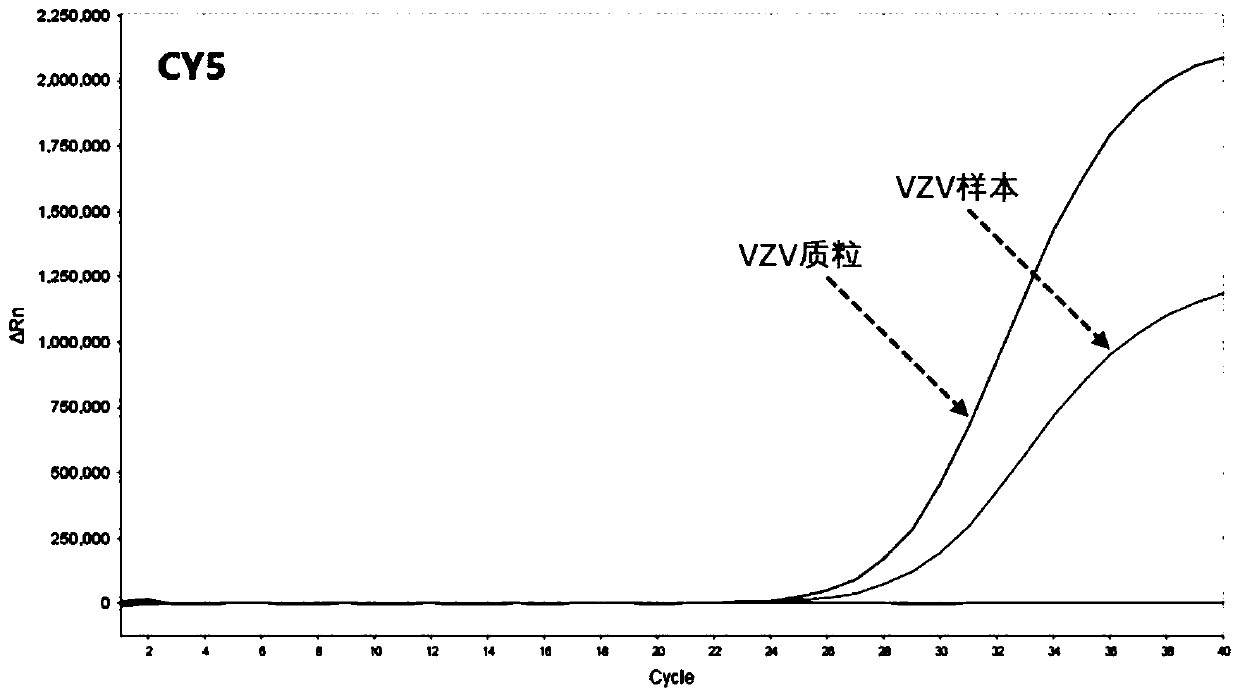
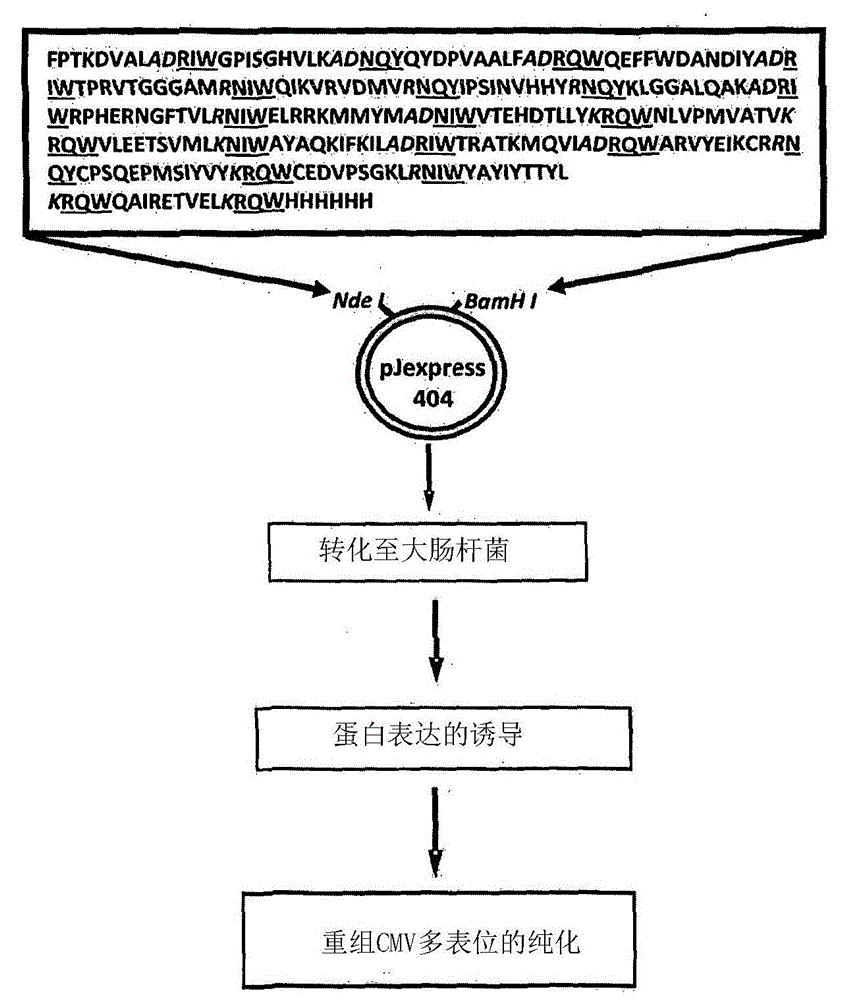
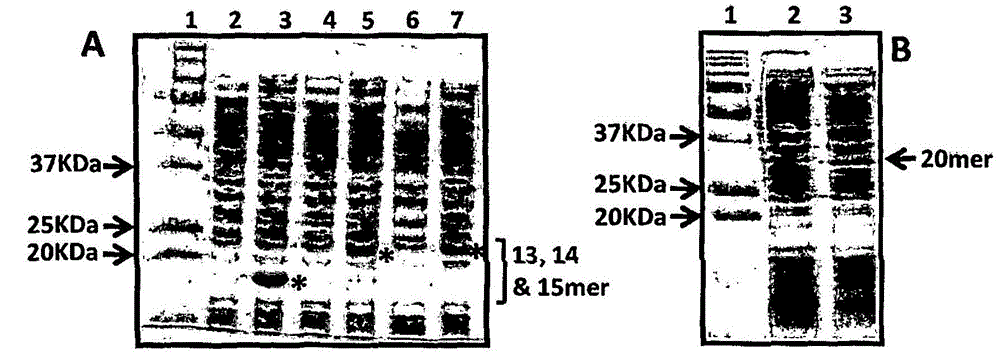
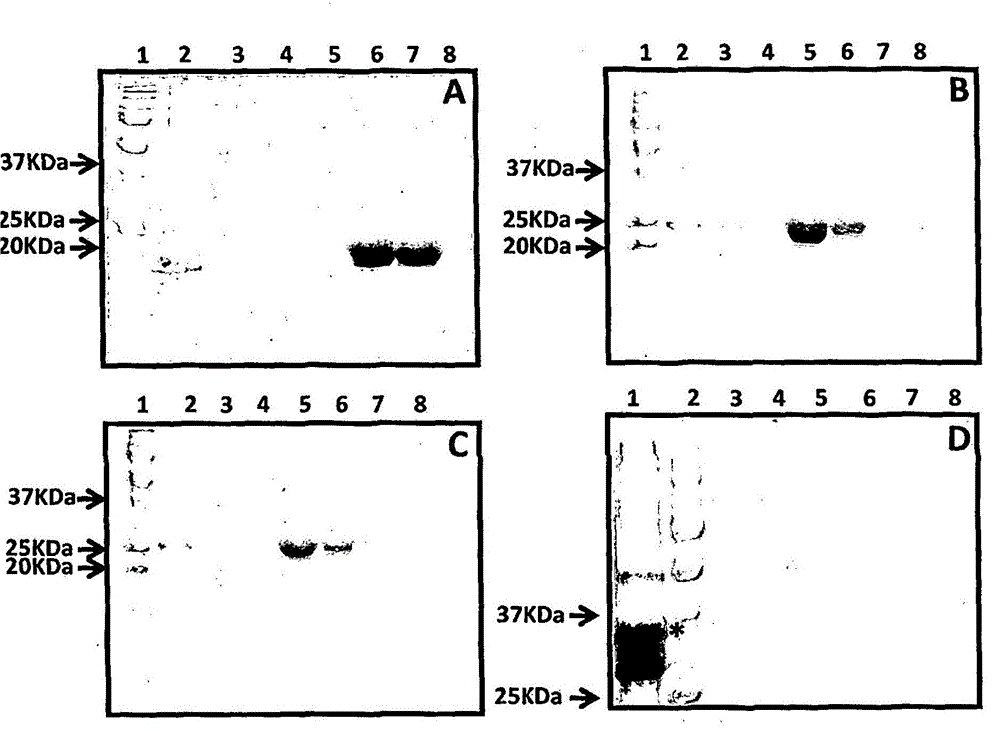
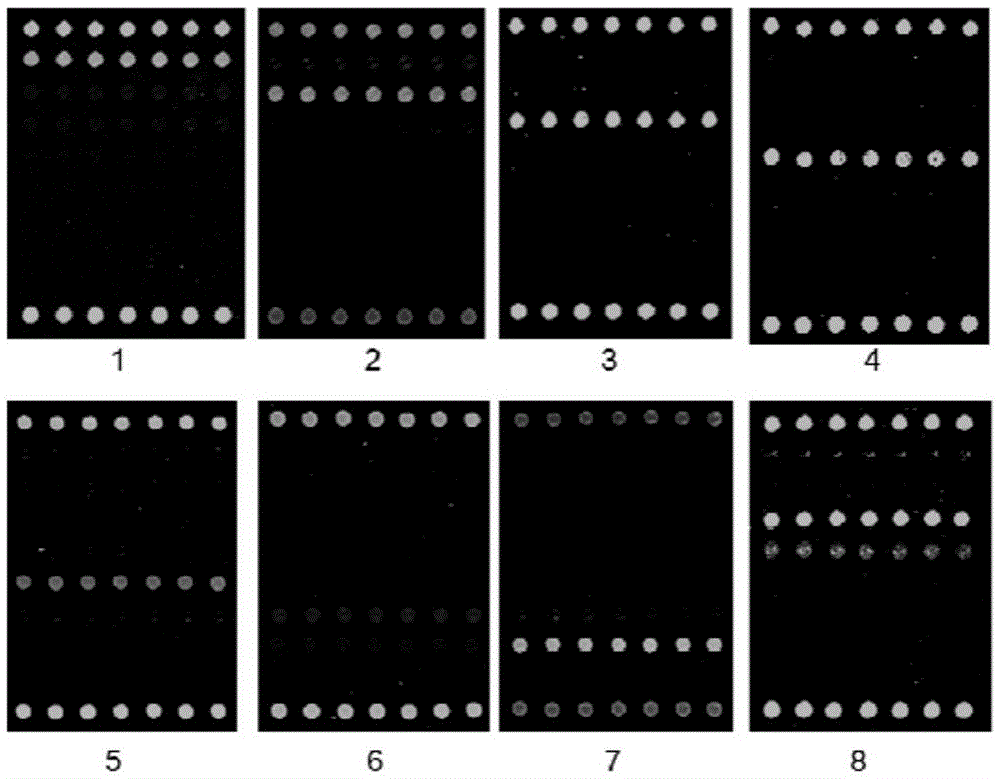
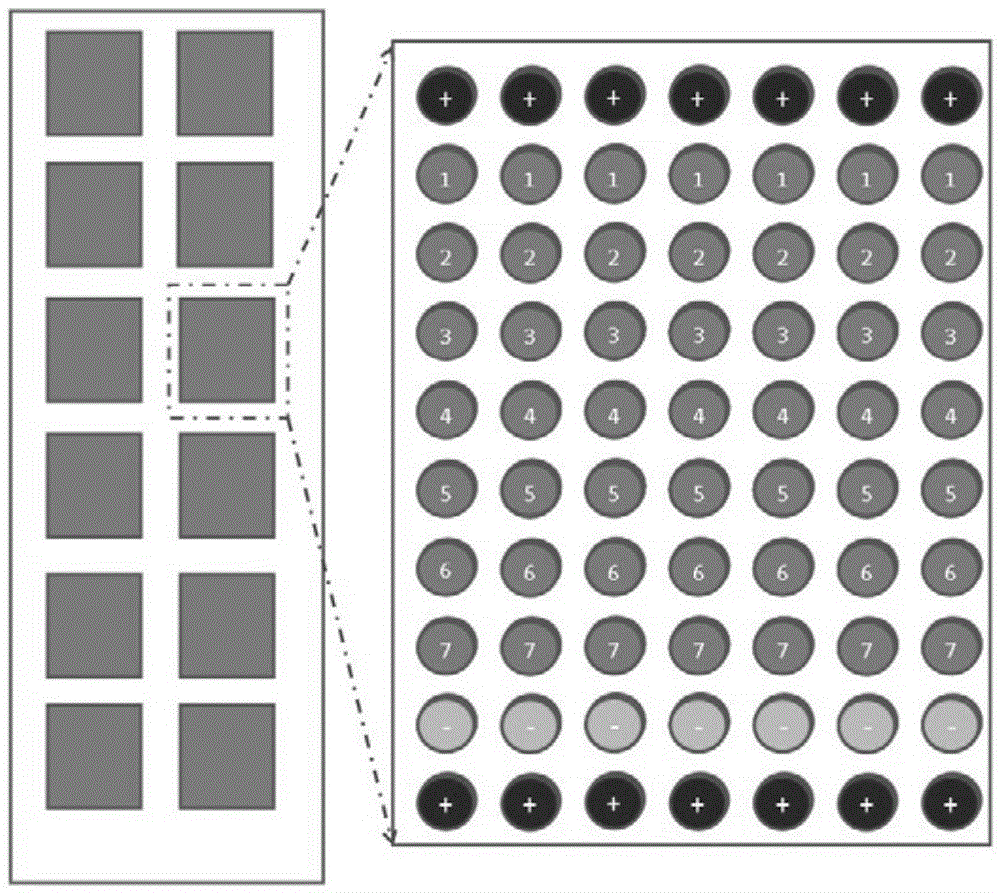
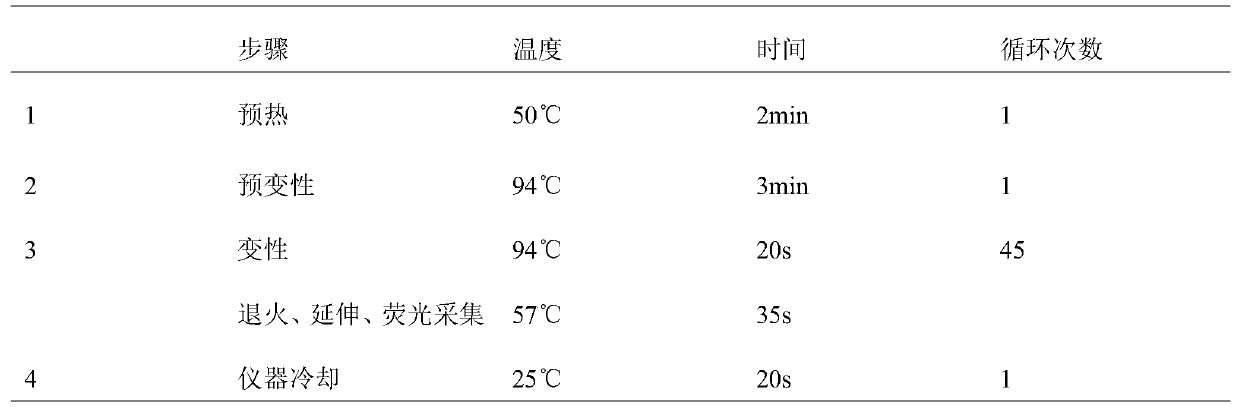
Patents
Literature
37 results about "Human herpesvirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quantitation method of virus
InactiveUS20110053145A1Sure easyHigh degreeMicrobiological testing/measurementBiological material analysisQuantitative determinationContinuous evaluation
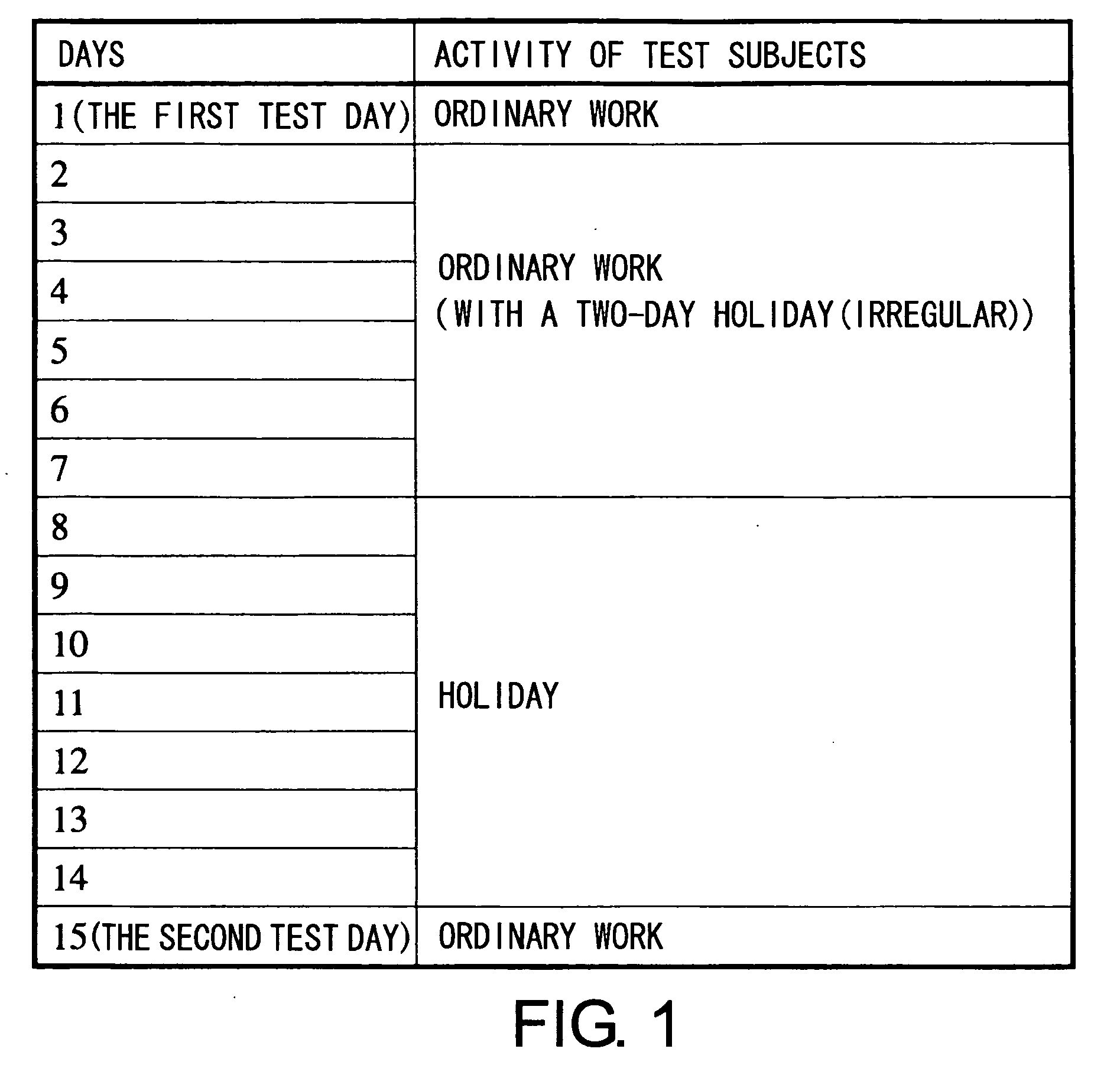
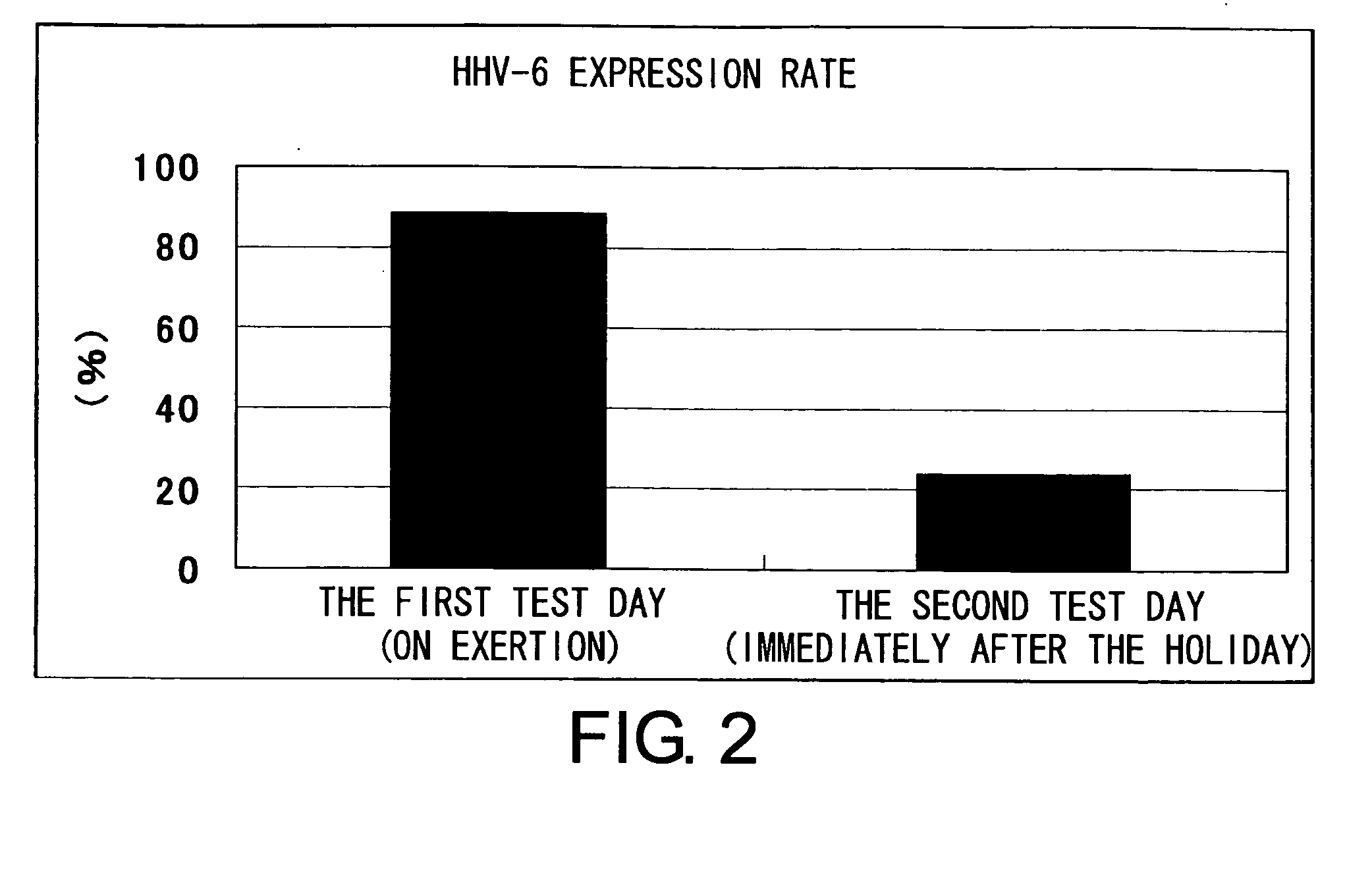
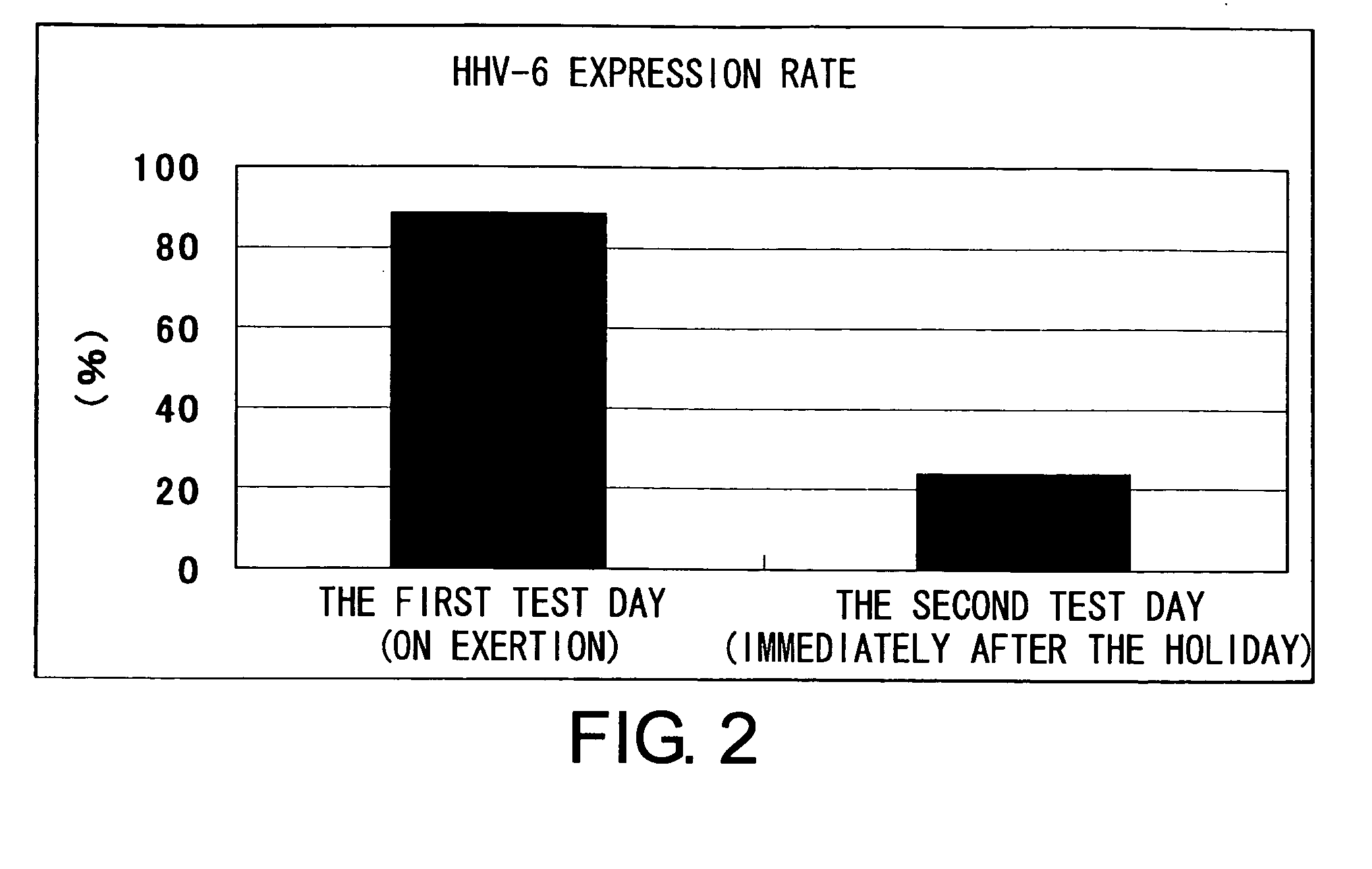
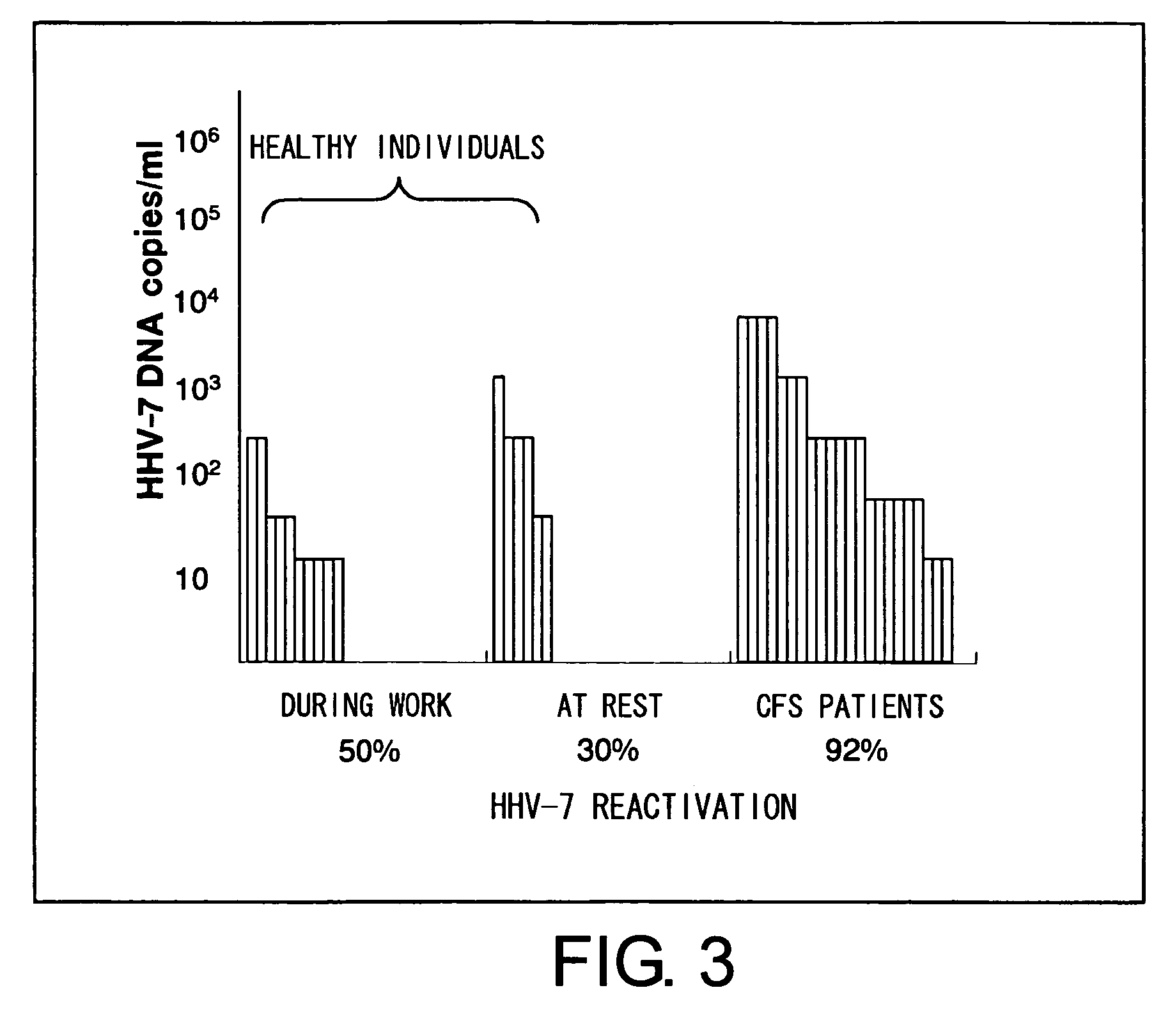
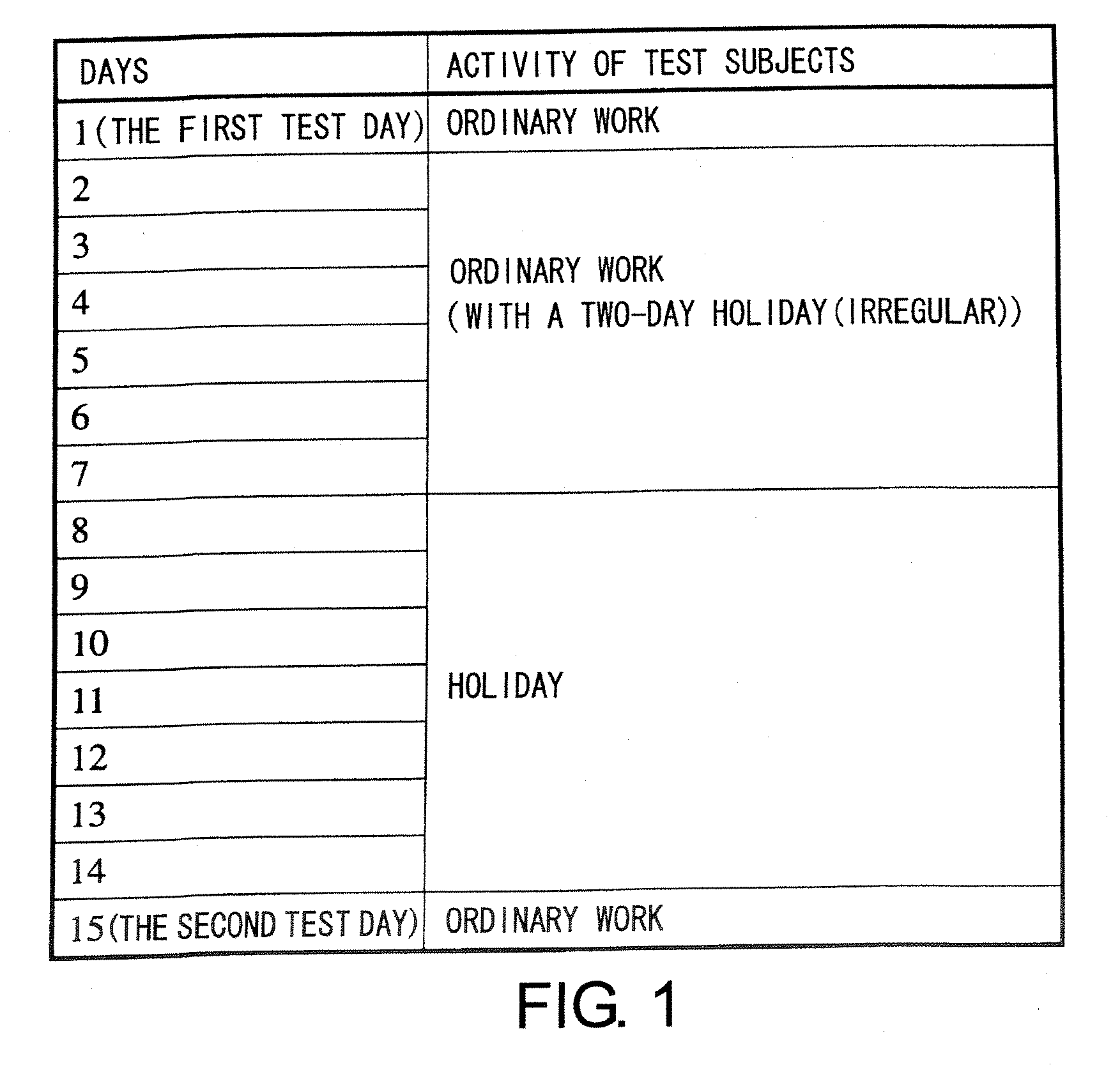
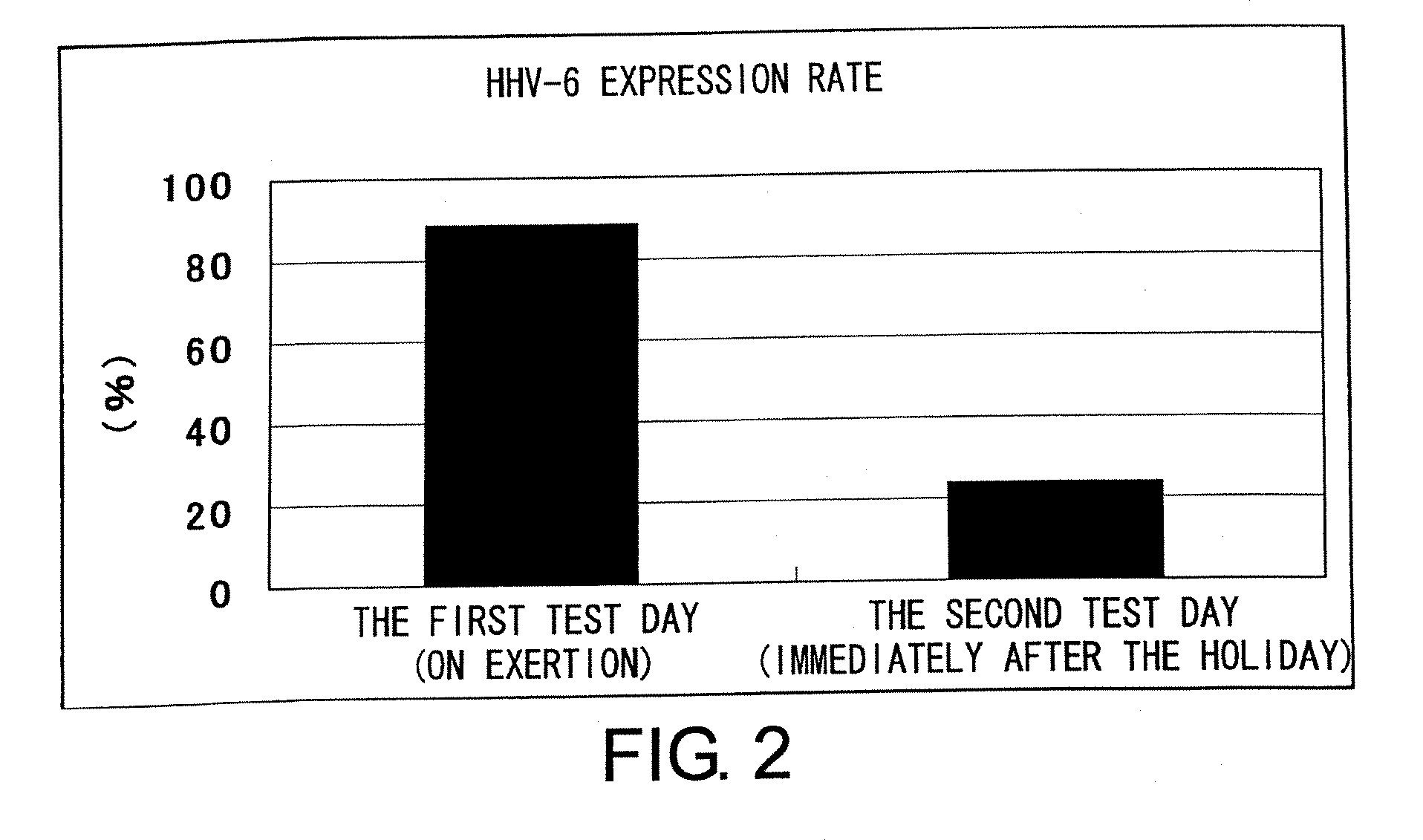
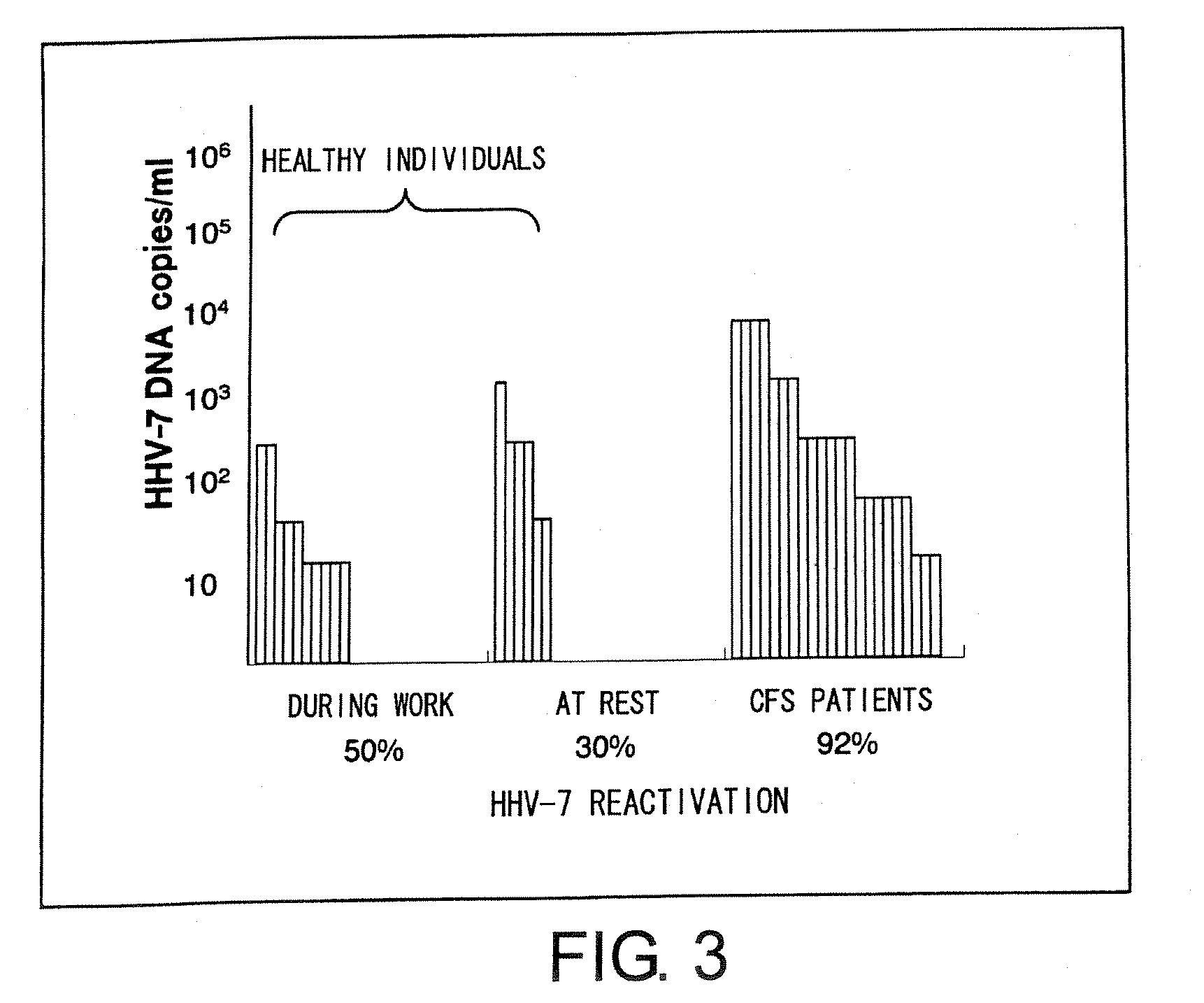
The present invention relates to a method of quantitatively determining the number of human herpesvirus (HHV) collected from a body fluid and a kit for performing the method. Conventionally, a trained technician has been required to accurately quantitatively determine a number of HHV collected from a body fluid. The method of the present invention is a novel method of quantitative determination that enables measurement of a number of HHV in a body fluid to be simply, accurately, and efficiently determined. The method of the present invention can enable continuous evaluation of the number of HHV in body fluids and, therefore, can be applied to quantitative evaluation of the accumulation of fatigue.
