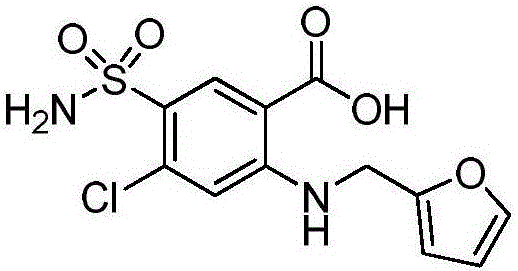
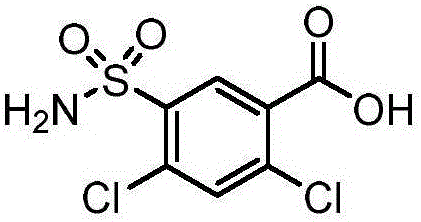
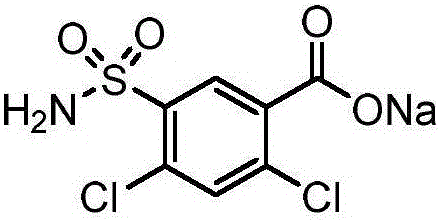
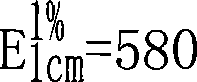Patents
Literature
79 results about "Furosemide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Furosemide is used to reduce extra fluid in the body (edema) caused by conditions such as heart failure, liver disease, and kidney disease.
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
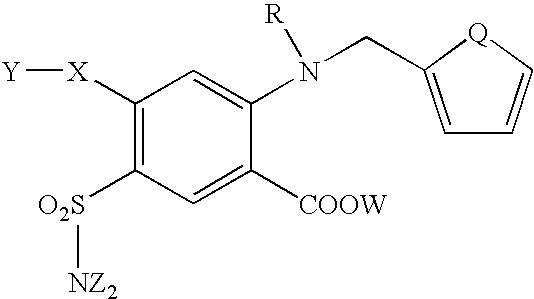
Furosemide modulators of HM74
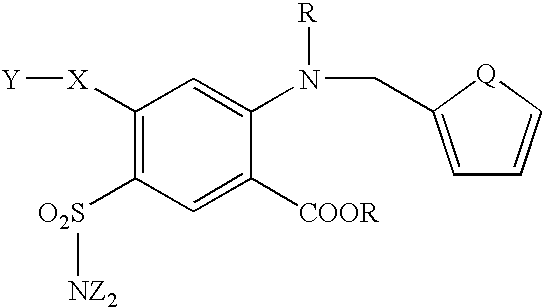
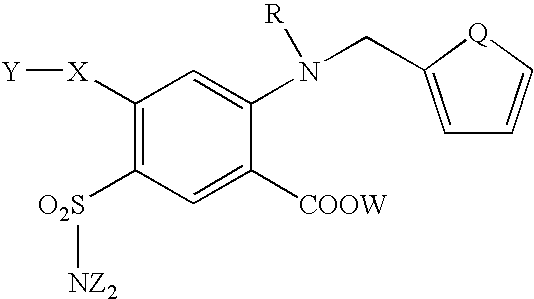
Host cells expressing HM74 were used to obtain furosemide-like molecules with agonist activity having the following structure formula:
Owner:AVENTIS PHARMA INC +1
Method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics
InactiveCN102901780AEasy to separateQualitatively accurateComponent separationPhenolphthaleinEphedrine
The invention relates to a method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics. According to the method, an appropriate mobile phase and a reasonable elution gradient are adopted during liquid-phase separation, so that the slimming components needing to be detected are effectively separated within 12 min, the analysis time of a sample instrument is shortened by 88%, and the work efficiency is greatly increased. Additionally, the advantages of high separation ability of the HPLC (High Performance Liquid Chromatography) technology, high sensitivity and stronger qualitative ability of the mass spectrum and the like are combined, so screening and confirmation can be simultaneously completed during one operation. By using the method disclosed by the invention, the seven chemical components including sibutramine, fenfluramine, phenolphthalein, ephedrine, caffeine, N,N-bi-demethylation sibutramine and furosemide can be effectively separated, the inspection time can be shortened, and the slimming chemical components which are illegally added to the traditional Chinese medicine, the health food or the cosmetics can be accurately and effectively distinguished.
Owner:HUNAN INST FOR FOOD & DRUG CONTROL
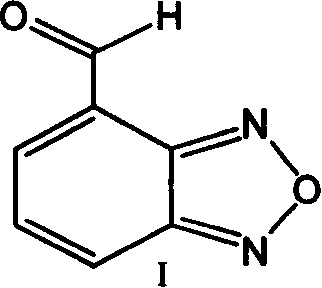
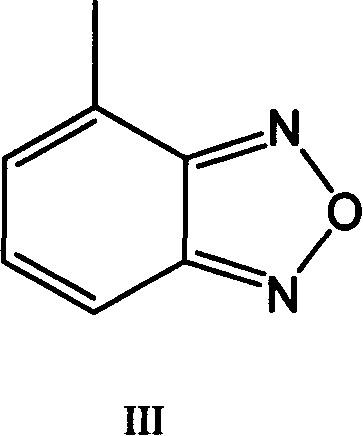
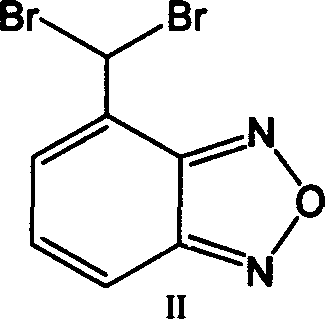
Method for preparing 4-formoxylbenzofuran
Improved, low-cost, scalable process for the preparation of 4-formylbenzofuran starting from 4-methylbenzofuran, wherein the novel 4-dibromobenzofuran is obtained as an intermediate . According to the method of the invention, 4-methylbenzofuran is brominated to obtain novel 4-dibromobenzofuran, which is then hydrolyzed to obtain 4-formylbenzofuran. 4-Formylbenzofura is a key intermediate in the production of isradipine for the treatment of hypertension and angina.
