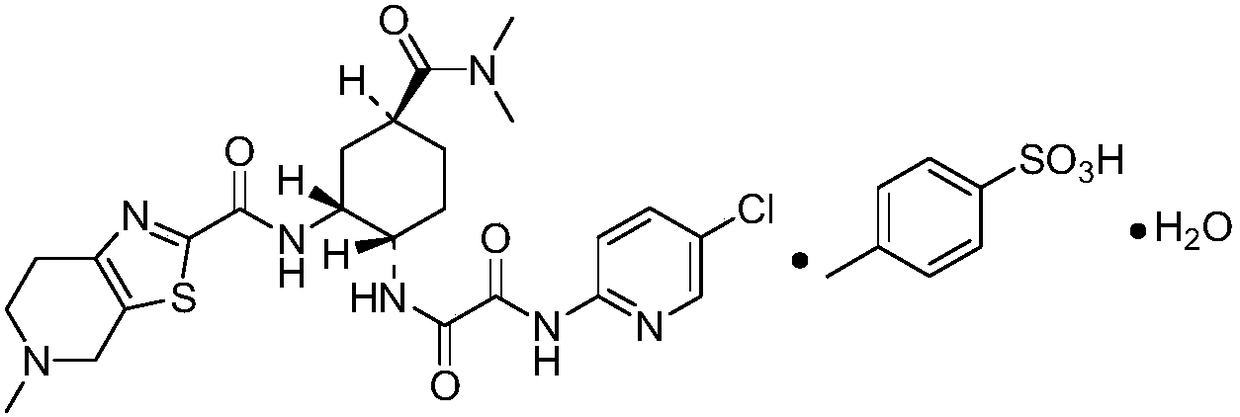
Pharmaceutical composition for edoxaban tablets
A technology of p-toluenesulfonic acid and edoxaban, applied in the field of medicine, can solve the problems of poor stability and suffering from swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Edoxaban p-toluenesulfonate sheet (1000)
[0035]
[0036] A total of 1000 tablets were produced, each tablet weighed 0.1g, and the specification was 30mg based on edoxaban.
[0037] Preparation process: The raw material drug is pulverized to make its particle size distribution reach D 50 ≤30μm and D 90 ≤60μm; crush the rest of the excipients through an 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVP K30 95% ethanol solution, granulate with a 18-mesh sieve, and dry at 40°C. Add the prescribed amount of magnesium stearate, mix evenly, pass through a 18-mesh sieve, measure the content of the main drug in the granules, determine the weight of the tablet, and compress the tablet to obtain final product. The dispersion time of the resulting tablet was 126s.
Embodiment 2
[0038] Embodiment 2: Edoxaban p-toluenesulfonate sheet (1000)
[0039]
[0040]
[0041] A total of 1000 tablets were produced, each tablet weighed 0.1g, and the specification was 15mg based on edoxaban.
[0042] Preparation process: The raw material drug is pulverized to make its particle size distribution reach D 50 ≤30μm and D 90 ≤60μm; crush the rest of the excipients through an 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVP K30 95% ethanol solution, granulate with a 18-mesh sieve, and dry at 40°C. Add the prescribed amount of talcum powder, mix evenly, pass through a 18-mesh sieve, measure the content of the main drug in the granules, determine the weight of the tablet, and compress the tablet to obtain the product. The dispersion time of the resulting tablet was 84s.
Embodiment 3
[0043] Embodiment 3: Edoxaban p-toluenesulfonate sheet (1000)
[0044]
[0045] A total of 1000 tablets were produced, each tablet weighed 0.1g, and the specification was 15mg based on edoxaban.
[0046] Preparation process: The raw material drug is pulverized to make its particle size distribution reach D 50 ≤30μm and D 90 ≤60μm; crush the rest of the excipients through an 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVP K30 95% ethanol solution, granulate with a 18-mesh sieve, and dry at 40°C. Add the prescribed amount of magnesium stearate, mix evenly, pass through a 18-mesh sieve, measure the content of the main drug in the granules, determine the weight of the tablet, and compress the tablet to obtain final product. The dispersion time limit of the obtained tablet was 60s.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com