Owner:JAPAN TOBACCO INC +1
Methods For Assessing Fatigue Level and Applications Thereof
InactiveUS20080280283A1Cause painCause to testMicrobiological testing/measurementDisease diagnosisMedicineBody fluid
Level of fatigue that accompanies everyday life or a disease can be simply, easily, and quantitatively assessed by obtaining a body fluid from a test subject and measuring the amount of human herpesvirus in the body fluid. Furthermore, the anti-fatigue potency of anti-fatigue substances and anti-fatigue food products can be measured.
Owner:VIRUS IKAGAKU KENKYUSHO
Real-time fluorescence nucleic acid isothermal amplification detection kit for human herpesvirus 1
ActiveCN105624329AAccurate measurementRapid determinationMicrobiological testing/measurementCerebrospinal fluidReverse transcriptase
The invention discloses a real-time fluorescence nucleic acid isothermal amplification detection kit for human herpesvirus 1 (HSV-1). The real-time fluorescence nucleic acid isothermal amplification detection kit specifically comprises a capturing probe, a T7 primer and an nT7 primer of HSV-1, an HSV-1 detection probe, M-MLV reverse transcriptase, T7RNA polymerase, and other reagent. The method provided by the invention can perform nucleic acid isothermal amplification detection on swab, scraping liquid and cerebrospinal liquid samples containing the HSV-1 in a high specificity, high sensitivity, low pollution and rapid manner; and the kit has the characteristics of high detection efficiency and high accuracy, so that the kit has bright application prospects.