Owner:圣玛精细化工有限责任公司
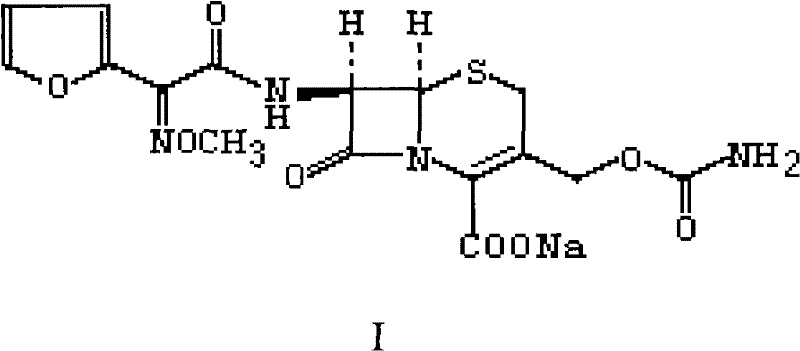
One-step recovery and preparation method of cefuroxime sodium
The invention relates to a one-step recovery and preparation method of cefuroxime sodium. The method comprises: dispersing cefuroxime sodium in a water-containing or non-aqueous mixed solvent of alcohol and ketone, adding hydrochloric acid or sulfuric acid at a temperature of 0-50°C, and reacting for 0.5-3 hours, and cefuroxime sodium is gradually converted into cefuroxime Fuuroctanoic acid is dissolved in the reaction system, and the by-product sodium chloride or sodium sulfate generated simultaneously is insoluble in the reaction system; Then, activated carbon is added for decolorization, filtered, and the by-product and activated carbon are filtered to obtain the cefuroxime acid solution; the obtained Cefuroxime sodium solution is mixed with sodium lactate or sodium isooctanoate aqueous or non-aqueous organic solvent solution to precipitate cefuroxime sodium crystals. The present invention adopts a one-step method for the recovery and preparation of cefuroxime sodium, without the need to separate and dry cefuroxime acid, thereby reducing the damage to cefuroxime acid, and the water content of the system is low during the recovery and preparation process, which is not suitable for unstable cefuroxime sodium Has a good protective effect.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
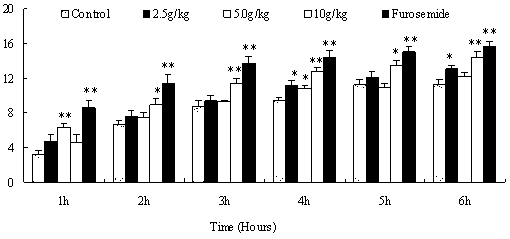
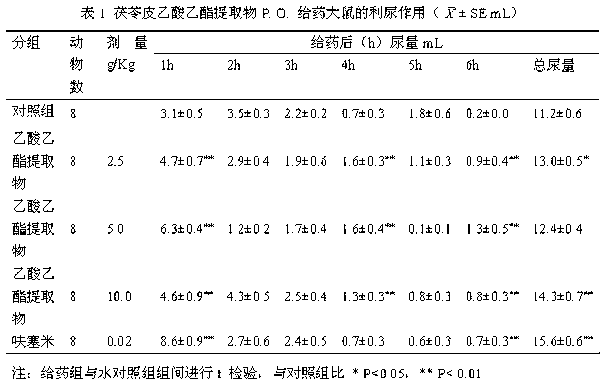
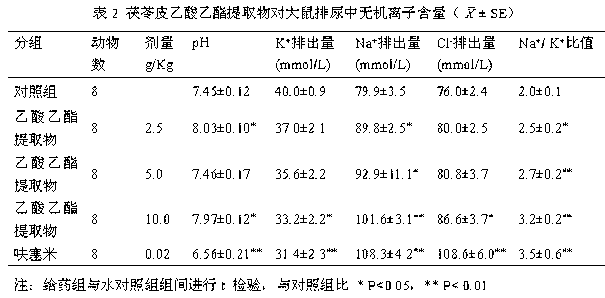
Poria peel extract and application of poria peel extract in preparation of diuretic medicines
The invention discloses a poria peel extract, prepared by the following steps: step (1) of drying and grinding poria peels, then dipping with ethanol and percolating, recovering ethanol, and concentrating to obtain brown pastes; step (2) of dissolving the obtained brown pastes with water, extracting with ethyl acetate, recovering ethyl acetate and concentrating to obtain pastes; step (3) of dissolving pastes obtained in the step (2) in ethanol, mixing with silica gel, loading in silica gel column with 100-200 meshes, eluting with petroleum ether-ethyl acetate eluent, collecting eluent, combining and concentrating to obtain the poria peel extract at the last. The extract provided by the invention has potassium-sparing and sodium excretion actions; diuretic action time of the extract is longer than that of furosemide; and the extract is featured with long-acting performance, low toxic side effects and so on, so that a new candidate diuretic medicine is provided for clinic.
