Improved human herpesvirus immunotherapy
A herpes virus and immune response technology, applied in the field of recombinant proteins, can solve the problems of not showing clinical efficacy and not entering clinical practice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
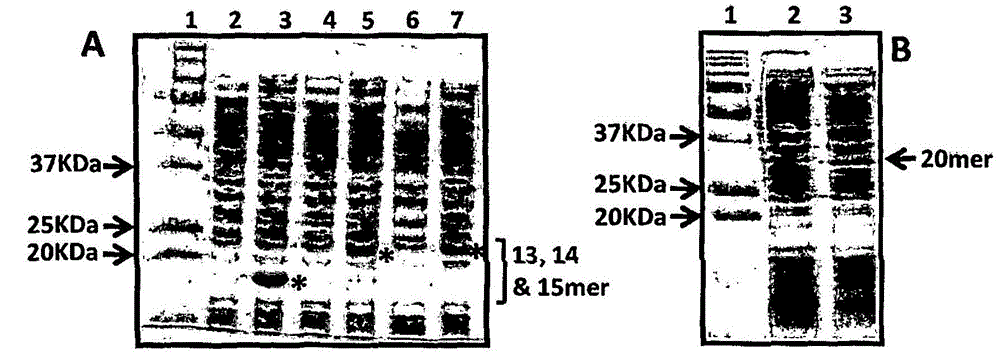
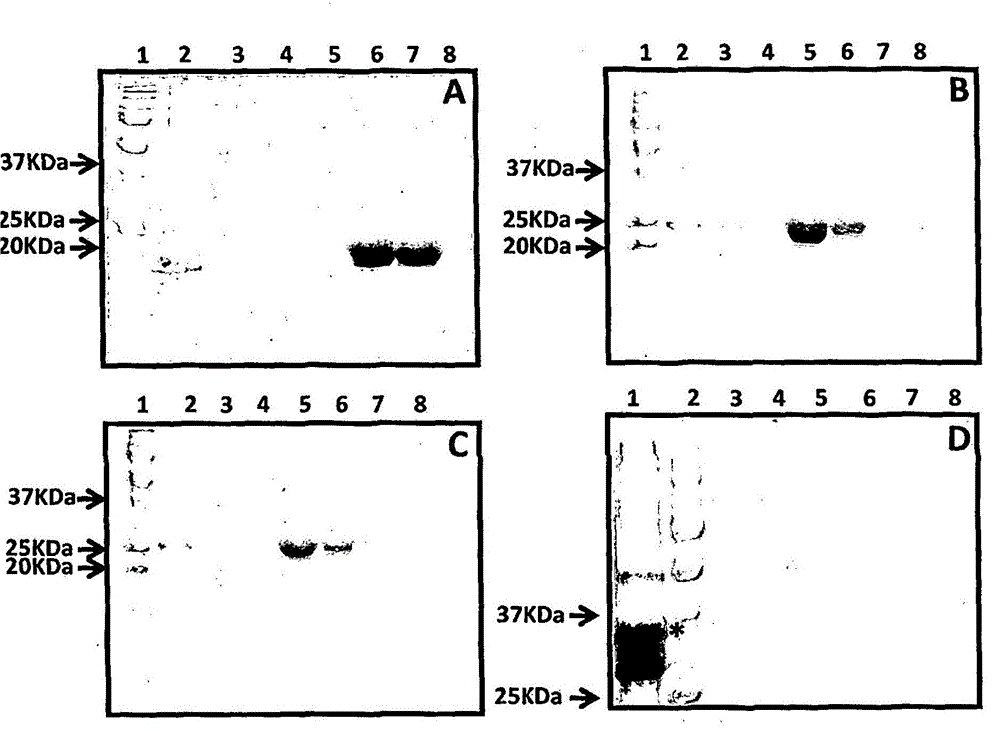
[0210] Purification and immunogenicity of CMV polyepitope protein
[0211] Materials and methods
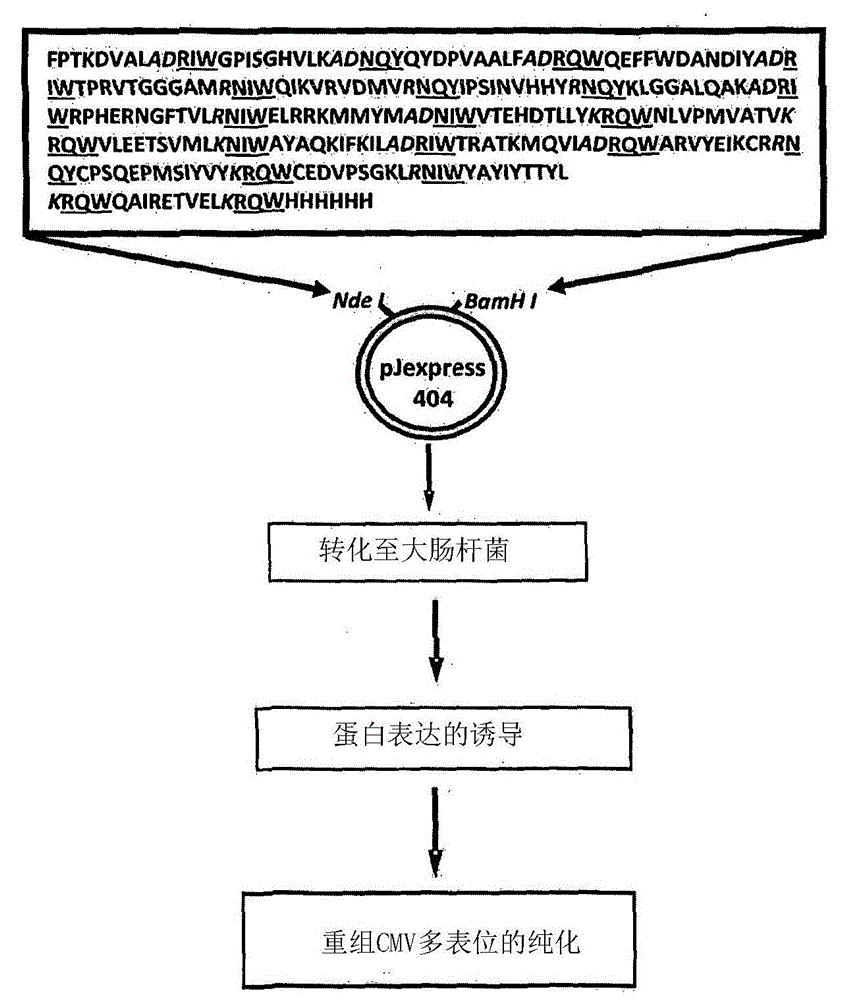
[0212] Construction of CMV multi-epitope vector
[0213] A series of CMV polyepitope inserts were designed to encode multiple HLA class I-restricted T-cell epitopes from five different antigens (pp65, IE-1, pp50, pp150 and gB). These polyepitopic sequences encode 13, 14, 15 or 20 different HLA class I-restricted CD8 + Epitopes (see Table 1).
[0214] Multi-epitope sequences were designed in such a way that each epitope sequence was preceded by a proteasome-released amino acid sequence (AD or K or R) and a TAP recognition motif ( RIW , QUR , NIW or NQY). In addition, a hexahistidine tag was inserted at the C-terminus of each polyepitopic protein to allow purification using a nickel-nitrilotriacetic acid (Ni-NTA) column. The amino acid sequence of each construct was translated into a DNA sequence based on codon utilization in Escherichia coli, and the insert was construc...
Embodiment 2
[0248] Immunogenicity of Adjuvanted CMV Polyepitope Proteins
[0249] Materials and methods
[0250] CMV multi-epitope vaccine formulation with MPL and CpG ODN1826
[0251] The CMV polyepitope vaccine was formulated by mixing 20 μg of CMVpoly, CMVpoly-PL or CMVpoly-PTL with 25 μg of MPL (TLR4 agonist) and 50 μg of CpG ODN1826 (TLR9 agonist) per dose in a volume of 100 μL. TLR agonists were purchased from InvivoGen (San Diego, CA, USA).