Owner:SHANGHAI RENDU BIOTECH
Compositions and methods for detection and treatment of human herpesvirus (HHV)-6
Disclosed herein are compositions and methods for detection and treatment of human herpesvirus (HHV)-6.
Owner:UNIVERSITY OF ROCHESTER
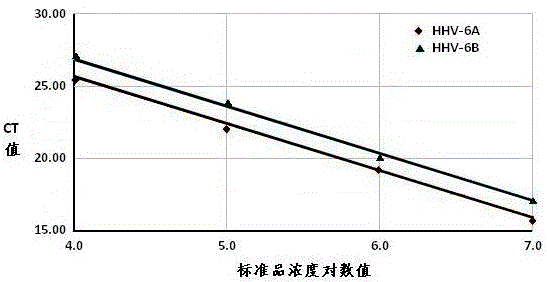
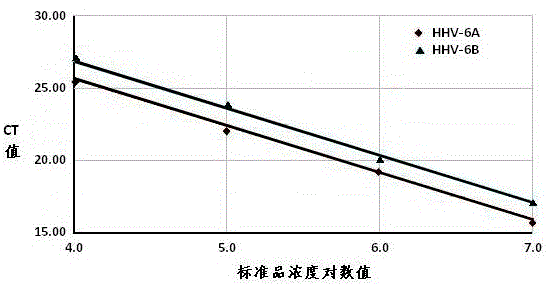
Real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6
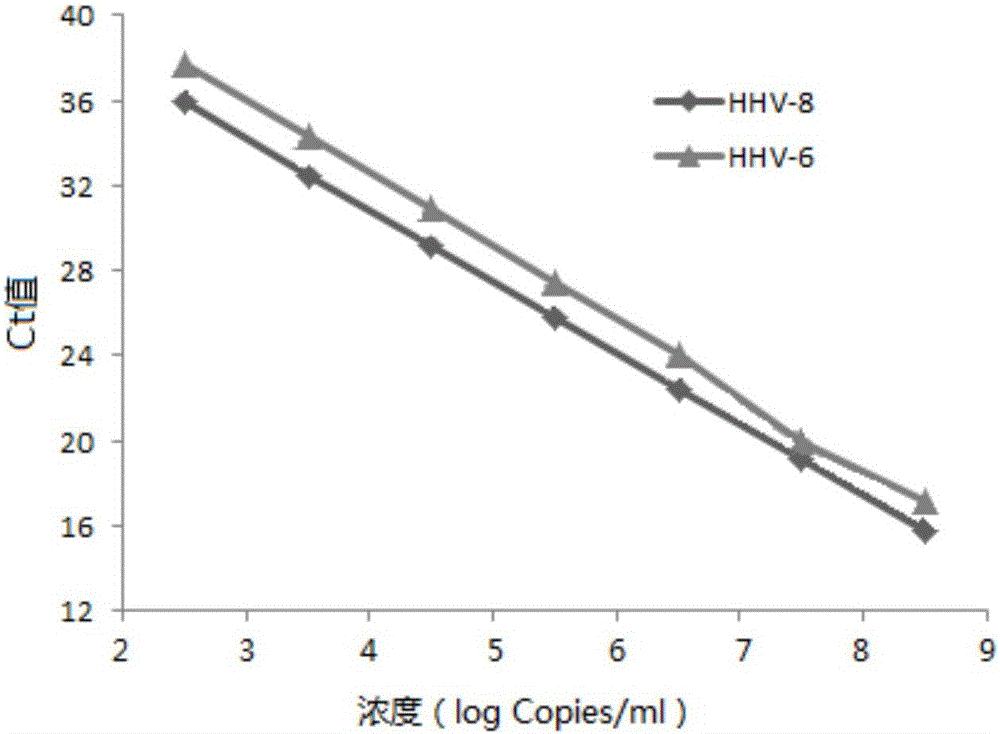
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6 (HHV-6). The real-time fluorescent quantitation PCR parting detection kit consists of quantitation PCR reaction liquid, an HHV-6A standard substance, an HHV-6B standard substance, an HHV-6A positive reference substance, an HHV-6B positive reference substance, a negative reference substance, a specification and a kit body, wherein the quantitation PCR reaction liquid contains a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HHV-6A fluorescent probe and an HHV-6B fluorescent probe. By the adoption of a real-time fluorescent quantitation PCR technology and a double-color fluorescent probe, the kit disclosed by the invention can detect two subtypes of the HHV-6 by a one-step method; the HHV-6A and HHV-6B in the sample can be simultaneously parted; a positive virus subtype can be accurately quantified in real time; the urgent need of early and acute parting diagnosis on the HHV-6 infection can be met; a basis is supplied to epidemiological investigation and targeted treatment on the HHV-6 infection.