Owner:NORTHWEST UNIV
Pharmaceutical composition for preparing oral solid preparation of indissolvable drugs
InactiveCN107095844ASmall particle sizeReduce aggregationOrganic active ingredientsGranular deliveryDissolutionHot melt
The invention discloses a pharmaceutical composition for preparing an oral solid preparation of indissolvable drugs, wherein the indissolvable drugs include telmisartan, loratadine, berberine hydrochloride and furosemide. Solid granules of the indissolvable drugs can be prepared by mixing the indissolvable drugs with a certain amount of stabilizing agents, and then performing hot-melting extrusion, melting granulation or spray drying. By adopting the technical scheme, the prepared granules are reduced in particle size, reduced in drug granule gathering, and easy to infiltrate and disperse, so that the dissolution rate and dissolution of telmisartan, loratadine, berberine hydrochloride and furosemide in dissolution medium can be increased, and meanwhile the chemical stability cannot be reduced, and the quality of the product is good.
Owner:JIANGSU YABANG AIPUSEN PHARMA
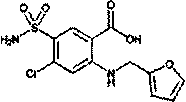
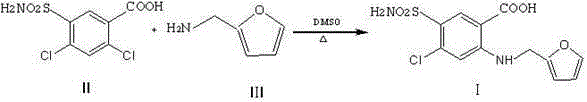
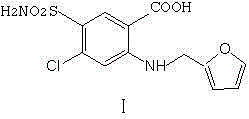
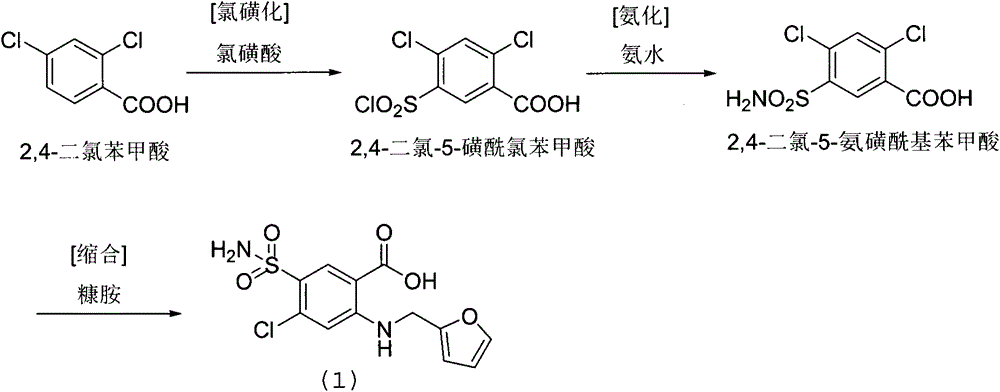
Preparation method of furosemide
The invention provides a preparation method of furosemide and relates to the technical field of pharmaceutical synthesis. The preparation method comprises the following steps: (1) 2,4-dichloro-5-sulfamoylbenzoic acid and alkali are subjected to a reaction in presence of an organic solvent, a reaction liquid is obtained and subjected to aftertreatment, and sodium 2,4-dichloro-5-sulfamoylbenzoic acid is obtained; (2) sodium 2,4-dichloro-5-sulfamoylbenzoic acid and furfurylamine are subjected to a reaction in presence of an organic solvent, furfurylamine and the solvent are recovered through reduced pressure distillation after the reaction, a reaction liquid is obtained and mixed with isopropyl alcohol, a mixture is stirred, crystallized and filtered, and sodium furosemide is obtained; (3) sodium furosemide is subjected to water dissolution, activated carbon decoloration and glacial acetic acid acidification, and a finished furosemide product is obtained. The preparation method has the advantages that raw materials are cheap and available, the cost is low, short time is consumed, reaction steps are short, operation is simple, product quality is good, yield is high, solvents can be recycled and reused, environmental pollution is small and the like, and the preparation method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
Sodium sulfadiazine compound preparation for treating pig brain hydropsy, and preparation method and application thereof
InactiveCN102580096APrescription is scientific and reasonableExcipient safetyAntibacterial agentsHydroxy compound active ingredientsMANNITOL/SORBITOLSulfadiazine
The invention discloses a sodium sulfadiazine compound preparation for treating pig brain hydropsy, and a preparation method and application thereof, and belongs to the technical field of veterinary medicines. The sodium sulfadiazine compound preparation comprises sodium sulfadiazine, mannitol, furosemide, trimethoprim and the like. The sodium sulfadiazine compound preparation is scientific and reasonable in formula, safe in auxiliary material, controllable in quality, high in stability and low in cost, and a preparation process is simple and is suitable for industrial production.
Owner:河南省针剂兽药工程技术研究中心
Preparation method of furosemide freeze-dried powder injection
InactiveCN105030700AControl UniformityShort reconstitution timePowder deliveryOrganic active ingredientsMass ratioFreeze-drying
The invention provides a preparation method of furosemide freeze-dried powder injection. The method comprises the following steps: a, mixing furosemide, an alkaline solution and first water for injection, and then mixing with an excipient after performing pH value adjustment, so as to obtain a mixed solution A; b, mixing the mixed solution A with second water for injection, and sequentially filtering, freezing and drying, so as to obtain the furosemide freeze-dried powder injection; the mass ratio of the first water for injection and the second water for injection is 5:(4 to 6). Compared with the prior art, the preparation method provided by the invention can effectively control the uniformity of the furosemide in the furosemide freeze-dried powder injection, and the redissolving time of products can be reduced. Experimental results show that, the redissolving time of furosemide freeze-dried powder injection obtained by adopting the preparation method provided by the invention is within 3 seconds.
