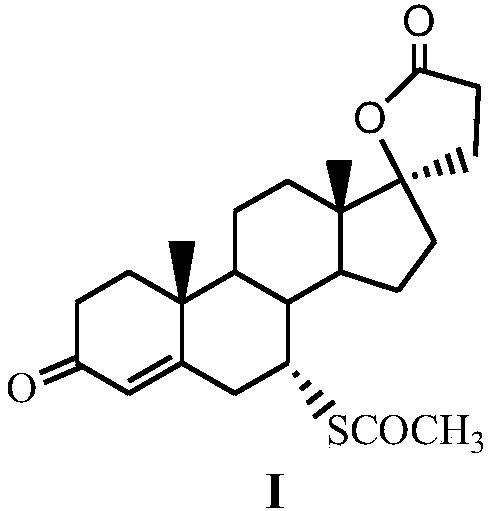
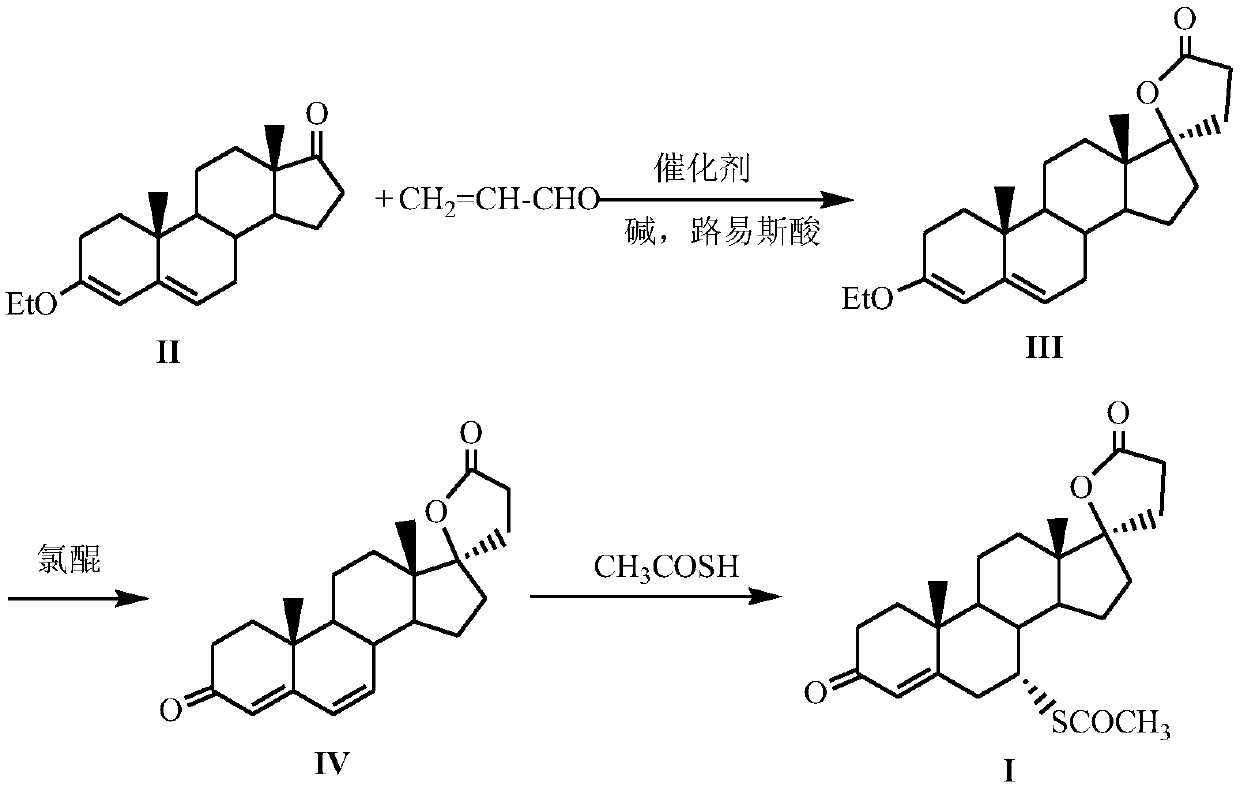
A kind of method for preparing spironolactone
A technology of spironolactone and lactone, which is applied in the field of preparation of spironolactone, can solve the problems of harsh reaction conditions, high production cost, long reaction route, etc., and achieve the effect of short synthesis route, high total yield and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Preparation of formula (III) compound 17β-hydroxyl-3-ethoxy-17α-pregna-3,5-diene-21-carboxylic acid-γ-lactone
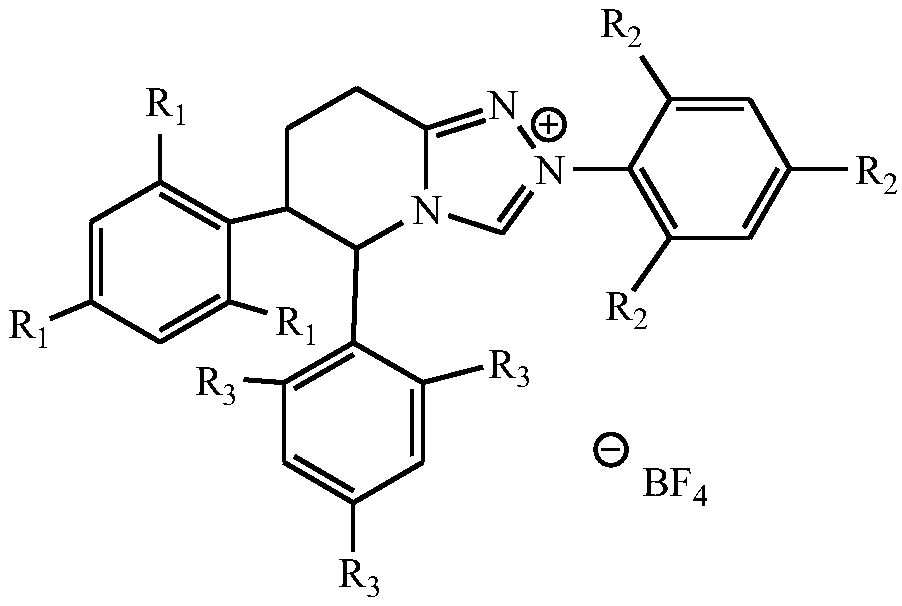
[0025] Under nitrogen protection, 20mmol formula (II) compound 3-ethoxy-androst-3,5-dien-17-one, 24mmol acrolein, 24mmol lithium bromide, 1.2mmol tetrahydropyridotriazole carbene salt Catalyst (R 1 =R 2 =CH 3 , R 3 =Cl) and 2 mmol triethylamine were added to a strictly dry reaction flask, then 20 mL of absolutely anhydrous dichloroethane was injected into the reaction flask, the stopper was sealed, and the reaction was stirred at room temperature for 5 h, then the stirring was stopped, and 20 mL of water was added to the reaction flask. , and then stirred for 1 h; filtered, the organic layer was separated, the aqueous layer was washed twice with dichloroethane, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid with a yield of 89%.
Embodiment 2