Owner:ZHEJIANG UNIV
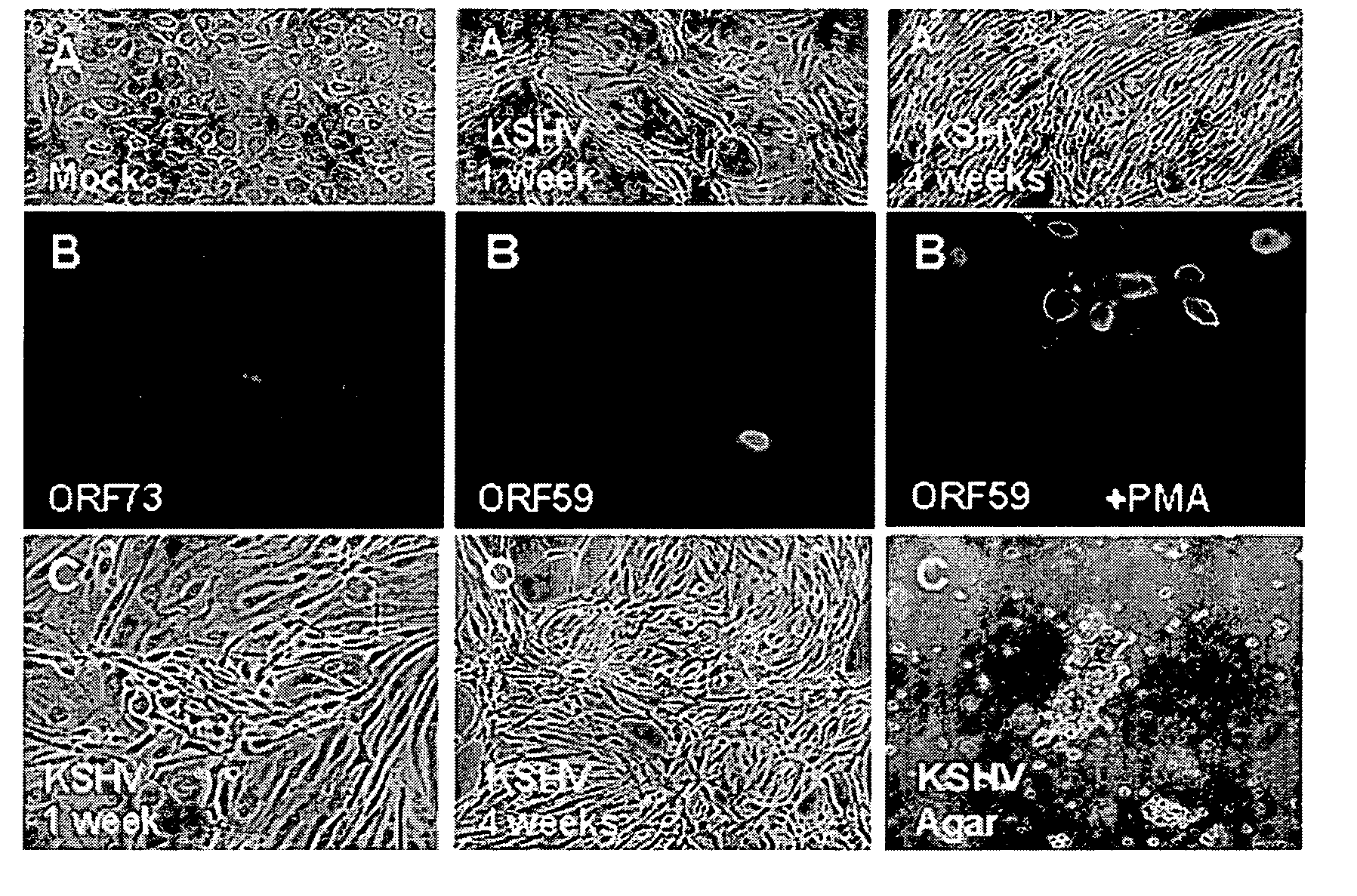
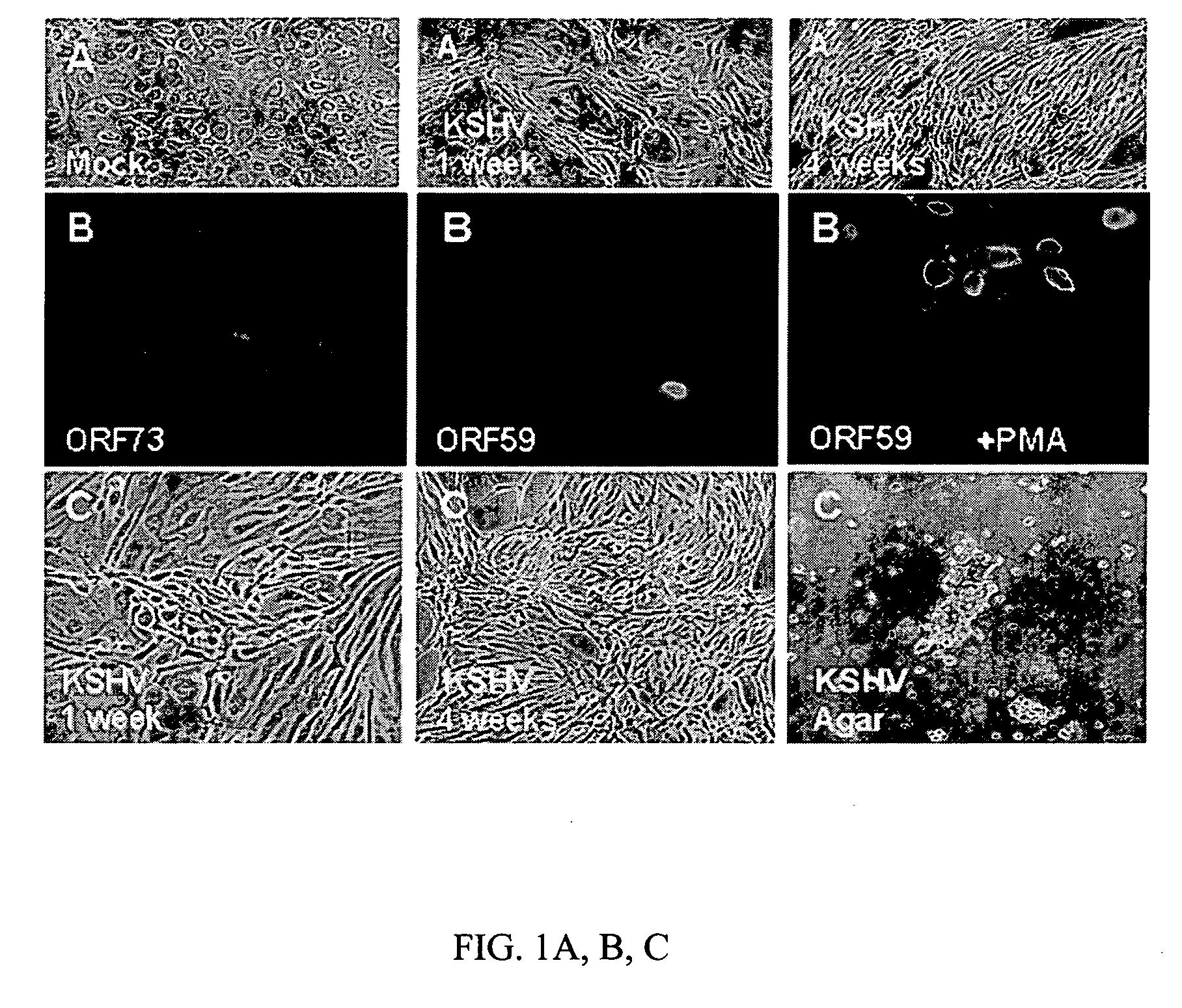
Methods of treatment and diagnosis of Kaposi's sarcoma (KS) and KS related diseases
Aspects of the present invention use gene expression profiling, and gene silencing methods to identify and provide a plurality of ‘validated’ KSHV-induced cellular gene sequences and pathways useful as targets for modulation of KSHV-mediated effects on cellular proliferation and phenotype (e.g., cancer) associated with latent and lytic phases of the Kaposi's sarcoma-associated herpesvirus (KSHV; Human herpesvirus 8; HHV8) life cycle. Particular embodiments provide therapeutic compositions, and methods for modulation and treatment of KSHV infection or KSHV-mediated effects on cellular proliferation and phenotype, comprising inhibition of KSHV-induced gene sequences or products thereof. Additional embodiments provide screening assays for compounds useful to modulate KSHV infection or KSHV-mediated effects on cellular proliferation and phenotype. Further embodiments provide diagnostic and / or prognostic assays for KSHV infection or related conditions. Additional embodiments provide novel in vivo models for KSHV infection or related conditions. Yet further aspects provide novel methods for transforming a mammalian cell, comprising expressing, by recombinant means, a transforming amount of RDCI, Neuritin, or both, and further provide cells transformed thereby.
Owner:OREGON HEALTH & SCI UNIV
Detection and quantification of human herpes viruses
InactiveUS7338761B2Rapid and sensitiveRapid and sensitive methodSugar derivativesMicrobiological testing/measurementClinical settingsInformatics
In clinical settings as well as in a drug development context, human herpes viruses can be detected, and even quantified, by the use of a real time PCR-based assay. An informatics analysis of existing gene sequences from different HHV types or strains is used to identify a target segment within a gene. A probe oligonucleotide and at least two primer oligonucleotides are then designed for selectively directing the amplification, in the course of a single amplification reaction, of the target segment of a particular HHV type or strain. This method is capable of an unprecedented level of discrimination among the following HHV types and strains: HHV1, drug resistant HHV1, HHV2, drug resistant HHV2, HHV3, HHV4a, HHV4b, HHV5, HHV6a, HHV6b, HHV7, and HHV8.
Owner:VIGEN LAB
Methods for assessing fatigue level and applications thereof
InactiveUS7824888B2Cause painCause to testMicrobiological testing/measurementDisease diagnosisMedicineBody fluid
Level of fatigue that accompanies everyday life or a disease can be simply, easily, and quantitatively assessed by obtaining a body fluid from a test subject and measuring the amount of human herpesvirus in the body fluid. Furthermore, the anti-fatigue potency of anti-fatigue substances and anti-fatigue food products can be measured.
Owner:VIRUS IKAGAKU KENKYUSHO
Diagnosis of mood disorders by detecting latent infection with human herpesvirus-6
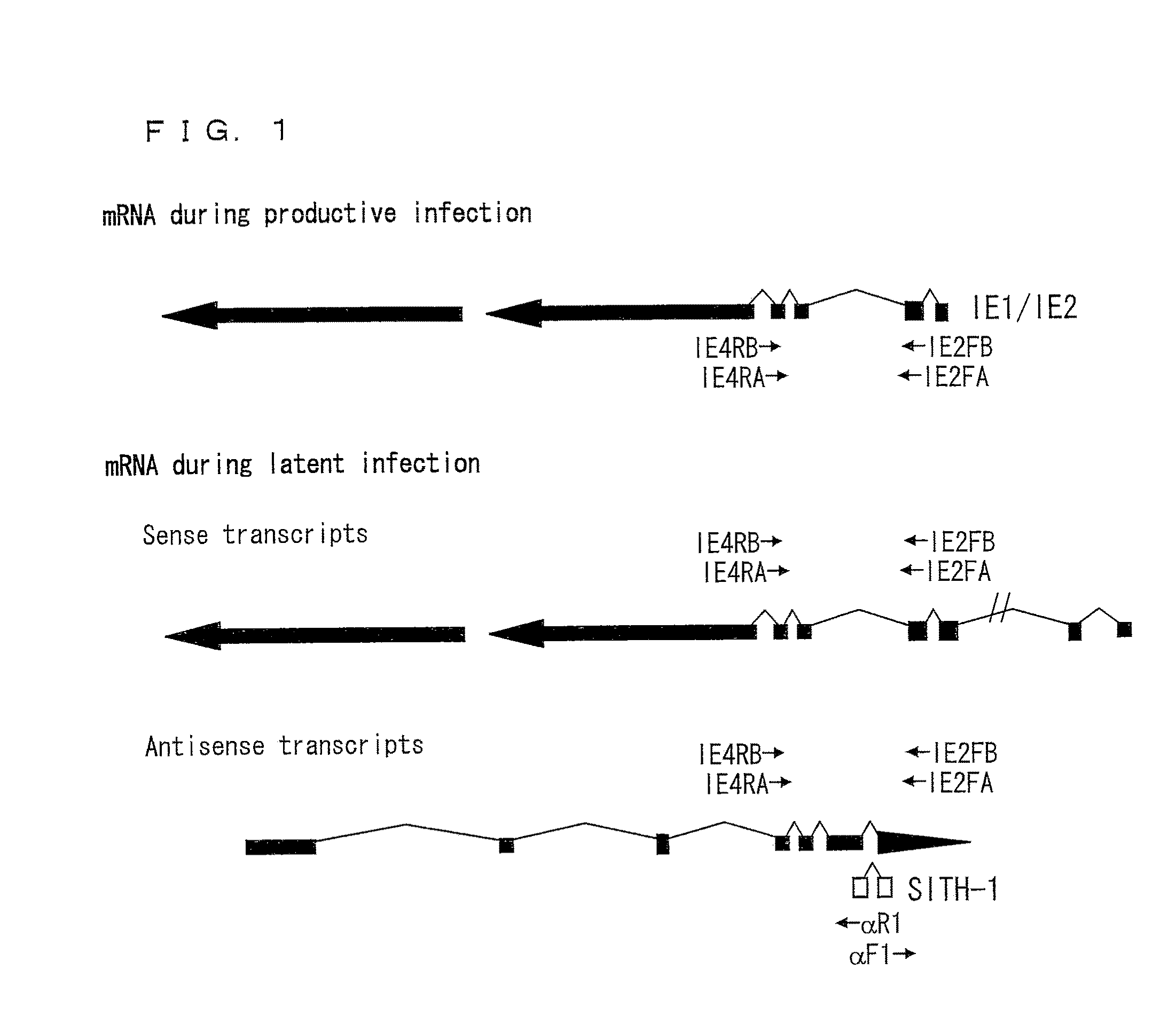
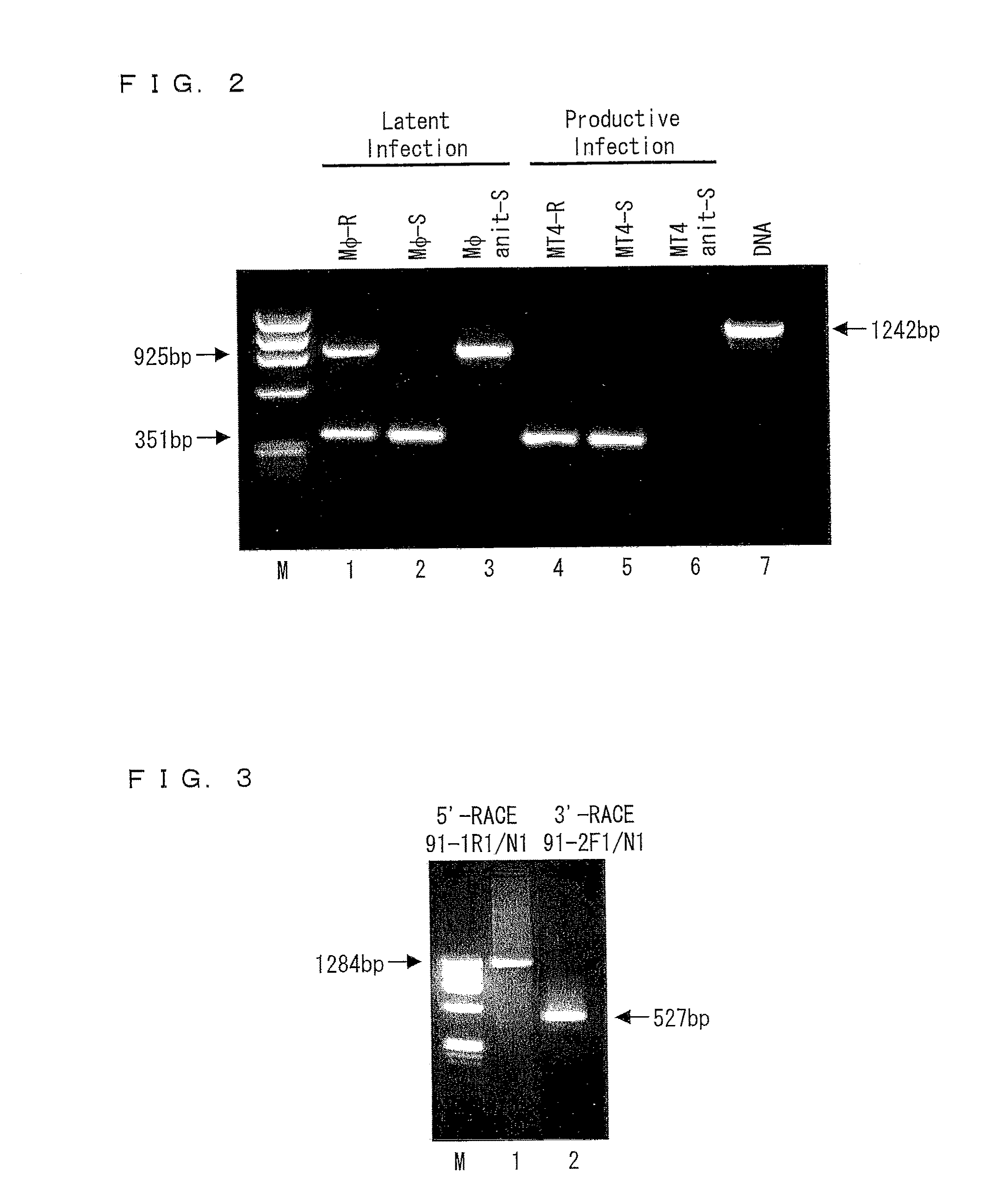
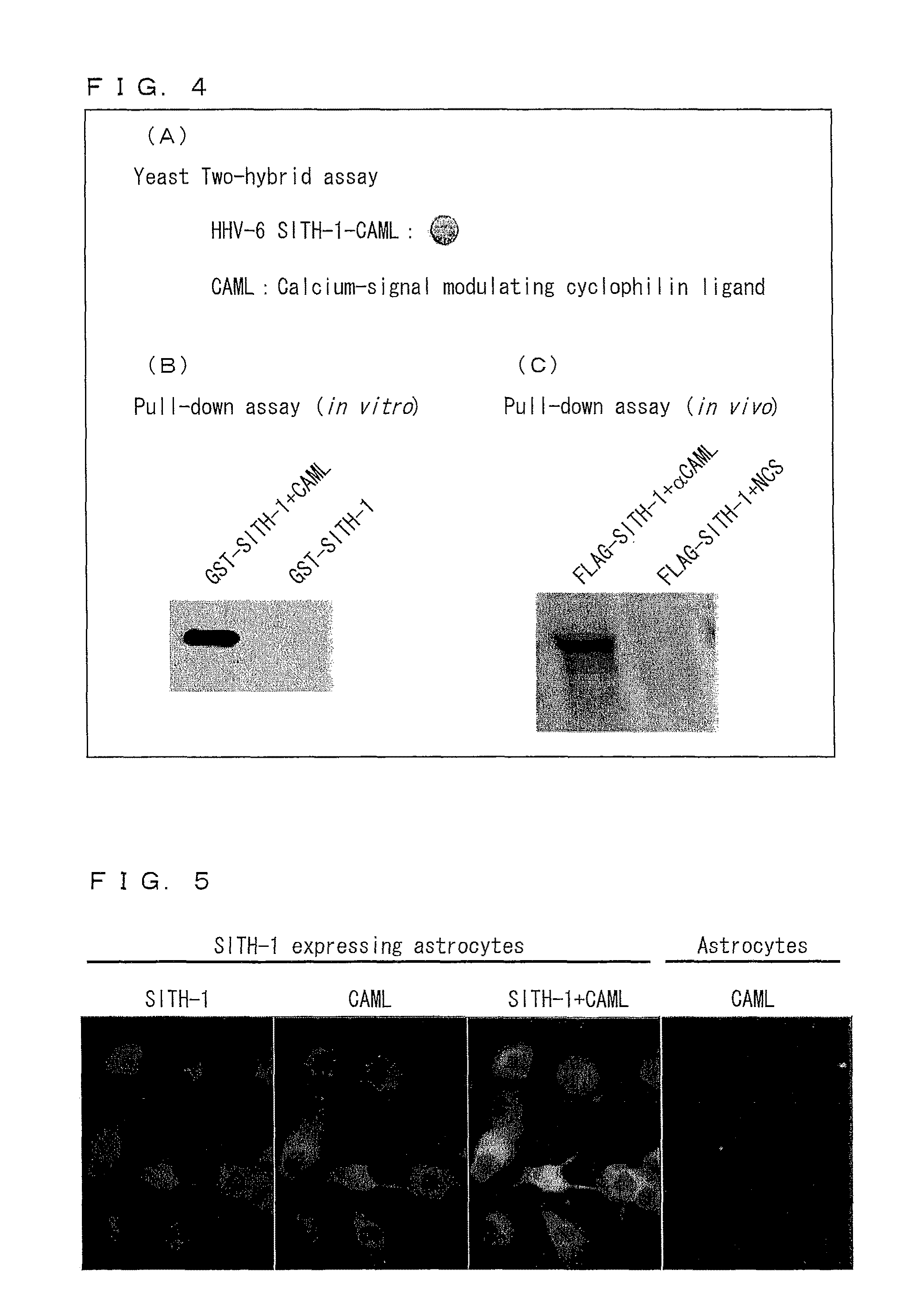
Disclosed are a protein and a gene each of which is a factor involved in latent infection with a herpesvirus. An antibody against the factor was detected in approximately 50% of patients suffering from mental disorders, whereas the antibody was hardly detected in healthy persons. Further, a mouse having SITH-1 introduced therein developed a mental disorder such as a manic-depressive illness or depression-like disorder. Based on these findings, it is possible to provide a method for objectively determining a mental disorder and an animal model of a mental disorder.