Owner:HUNAN KELUN PHARMA
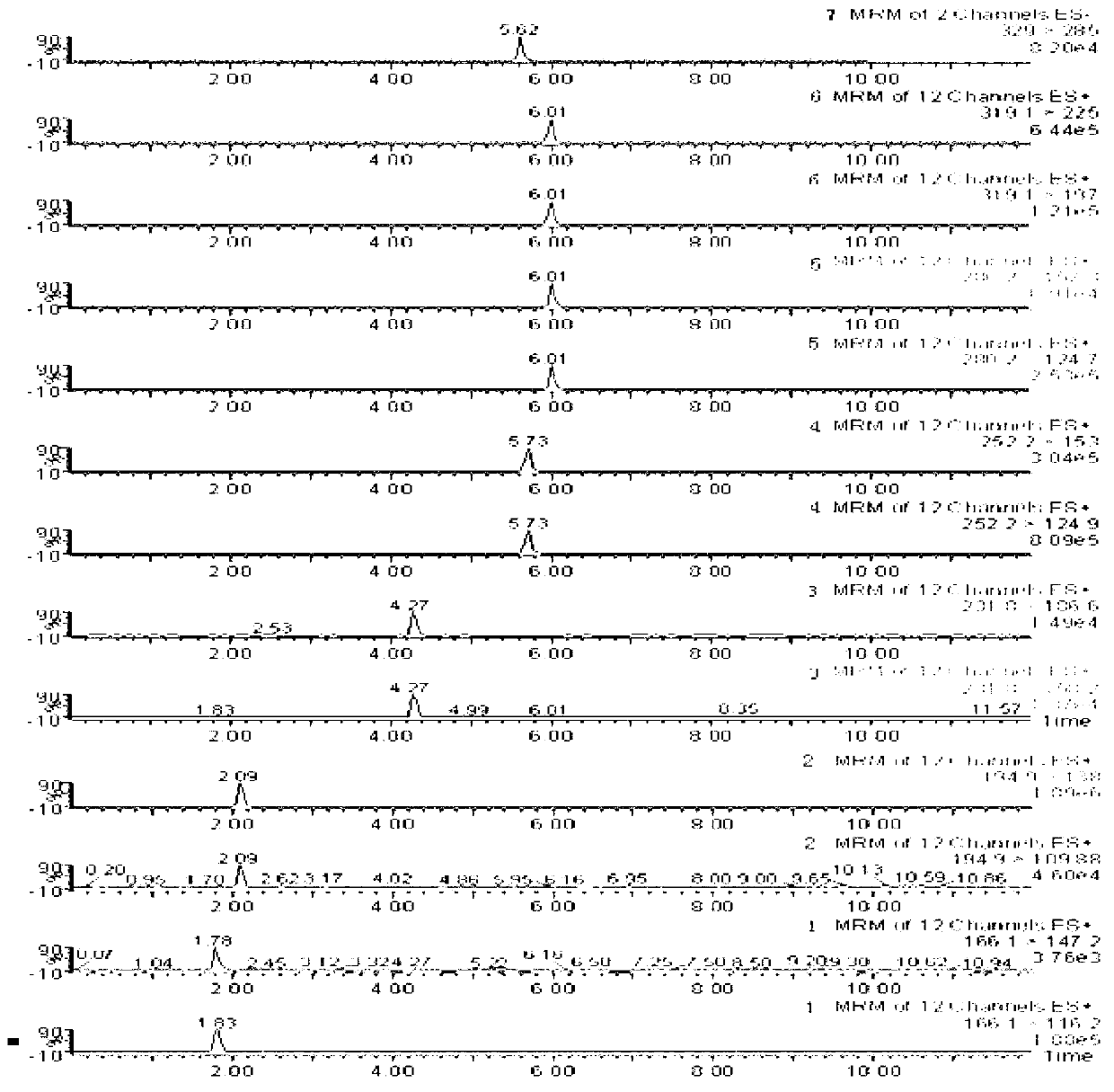
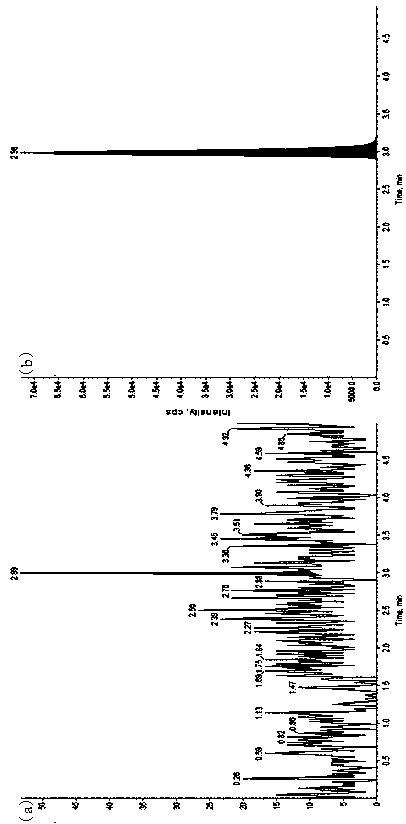
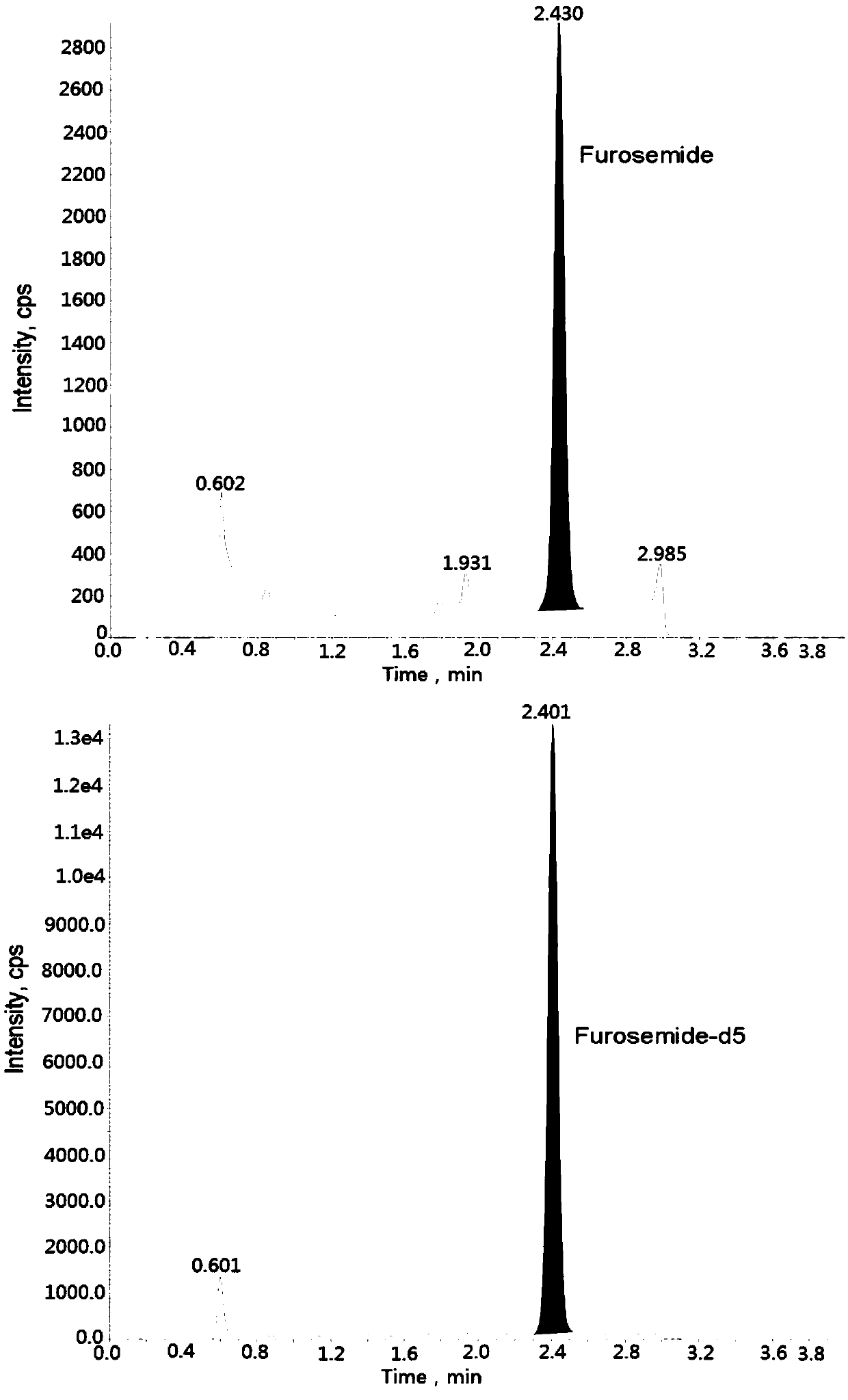
Method for detecting furosemide in human plasma by liquid chromatography-mass spectrometry and application thereof
InactiveCN110133169AGood reproducibilityImprove accuracyComponent separationPretreatment methodEffect factor
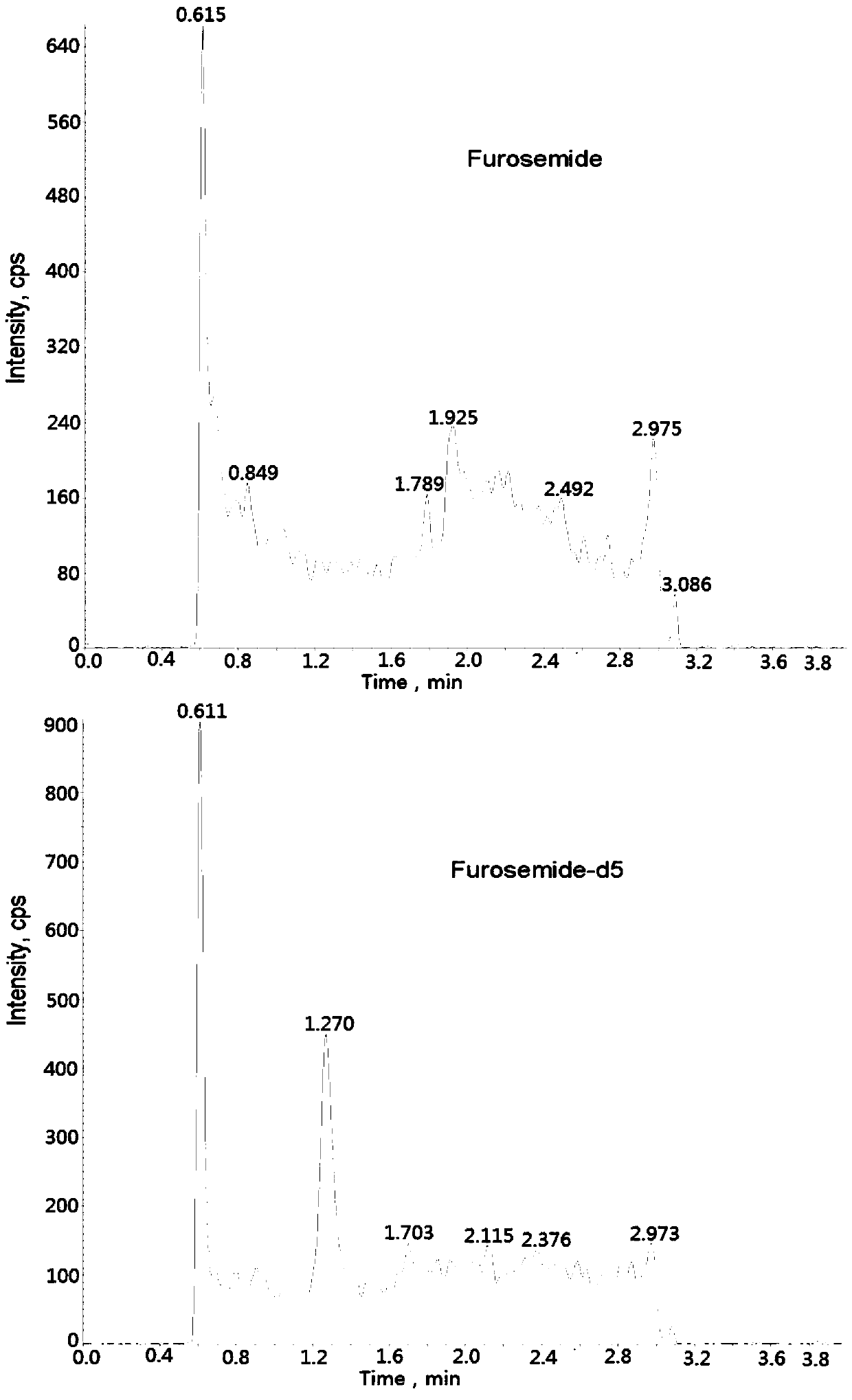
The invention relates to a method for detecting furosemide in human plasma by liquid chromatography-mass spectrometry and application thereof, belonging to the field of biological analysis. The methodadopts a protein precipitation pretreatment method using a deuterated internal standard substance as a precipitating agent, adopts Inertsil C8-3 column isocratic elution, and adopts ESI (ElectroSprayIonization) tandem mass spectrometry detection. Through adoption of the method of the invention, the furosemide has a good linear range of 7.5-1500ng / mL, the furosemide matrix effect factor is 107%,the plasma matrix does not influence the accurate quantification of furosemide, and the extraction recovery rate is 99.2%. The method has the advantages of strong specificity and selectivity, high sensitivity, rapid detection and small