[0026] Example 2 Preparation of compound 17β-hydroxyl-3-ethoxyl-17α-pregna-3,5-diene-21-carboxylic acid-γ-lactone of formula (III) Under nitrogen protection, 20mmol of formula ( II) compound 3-ethoxy-androst-3,5-dien-17-one, 24mmol acrolein, 24mmol lithium chloride, 1.5mmol tetrahydropyridotriazole carbene salt (R 1 =CH 3 , R 2 =R 3 =OCH 3 ) and 3mmol potassium carbonate were added to a strictly dry reaction flask, then 20mL of absolutely anhydrous toluene was injected into the reaction flask, the stopper was sealed, the reaction was stirred at room temperature for 6h, the stirring was stopped, 20mL of water was added, and the reaction was stirred for 1h; After filtration, the organic layer was separated, the aqueous layer was washed twice with toluene, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid with a yield of 78%.
Embodiment 3
[0027] Embodiment 3 Preparation of formula (III) compound 17β-hydroxyl-3-ethoxyl-17α-pregna-3,5-diene-21-carboxylic acid-γ-lactone
[0028] Under nitrogen protection, 20mmol formula (II) compound 3-ethoxy-androst-3,5-dien-17-one, 24mmol acrolein, 24mmol potassium bromide, 1.4mmol tetrahydropyridotriazole Carbene salt catalyst (R 1 =R 2 =CH 2 Cl, R 3 =H) and 3.4mmol sodium acetate were added to a strictly dry reaction flask, and then 20mL of absolutely anhydrous dichloroethane was injected into the reaction flask, the stopper was sealed, and the reaction was stirred at room temperature for 6h, then the stirring was stopped, and 20mL of water was added to the reaction flask. , and stirred for another 1 h; filtered, the organic layer was separated, the aqueous layer was washed twice with dichloroethane, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid, yield: 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com