Owner:VIRUS IKAGAKU KENKYUSHO
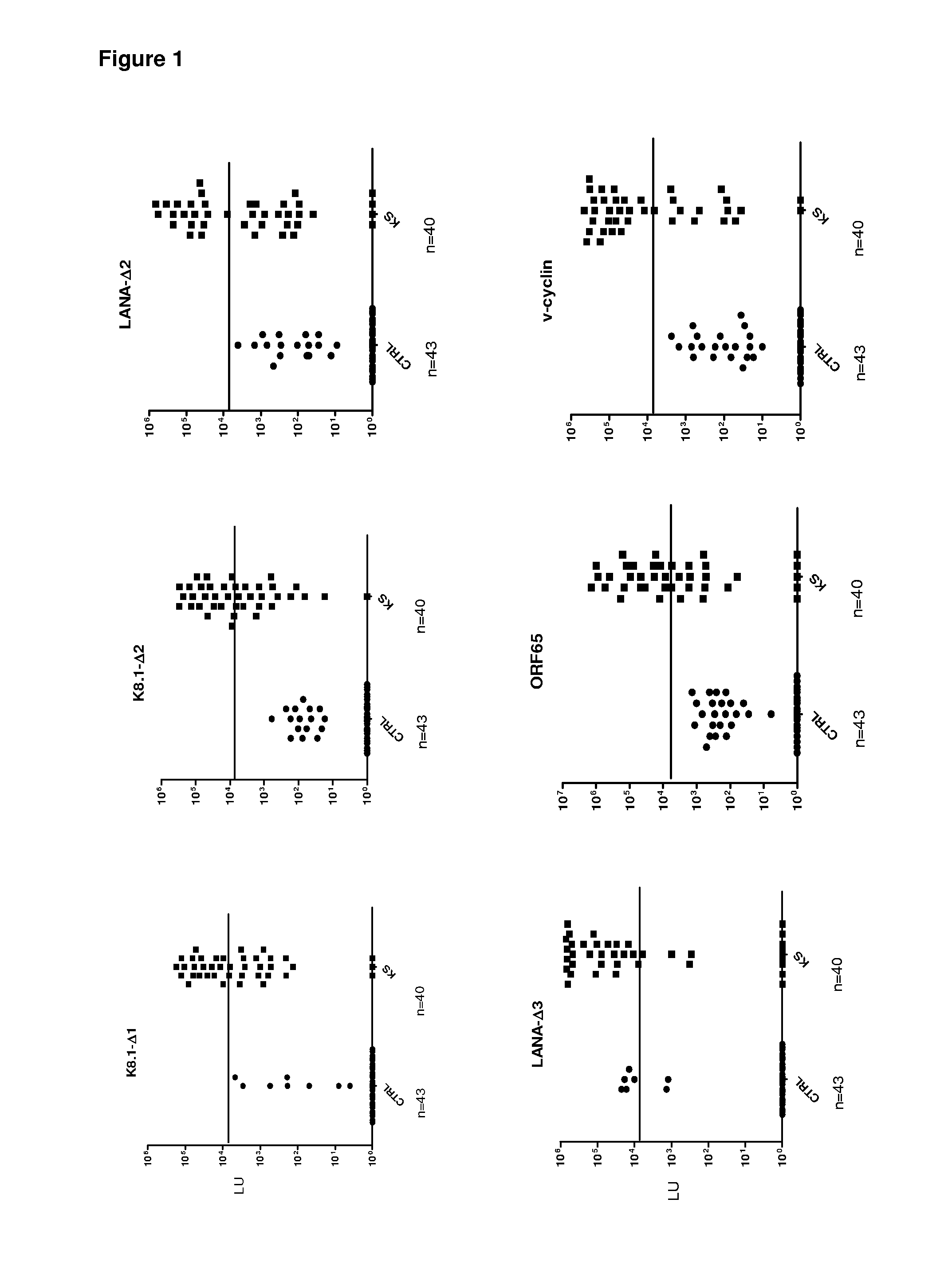
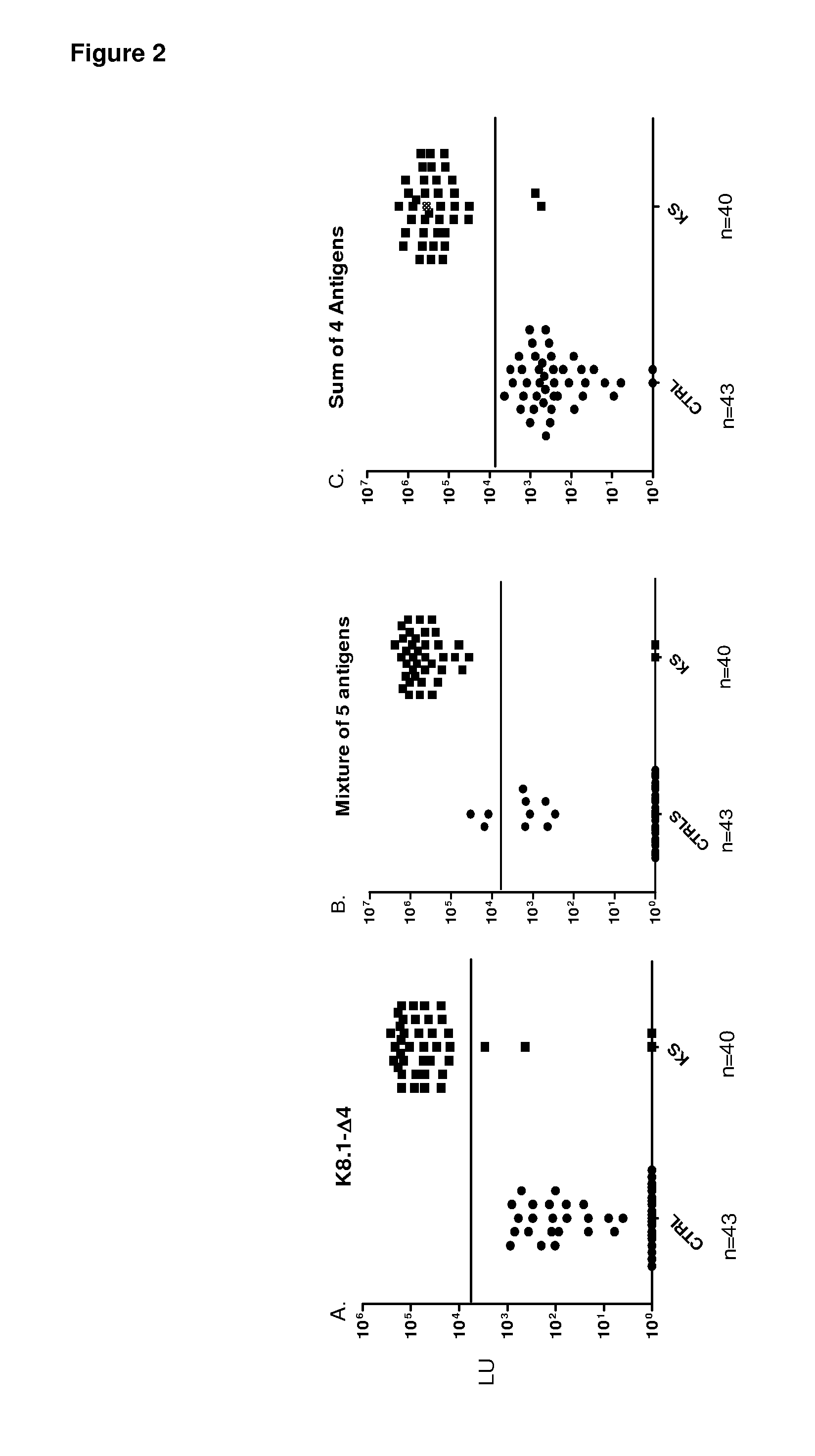
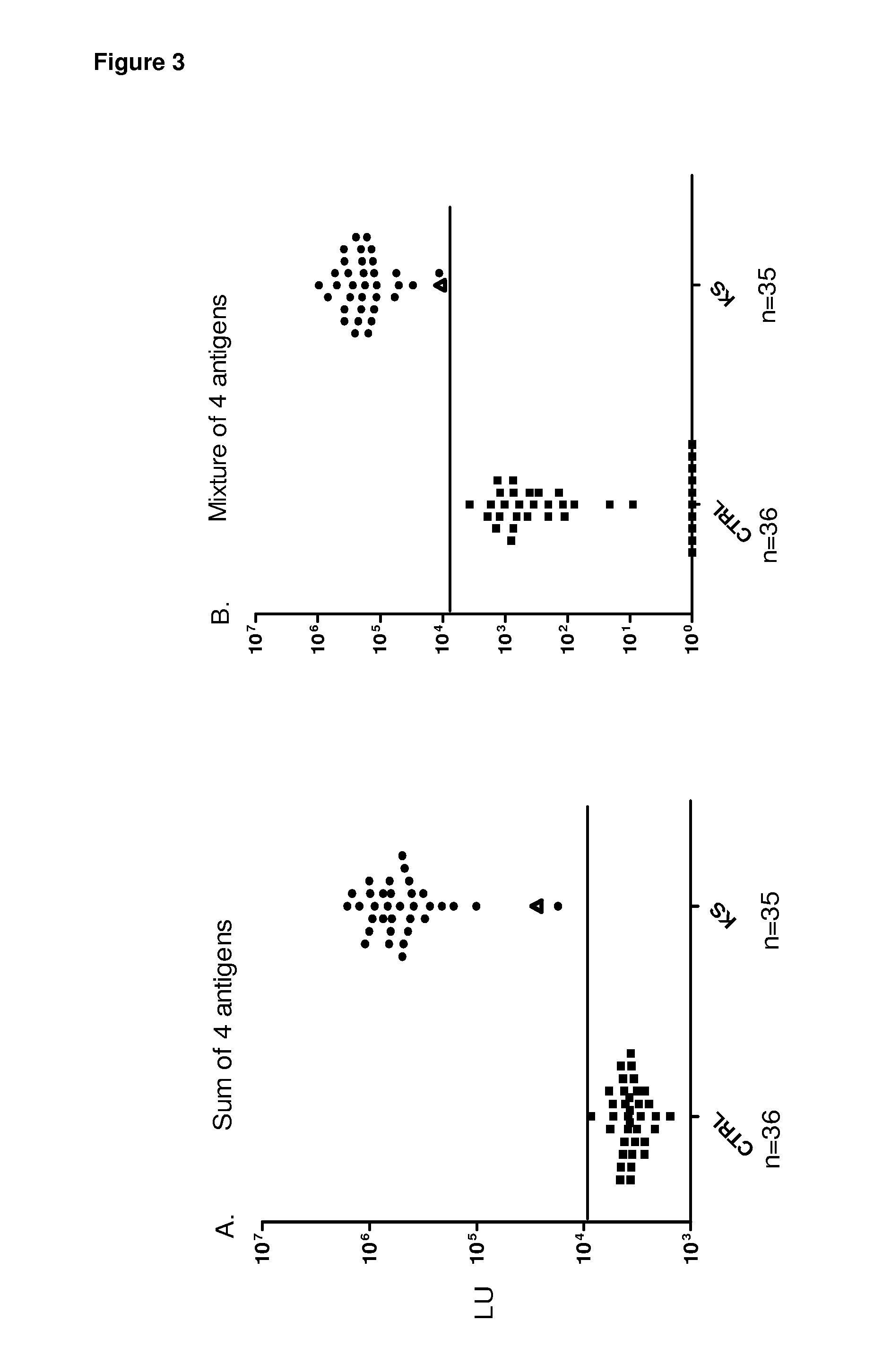
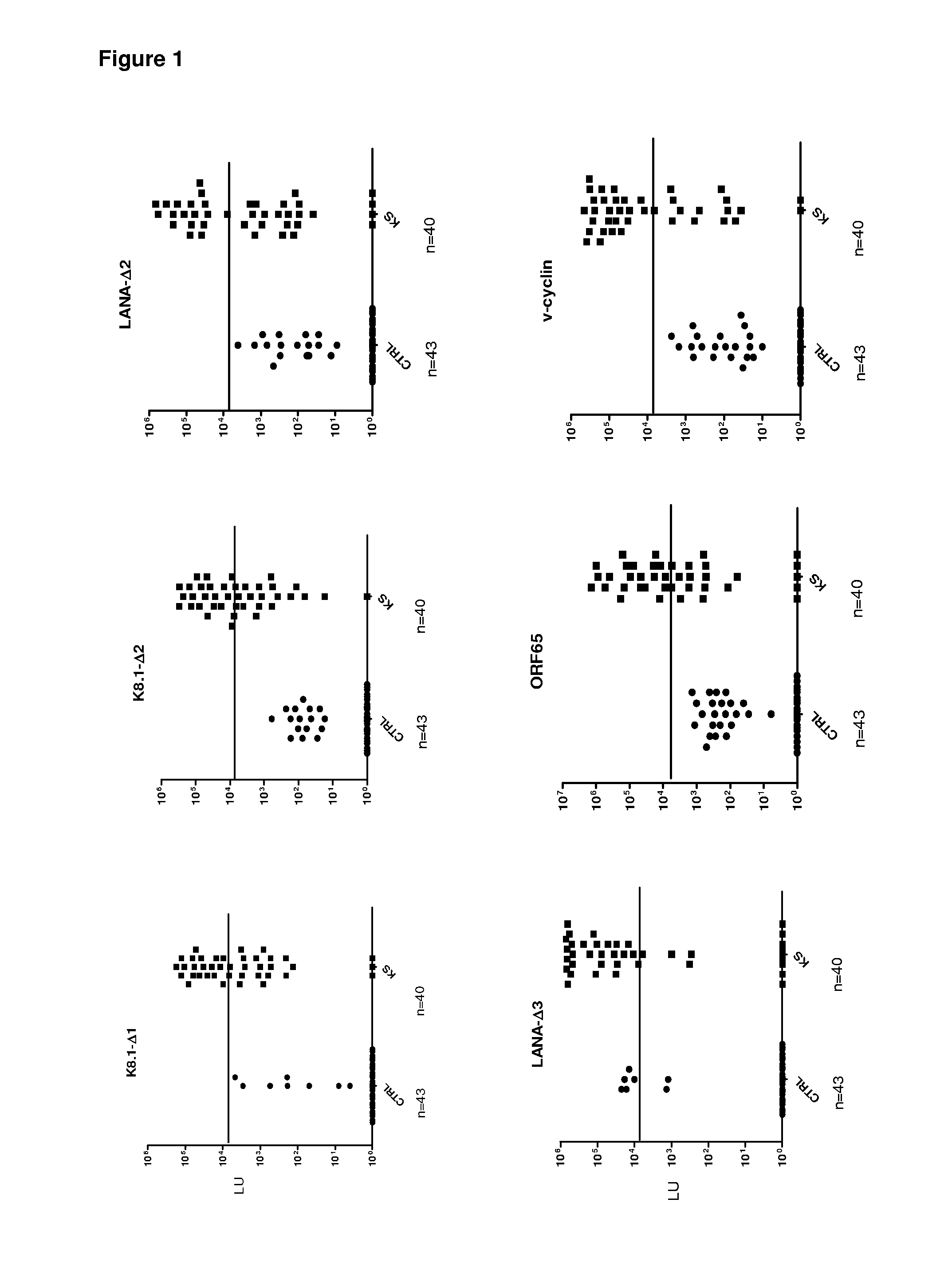
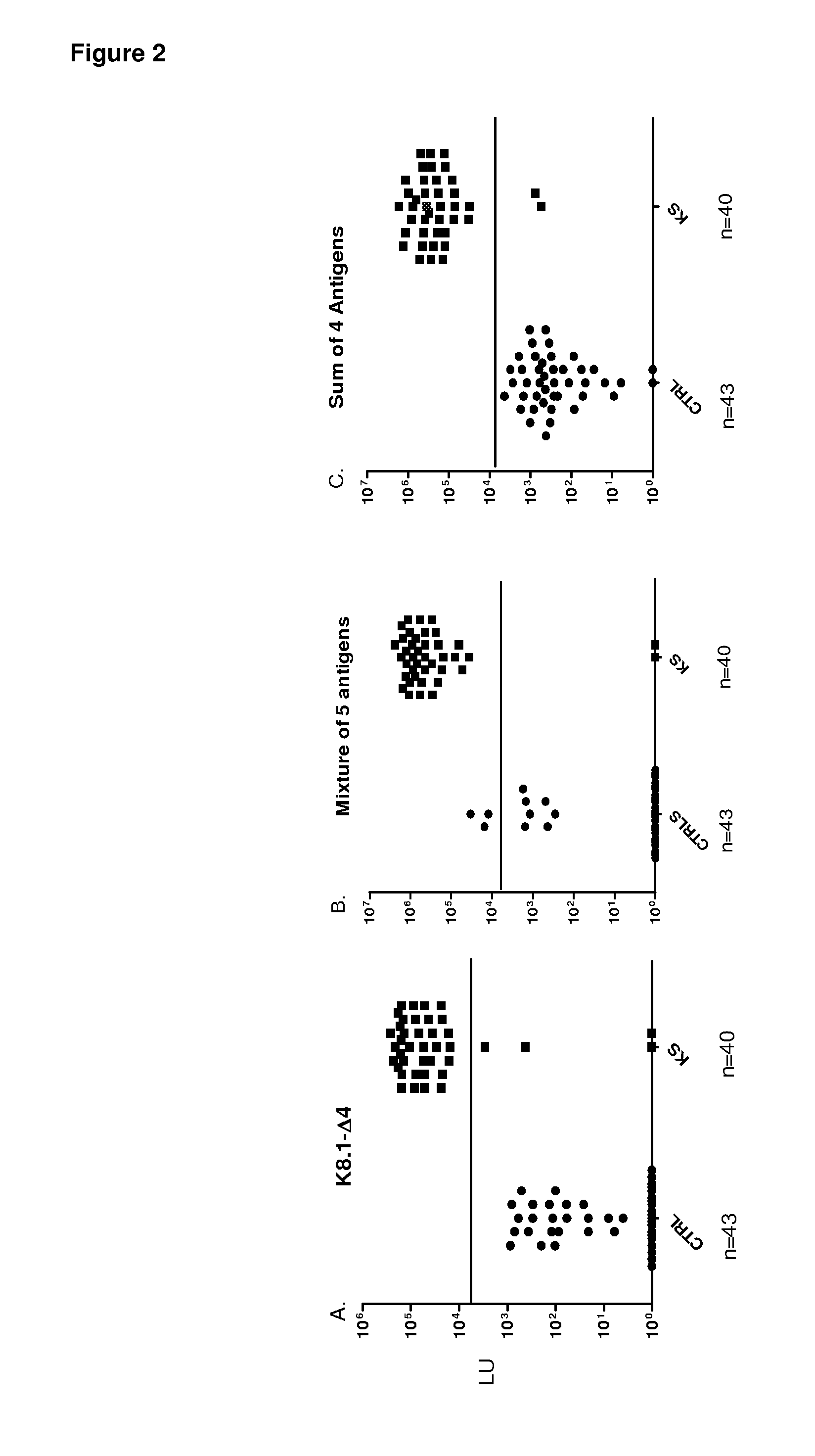
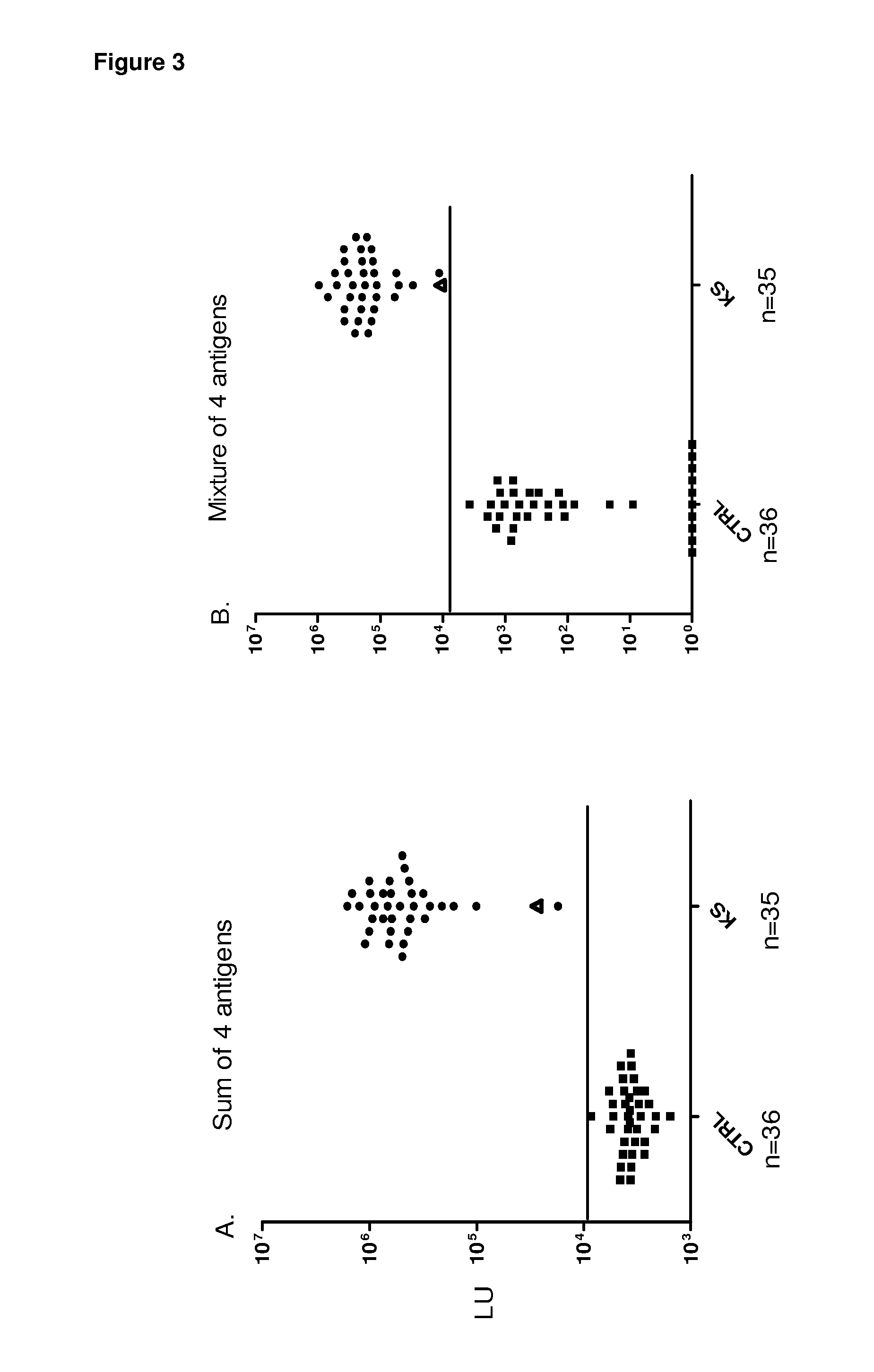
Serological screening for hhv-8 infection using antigen mixtures
The invention provides compositions, methods, and kits for the diagnosis or detection of active, latent, or prior infection with human herpesvirus 8 in a subject sample.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
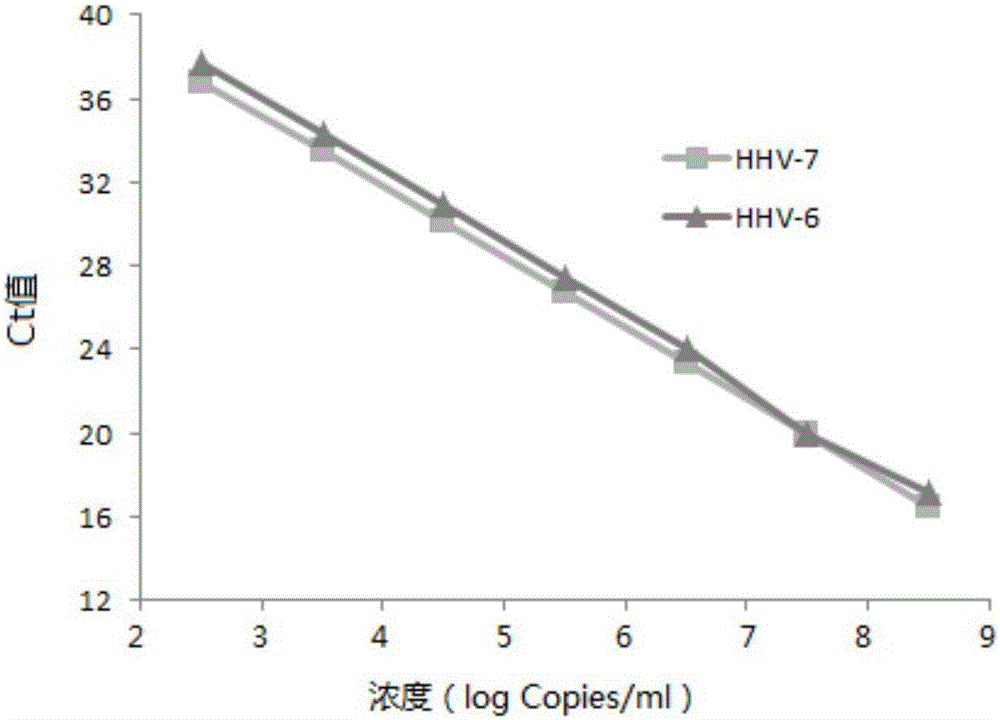
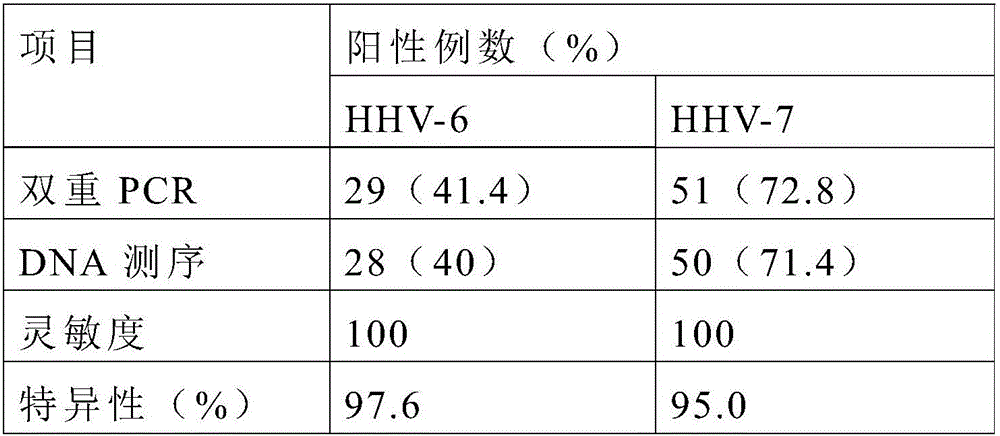
Primers, probes and kit for synchronously detecting human herpesvirus-6 and human herpesvirus-7