dosage, and capacity for meeting the requirements of simple, reliable, high-flux and condition-controllable clinical mass sample analysis. The method of the invention can be used for evaluating the bio-equivalence of each formulation of furosemide.
Owner:TIANJIN LISHENG PHARM CO LTD
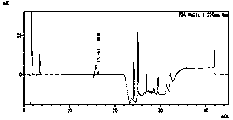
Control method for determining content of furfurylamine in furosemide
InactiveCN110954629AImprove stabilityStrong specificityComponent separationFurfurylaminePhosphoric acid
The invention discloses an analysis method for determining the content of furfurylamine in furosemide, which comprises the following steps: dissolving furosemide with sodium hydroxide, neutralizing furosemide with hydrochloric acid, determining furosemide by high performance liquid chromatography, and taking a mixed solution of phosphate buffer salt and acetonitrile as a mobile phase by using a C18 chromatographic column. The invention provides a method for quickly determining furfurylamine in furosemide, which is strong in specificity, high in sensitivity, high in accuracy, simple and convenient to operate and capable of more effectively controlling the quality of furosemide.
Owner:南京科宁检测科技有限公司
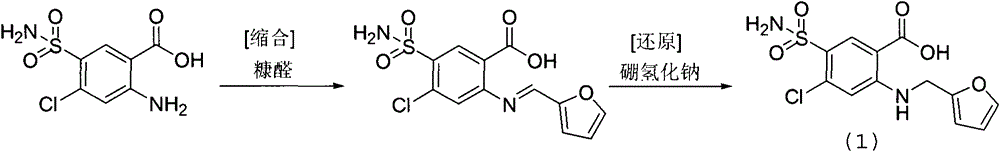
Preparation method of furosemide
The invention belongs to the technical field of pharmacy and discloses a preparation method of furosemide. The preparation method comprises putting 2, 4-dichloro-5-sulfamoylbenzoic acid and an acid-binding agent into an appropriate solvent, heating the solution to a certain temperature in an inert gas protective atmosphere, dropwisely adding furfuryl amine into the solution, after the reaction, adjusting pH through an acid so that crystals are precipitated, filtering the crystals to obtain a furosemide crude product, refining the crude product through an organic solvent-water mixed solvent, adjusting pH to greater than 7, carrying out heating so that the crude product is dissolved, carrying out decoloration through activated carbon, carrying out hot filtration, adjusting pH of the filtrate through an acid so that solids are precipitated and carrying out filtration and drying to obtain furosemide. The preparation method utilizes cheap and easily available raw materials, has a high conversion rate, less side products and high product purity, has simple processes and is suitable for industrial production. A HPLC analysis result shows that purity is greater than or equal to 99.0% and a total yield is 71.2%.