[0252] mouse immunization
[0253] HHD 1 mice containing human HLA-A*0201 with disrupted mouse MHC class I were bred and maintained under specific pathogen-free conditions under QIMR. All protocols were in compliance with the QIMR Animal Ethics Committee. At least 5 (M1-5) six- to eight-week-old mice per group were immunized subcutaneously (s.c.) at the base of the tail using the CMV multi-epitope vaccine formulated with the specific adjuvant combinations described above. Mice were boosted on day 21 with the same vaccine formulation and...
Embodiment 3
[0278] Immunogenicity of EBV polyepitope proteins combined with adjuvants
[0279] Materials and methods
[0280] Construction of EBV multi-epitope construct
[0281] EBV polyepitopes were designed to encode multiple HLA class I-restricted T cell epitopes from nine different antigens (BMLF1, BRLF1, BZLF1, LMP2, LMP2A, EBNA1, EBNA3A, EBNA3B and EBNA3C). Epitope HLA restrictions, amino acid sequences and amino acid positions of these epitopes are listed in Table 3, and in Figure 13 Illustration in A.
[0282] The polyepitope sequences were designed in such a way that each epitope sequence was preceded by a proteasome released amino acid sequence (AD or K or R), and a hexahistidine tag insert at the C-terminus of each polyepitope protein to allow purification with a nickel-nitrilotriacetic acid (Ni-NTA) column. The amino acid sequence of each construct was translated into a DNA sequence according to the codon utilization of Escherichia coli, and then the insert was construct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com