ActiveCN106119425ALittle interactionStrong specificityMicrobiological testing/measurementMicroorganism based processesSynchronous detectionBiology
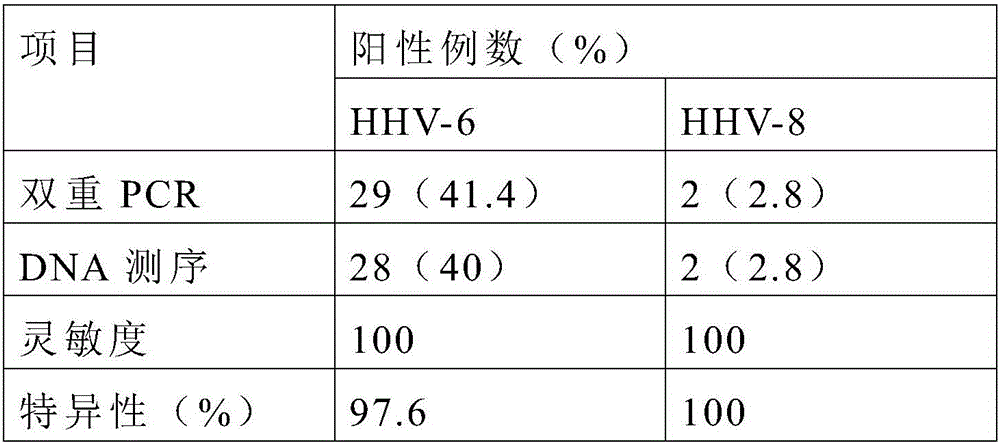
The invention discloses primers, probes and kit for synchronously detecting human herpesvirus-6 and human herpesvirus-7. According to the primers, the probes and the kit, through selecting a novel target gene and redesigning the primers and the probes, the reaction system is optimized, the false positive incidence of clinical synchronous detection on human herpesviruses is substantially lowered, the sensitivity is high, the specificity is good, and the time for detection is shorter, so that the clinical application and popularization of the technology can be achieved.