Owner:HEZE CITY FANGMING PHARMA
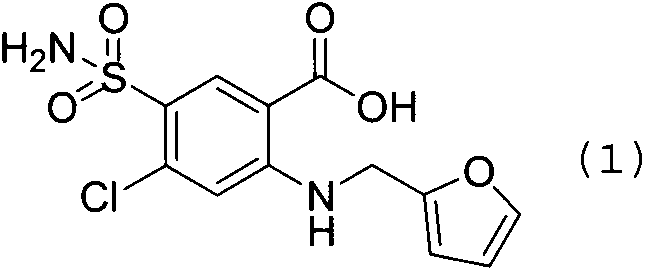
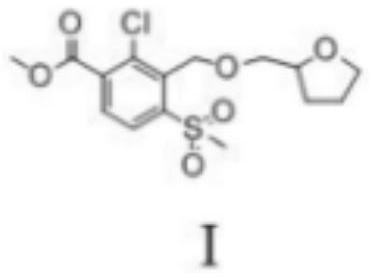
Furosemide preparation method
The invention belongs to the field of pharmaceutical chemicals and particularly relates to a furosemide preparation method. A chemical formula of furosemide is shown as a formula (1) in the specification. The preparation method includes: heating a tetrahydrofuran compound shown as a formula (2) and a 2-aminobenzoic acid compound shown as a formula (3) and / or salts of 2-aminobenzoic acid compound in a reaction solvent, wherein the temperature is controlled to be 80-150 DEG C, and in the formula (2), X refers to halogen, lower alkyl sulfonyloxy, arylsulfonyloxy or araklyl sulfonyloxy; performing nucleophilic reaction under the action of an acid-binding agent and / or catalyst until the reaction of the formula (3) is finished completely, wherein the reaction time is 5-36h; separating and purifying reaction liquid to obtain the furosemide shown as the formula (1). By the furosemide preparation method, high-purity high-yield furosemide preparation can be realized through simple steps without any complex purification steps, the yield is up to 97.0%, and the purity is up to 99.8%. Therefore, the furosemide preparation method has remarkable economic benefits and is pretty advantageous.
Owner:CHANGZHOU YABANG PHARMA
Compound sarafloxacin hydrochloride injection, preparing method and use thereof
InactiveCN101015523AImprove survival rateReduce morbidityAntibacterial agentsOrganic active ingredientsCurative effectSodium hydroxide
The invention discloses a compound sarafloxacin hydrochloride injection, its preparing process and use thereof, wherein the injection comprises (by weight portions) sarafloxacin hydrochloride 5-20 parts, maize flour 0.25-5 parts, ethanol 50-300 parts, methyl glycol 50-300 parts. The preparing process consists of steps of weighing each constituents, charging water for injection and stirring, adjusting acidity to 9.5-11 with hydrochloric acid or sodium-hydroxide, and charging water for injection to 1000ml.
Owner:TIANJIN SHENGJI GRP CO LTD
Preparation method of and application of compound Haqing injection in preparing preparation for atomizing and rectal administration
InactiveCN105287854ASignificant effectSignificant clinical effectAmphibian material medical ingredientsOrganic active ingredientsDiseaseBronchial epithelium