Owner:武汉光谷创鑫医药孵化服务有限公司
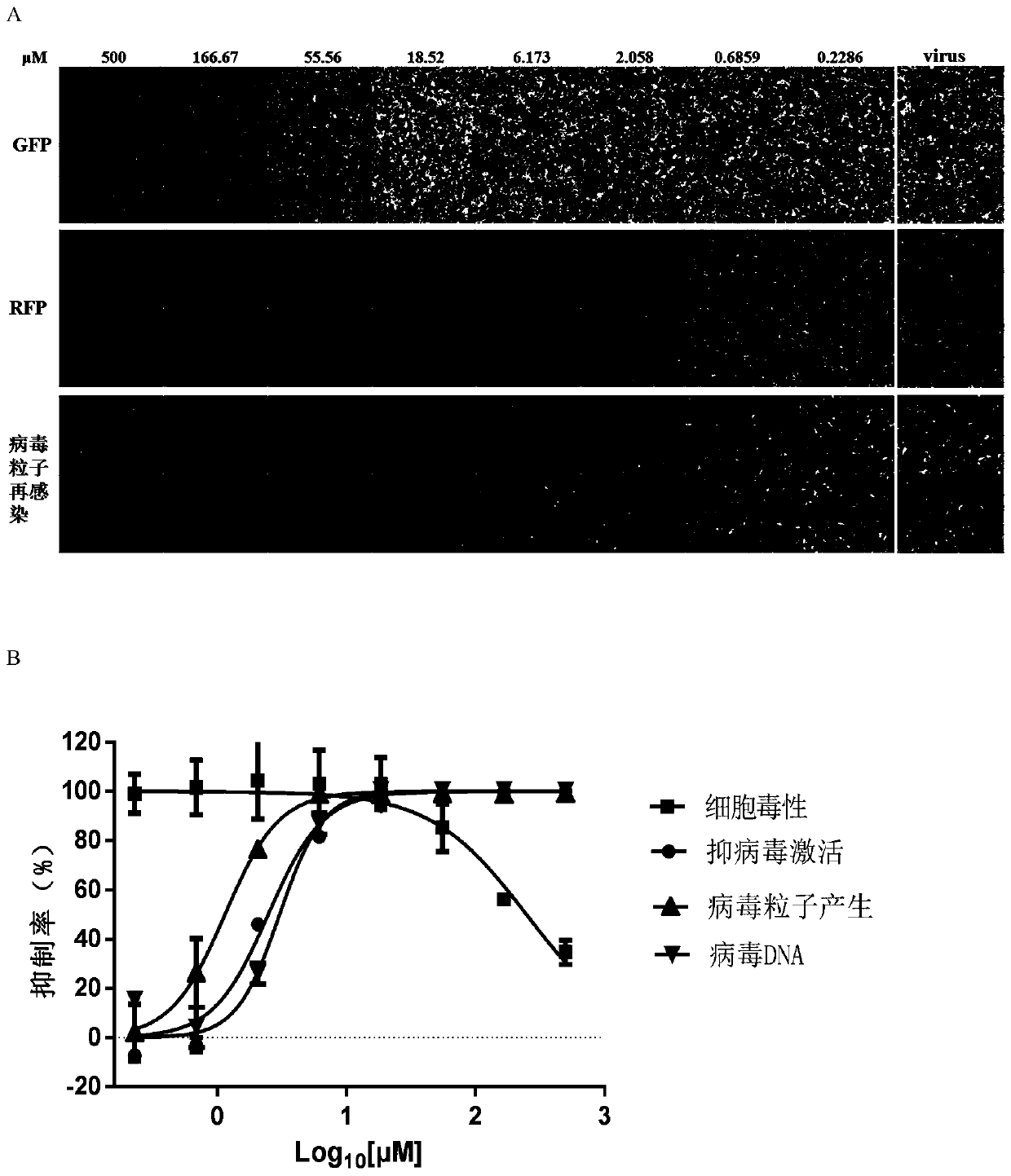
Application of spironolactone in preparation of drug for treating human herpesvirus infection
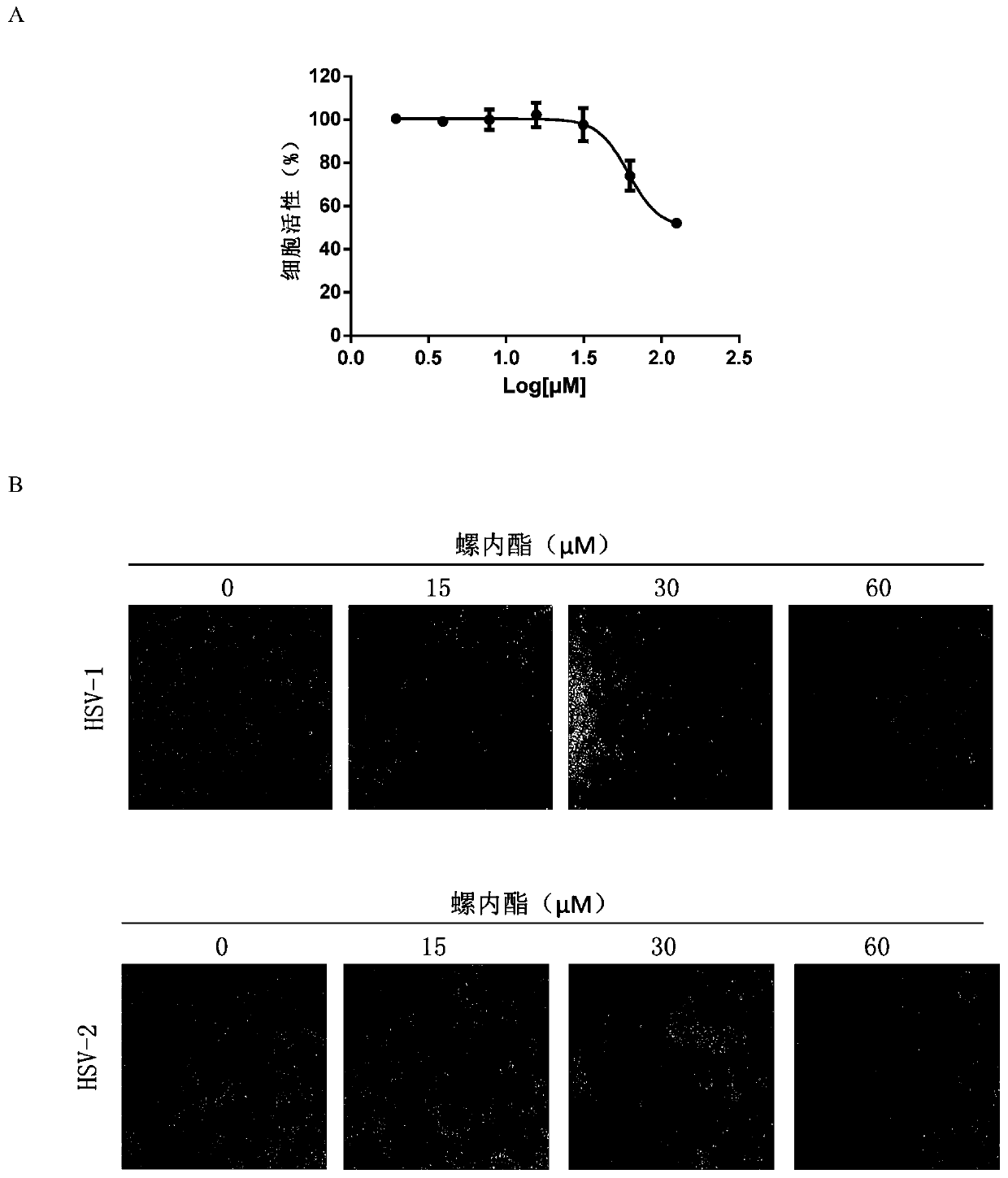
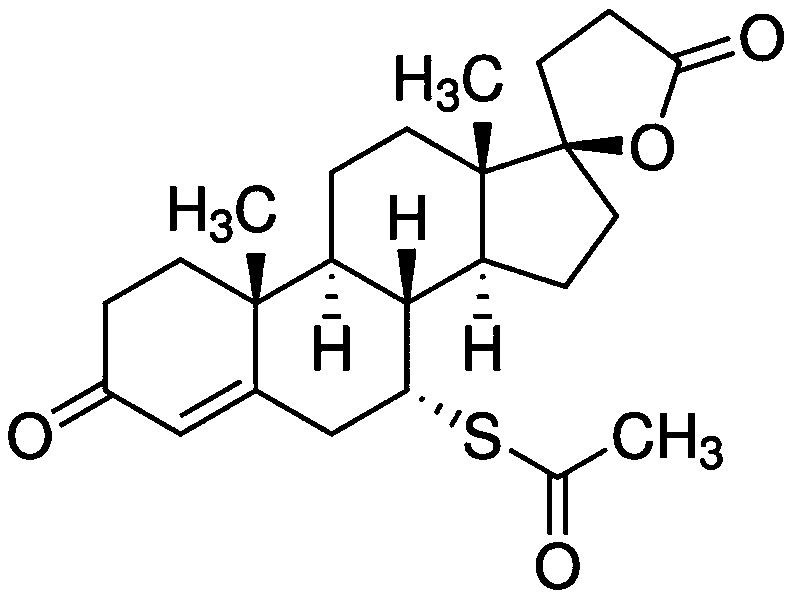
InactiveCN109925317AActive Broad SpectrumHigh activityOrganic active ingredientsAntiviralsSide effectCytopathic effect
The invention discloses application of spironolactone in preparation of a drug for treating human herpesvirus infection. We evaluate the effect of resisting Kaposi's sarcoma-associated herpes virus KSHV and herpes simplex viruses HSV-1 and HSV-2 of the spironolactone by measuring the level of infectious virion production, the level of DNA replication during lysis, or the degree of cytopathic effect caused by the virus. The spironolactone has significant antiviral activity and a dose-dependent effect, can inhibit the activation of replication during KSHV lysis with a half inhibitory concentration (IC50) of 1.145 microM to KSHV infectious virions. At non-toxic concentration, the spironolactone is effective in reducing cytopathic effect caused by HSV-1 and HSV-2. The spironolactone is a complete inhibitor of mineralocorticoids (such as aldosterone). The spironolactone is clinically used for inhibiting sodium reabsorption, potassium excretion, diuresis and the like of organisms and has thesmall toxic and side effects on the human body. The compound can inhibit replication of herpes virus in the lysis phase, and has a wide prospect of being developed into the drug for treating human herpes virus infection.
Owner:武汉威立得生物医药有限公司
Human herpesvirus I/II/III/V type nucleic acid typing detection kit and detection method
PendingCN110317902ARapid diagnosisEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionTyping
The invention discloses a human herpesvirus I / II / III / V type nucleic acid typing detection kit. The kit comprises a rapid virus nucleic acid extraction reagent, a PCR amplification reagent, a human herpesvirus I / II / III / V type nucleic acid detection reagent, a positive reference substance and a negative reference substance. According to the kit, multiple channel primer probes with human herpesvirusI / II / III / V type specificity are introduced so that the workload can be decreased, the problem is also avoided that other detection methods are not high in specificity and easily cause missed diagnosisand misdiagnosis, and it can be achieved that the same system can multiply and simultaneously detect four pathogens without mutual interference of the primer probes of all channels. The invention notonly discloses a real-time fluorescence quantitative PCR method aiming at human herpesvirus I / II / III / V type typing detection for the first time, but also has the advantages of good sensitivity, highspecificity, accuracy, reliability, rapidness, convenience and the like, thereby realizing rapid diagnosis of the infection situation of a herpesvirus I / II / III / V type. The invention also provides a human herpesvirus I / II / III / V type nucleic acid typing detection method.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
Improved human herpesvirus immunotherapy
An isolated protein comprises respective amino acid sequences of each of a plurality of CTL epitopes from two or more different herpesvirus antigens and further comprises an intervening amino acid or amino acid sequence between at least two of said CTL epitopes comprising proteasome liberation amino acids or amino acid sequences and, optionally, Transporter Associated with Antigen Processing recognition motifs. The isolated protein is capable of rapidly expanding human cytotoxic T lymphocytes (CTL) in vitro and eliciting a CTL immune response in vivo upon administration to an animal as an exogenous protein. Typically, the isolated protein comprises no more than twenty (20) CTL epitopes derived from cytomegalovirus and / or Epstein-Barr virus antigens.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Methods for assessing fatigue level and applications thereof
InactiveUS20110020789A1Cause painCause to testMicrobiological testing/measurementDisease diagnosisDiseaseDaily living
Level of fatigue that accompanies everyday life or a disease can be simply, easily, and quantitatively assessed by obtaining a body fluid from a test subject and measuring the amount of human herpesvirus in the body fluid. Furthermore, the anti-fatigue potency of anti-fatigue substances and anti-fatigue food products can be measured.
Owner:VIRUS IKAGAKU KENKYUSHO
Human herpesvirus type 6 real-time fluorescent quantitative PCR typing detection kit
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6 (HHV-6). The real-time fluorescent quantitation PCR parting detection kit consists of quantitation PCR reaction liquid, an HHV-6A standard substance, an HHV-6B standard substance, an HHV-6A positive reference substance, an HHV-6B positive reference substance, a negative reference substance, a specification and a kit body, wherein the quantitation PCR reaction liquid contains a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HHV-6A fluorescent probe and an HHV-6B fluorescent probe. By the adoption of a real-time fluorescent quantitation PCR technology and a double-color fluorescent probe, the kit disclosed by the invention can detect two subtypes of the HHV-6 by a one-step method; the HHV-6A and HHV-6B in the sample can be simultaneously parted; a positive virus subtype can be accurately quantified in real time; the urgent need of early and acute parting diagnosis on the HHV-6 infection can be met; a basis is supplied to epidemiological investigation and targeted treatment on the HHV-6 infection.
Owner:ZHEJIANG UNIV
Corroles for treating human cytomegalovirus infections
ActiveUS20210369671A1Good selectivity indexGood indexAntiviralsHeterocyclic compound active ingredientsHerpesvirus infectionHuman cytomegalovirus
Disclosed is a family of corroles for its use in the treatment of an infection by human herpesvirus, especially in the treatment of an infection by human cytomegalovirus.
Owner:UNIV DE BOURGOGNE (FR)
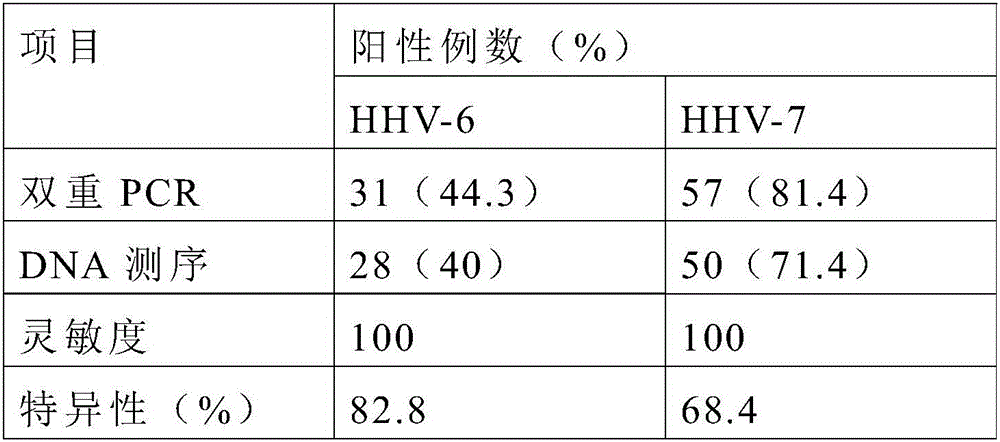
Primer, probe and kit for simultaneously detecting human herpesvirus 6 and 8
InactiveCN106244731ALittle interactionStrong specificityMicrobiological testing/measurementMicroorganism based processesRe engineeringBiology
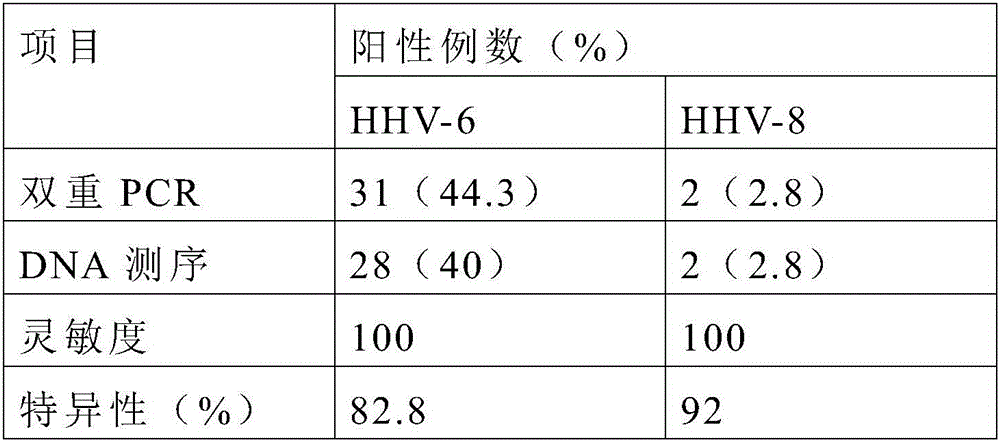
The invention discloses a primer, probe and kit for simultaneously detecting human herpesvirus 6 and 8. The primer, probe and kit has the advantages that the primer and the probe are redesigned by selecting new target genes, a reaction system is optimized, the false positive rate of simultaneous human herpesvirus detection is lowered greatly, the primer, probe and kit is high in sensitivity, good in specificity and short in detection time, and clinical application and popularization can be achieved.
Owner:武汉百格资产管理有限公司
Cyanobacterial extracts, processes for preparing the same and uses thereof
ActiveUS20200197458A1Reduce the amount of solutionSatisfying activitiesOrganic active ingredientsUnicellular algaeEnterovirusRespiratory syncytial virus (RSV)
The present disclosure provides various cyanobacterial extracts exhibiting antiviral activity to a wide spectrum of viruses, such as enterovirus (EV), respiratory syncytial virus (RSV), Human Herpesvirus (HHV), Ebola virus, porcine epidemic diarrhea virus (PEDV), and porcine reproductive and respiratory syndrome virus (PRRSV). The cyanobacterial extract is prepared from biomass of A. maxima (or Spirulina maxima). Also disclosed herein are process for preparing the cyanobacterial extract and uses of the cyanobacterial extract.
Owner:FAR EAST BIO TEC
Clinical assays for the detection and typing of human herpesviruses
InactiveUS7348145B2Quick checkTest cycle time superiorSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
The present invention provides methods of unambiguously identifying a human herpesvirus in a sample. The assays, which allow for the detection and typing of all ten human herpesviruses, involve multiplex PCR assays using consensus primers to amplify conserved regions of the herpesvirus DNA. A dot blot / chemiluminescence assay and real time PCR assay ideal for clinical setting were disclosed. A heteroduplex mobility assay suitable for uses in research laboratory was also presented.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for producing immune cells and method for inducing immune effector cells
Owner:VECTORITE BIOMEDICA +1
Serological screening for HHV-8 infection using antigen mixtures
Owner:UNITED STATES OF AMERICA
Early childhood infection with human herpes virus type 6 (hhv-6) as a protection against the development of ige sensitization, atopic disease and allergy in young children
InactiveUS20070207169A1Preventing atopy and atopic diseaseViral/bacteriophage medical ingredientsRespiratory disorderAtopic diseaseAllergy
The present invention discloses a use of human herpes virus 6 (HHV-6) or derivatives thereof, for the manufacture of a pharmaceutical composition intended to be administered during early childhood, for the prevention of IgE sensitization and allergy development in young children. Examples of viral compositions include, but are not limited to live human herpes virus 6 (HHV-6).
Owner:FORSKARPATENT I SYD AB
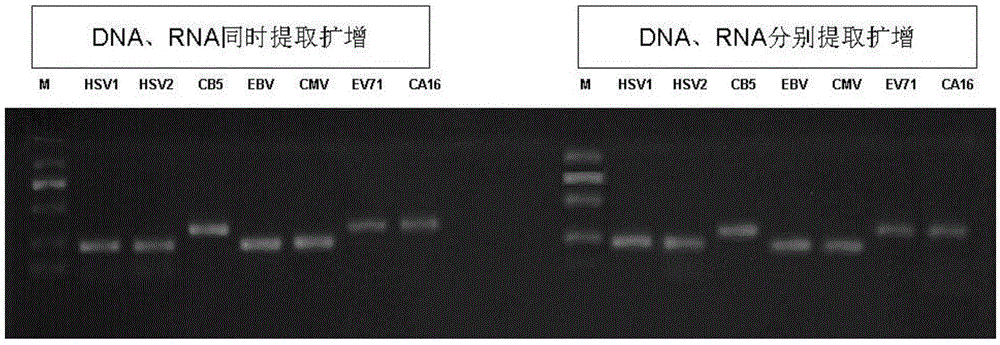
Gene chip for simultaneous detection of human herpesvirus and enterovirus
InactiveCN103981289BImplement extractionAchieve early diagnosisMicrobiological testing/measurementMicroorganism based processesEnterovirusNucleotide
The invention relates to a gene chip capable of simultaneously detecting human herpes virus and enterovirus. The gene chip comprises a nucleotide extraction kit, a simultaneous DNA (Deoxyribonucleic Acid) and RNA (Ribonucleic Acid) reverse transcription kit, a simultaneous DNA and RNA PCR amplification kit and a gene chip linked with a specific probe. According to the invention, the simultaneous extraction, amplification and detection of nucleic acid of a plurality of herpes viruses and intestinal viruses can be realized by only 100 mu l of cerebrospinal fluid. The gene chip has the advantages of less required sample amount, fast extraction speed, high amplification efficiency, reliable results and strong specificity. The early diagnosis and treatment of clinical viral encephalitis and meningitis induced by the viruses can be achieved and the current bottleneck problem of the detection of viruses in clinical cerebrospinal fluid is resolved.
Owner:山东大学齐鲁儿童医院
A kind of human herpesvirus type 4 detection kit and detection method
ActiveCN106755584BEasy to operateHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesHuman herpesvirusPolymerase chain reaction
The invention belongs to the field of biotechnology, and specifically relates to a human herpesvirus 4 detecting kit and a detecting method. The human herpesvirus 4 detecting kit comprises a nucleic releaser, magnetic bead containing nucleic extracting liquid and PCR (Polymerase Chain Reaction) reacting liquid, wherein the nucleic releaser comprises surfactin, chlorhexidine acetate, 2-6% of potassium chloride and hydrophilic graphene; the magnetic bead containing nucleic extracting liquid comprises 4-hydroxyethylpiperazine ethane sulfonic acid, sodium chloride and magnetic balls; the PCR reacting liquid comprises an upstream primer, a downstream primer, and a detector; the upstream primer and the downstream primer are used for target polynucleotide amplification; the detector is used for detecting target polynucleotide. The kit has the advantages of being simple to operate, high in detection sensitivity, high in accuracy, high in specificity and wide in detection range; reliable experiment basis is provided for clinically diagnose EBV (Epstein-Barr Virus) infection.
Owner:艾吉泰康(嘉兴)生物科技有限公司
Herpes simplex virus type 1 real-time fluorescent nucleic acid constant temperature amplification detection kit
ActiveCN105624329BAccurate measurementRapid determinationMicrobiological testing/measurementCerebrospinal fluidT7 RNA polymerase
Owner:SHANGHAI RENDU BIOTECH
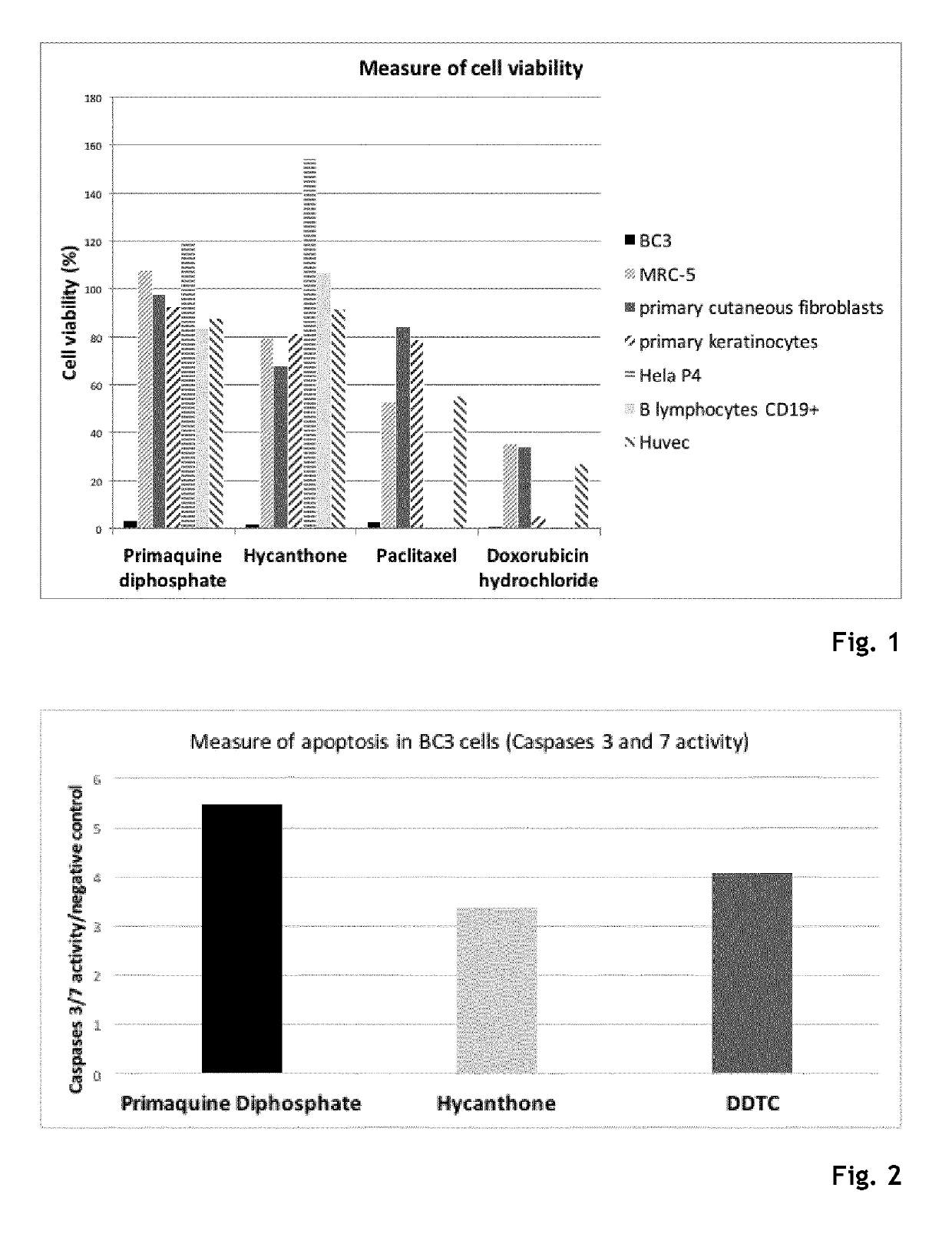
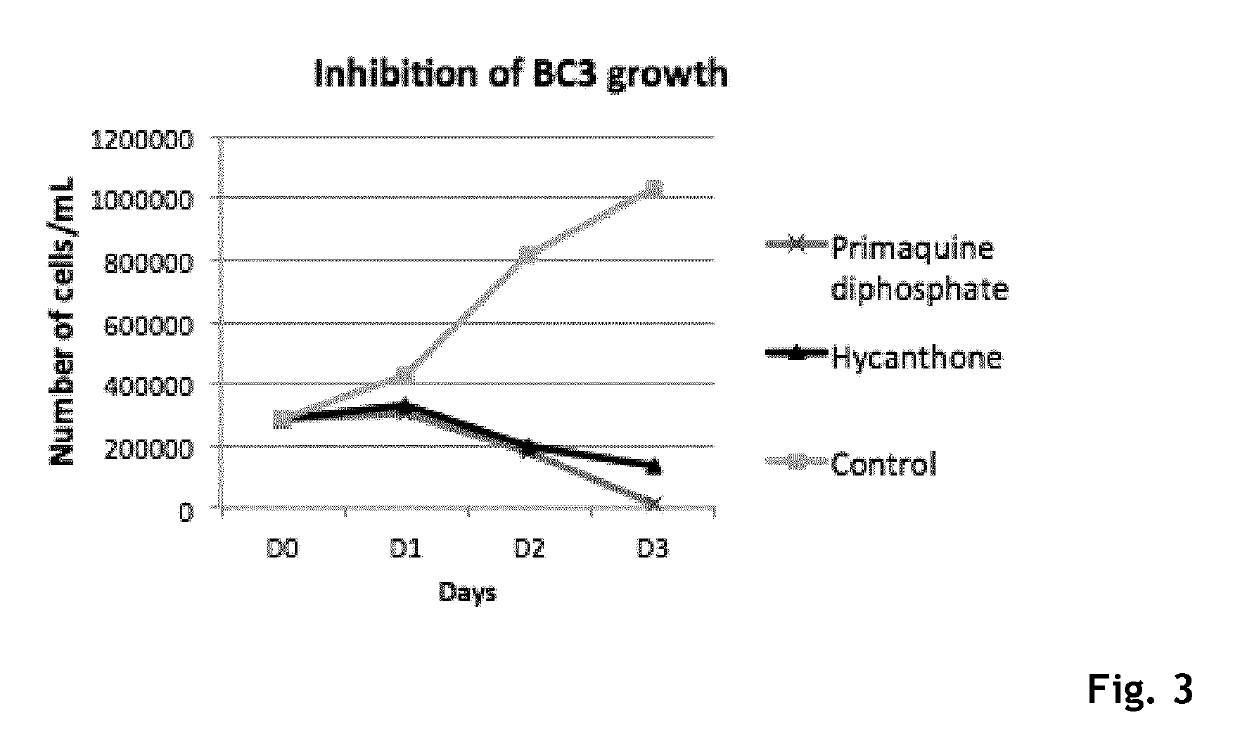
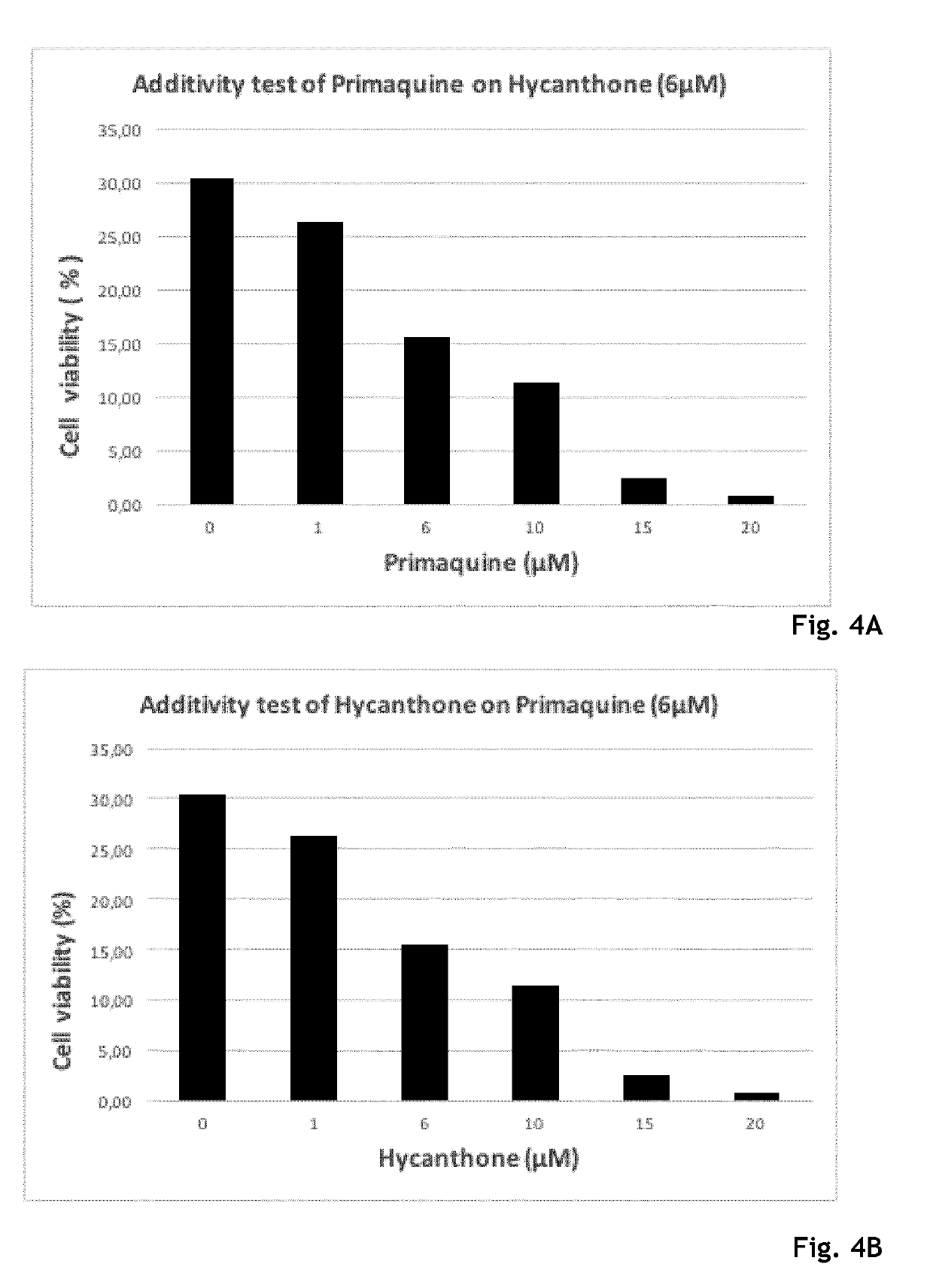
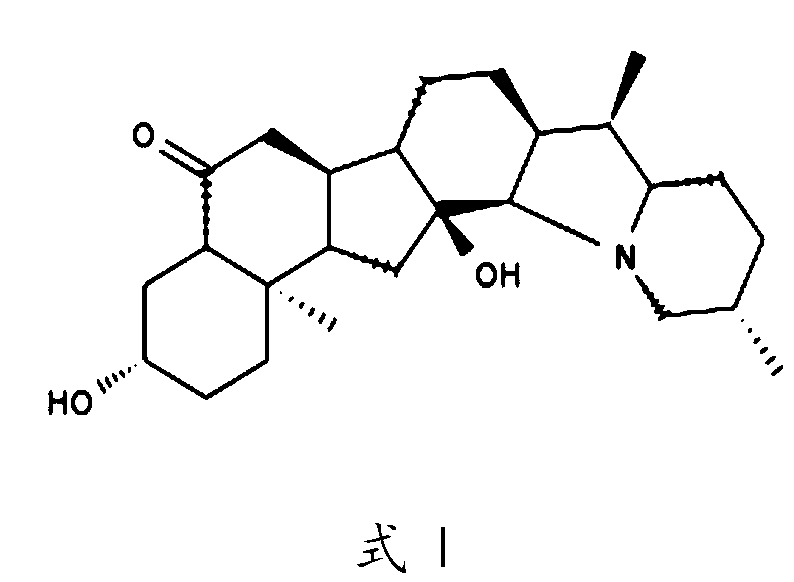
Hycanthone derivatives and primaquine derivatives for use in the prevention and/or the treatment of disorders associated to gammaherpesvirus
The present invention relates to compounds of the following general formula (I) or (II) or a pharmaceutically acceptable salt and / or solvate thereof, for use in the prevention and / or the treatment of disorders associated to gammaherpesvirinae, in particular to the human herpesvirus 8 (HHV8) or the human herpes virus 4 (HHV4), and pharmaceutical compositions containing such compounds.
Owner:UNIV DE PARIS +4
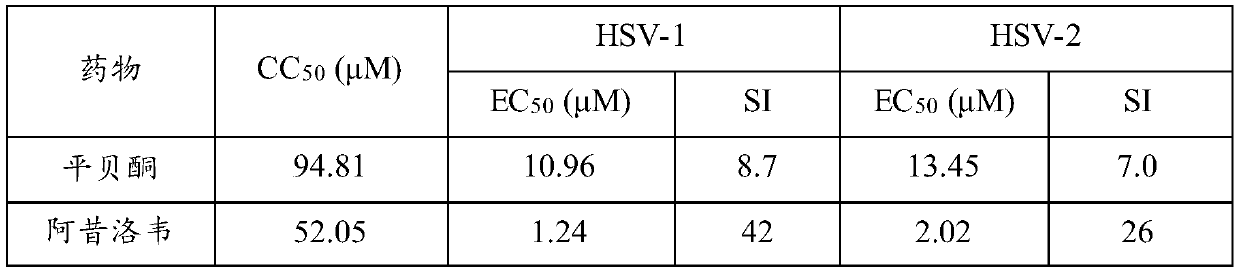
Application of pimbidone in the preparation of medicaments for preventing and/or treating human herpesvirus infection
ActiveCN106265658BImprove survival rateStrong antiviral activityOrganic active ingredientsAntiviralsMedicineDeath cause
Owner:JIANGSU KANION PHARMA CO LTD
Compositions and methods for detecting human herpesviruses
InactiveUS7442505B2Sugar derivativesMicrobiological testing/measurementMicrobiologyHuman herpesvirus
Owner:BOARD OF RGT UNIV OF NEBRASKA
Mucosal adjuvant composition
ActiveUS20130302360A1Antibacterial agentsSsRNA viruses negative-senseCulture fluidCanine distemper virus CDV
Problem to be Solved: The present invention provides a novel mucosal adjuvant.Solution: The present invention provides a mucosal adjuvant composition containing at least a composition comprising molecules having a molecular weight in a range of 100 to 300 kDa obtained from cells or culture fluid of Bordetella bronchiseptica. Administration of the mucosal adjuvant composition to a non-human animal at a surface of the mucous membrane can enhance immunity at the surface of mucous membrane. Therefore preventive effects against trans-mucosal infection can be increased by administering an inactivated vaccine against trans-mucosal infection and the mucosal adjuvant composition to a non-human animal at a surface of mucous membrane. The present invention is effective for preventing trans-mucosal infections, including one or more infections of e.g., canine parainfluenza, canine adenovirus, canine coronavirus, canine parvovirus, canine distemper virus, canine herpesvirus, reovirus and pneumovirus.
Owner:KYORITSU SEIYAKU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com