The invention provides a preparation method of and application of a compound Haqing injection in preparing a preparation for atomizing and rectal administration . The preparation is a combination of the compound Haqing injection and one or more of ambroxol, potassium dehydroandrogrpholide succinate, furosemide and dexamethasone, is mainly used for atomizing and rectal administration and has remarkable effect on treating respiratory tract diseases such as respiratory tract infection, bronchial asthma and bronchitis.
Owner:耿福能
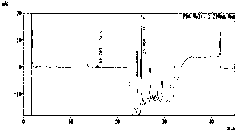
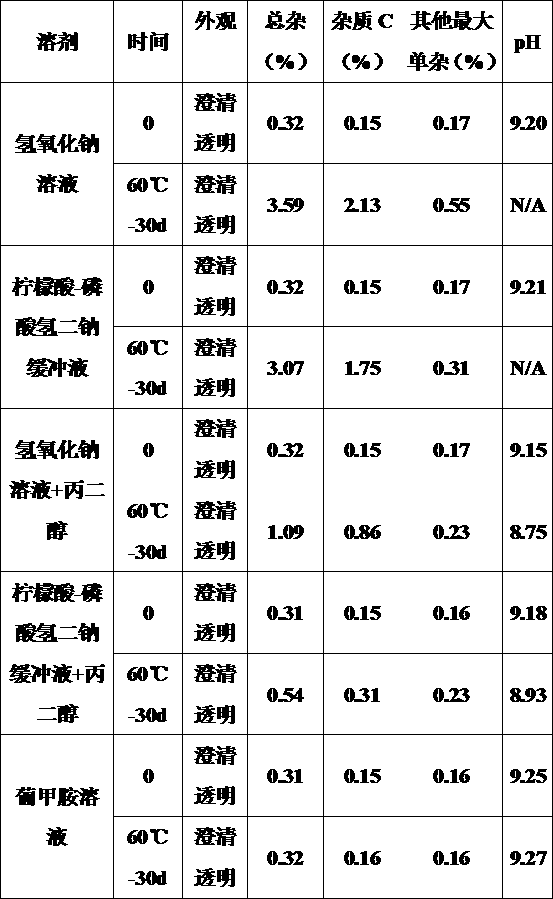
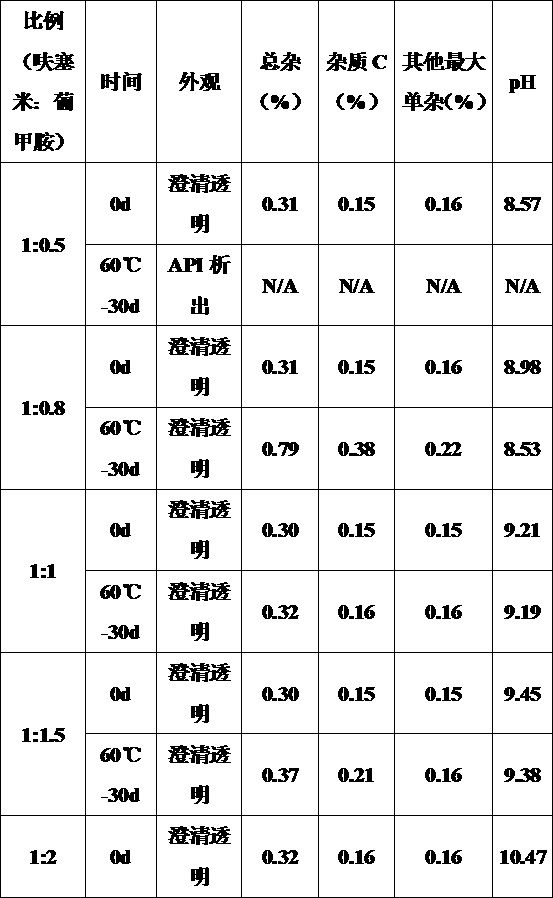
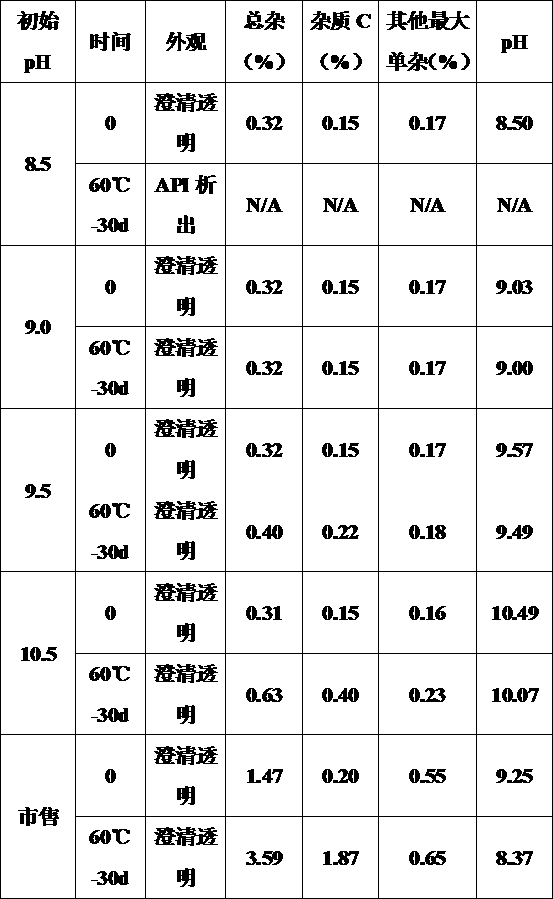
Method for measuring furosemide by high performance liquid chromatography and genetic toxic impurity in its preparation
InactiveCN110389190ARealize separation detectionGuaranteed validityComponent separationPhosphoric acidDiluent
The invention discloses a method for measuring genetic toxic impurity C in furosemide by high performance liquid chromatography. The method comprises using a carbon-18 chromatographic column and usinga mixed solution of phosphoric acid water and acetonitrile as a mobile phase, wherein the volume ratio of the phosphoric acid water to the acetonitrile in the mobile phase is (97 to 85): (3 to 15); and using a mixed solution of acetonitrile and water as a diluent. The invention provides a method for separating and measuring the genetic toxic impurity C in furosemide, which finally simplifies thecontrol method of the genetic toxic impurities, improves the quality control requirement of the furosemide, and ensures the effectiveness and safety of the furosemide.
Owner:南京科宁检测科技有限公司
Furosemide orally taking solution and the application in supersonic inspection
InactiveCN101032466AQuick effectEasy to takeOrganic active ingredientsPharmaceutical delivery mechanismMedicineUltrasonic imaging
The present invention is orally taken furosemide solution and its application in ultrasonic examination. Of the furosemide solution, each 100 ml contains furosemide in 10-30 mg. The orally taken furosemide solution is prepared through adding furosemide into distilled water, regulating pH value and other steps. The orally taken furosemide solution is applied in ultrasonic imaging examination, and can promote urination fast to fill bladder so as to obtain clear ultrasonic image.
Owner:贾恩礼
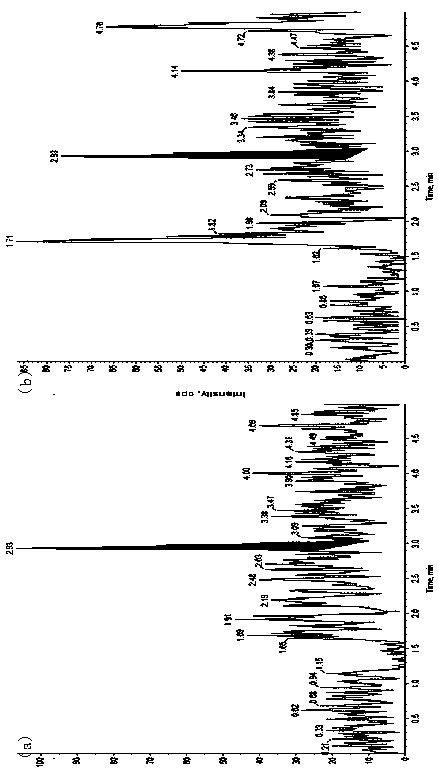
Method for determining concentration of furosemide in plasma by using liquid chromatography-mass spectrometry
InactiveCN109541106AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
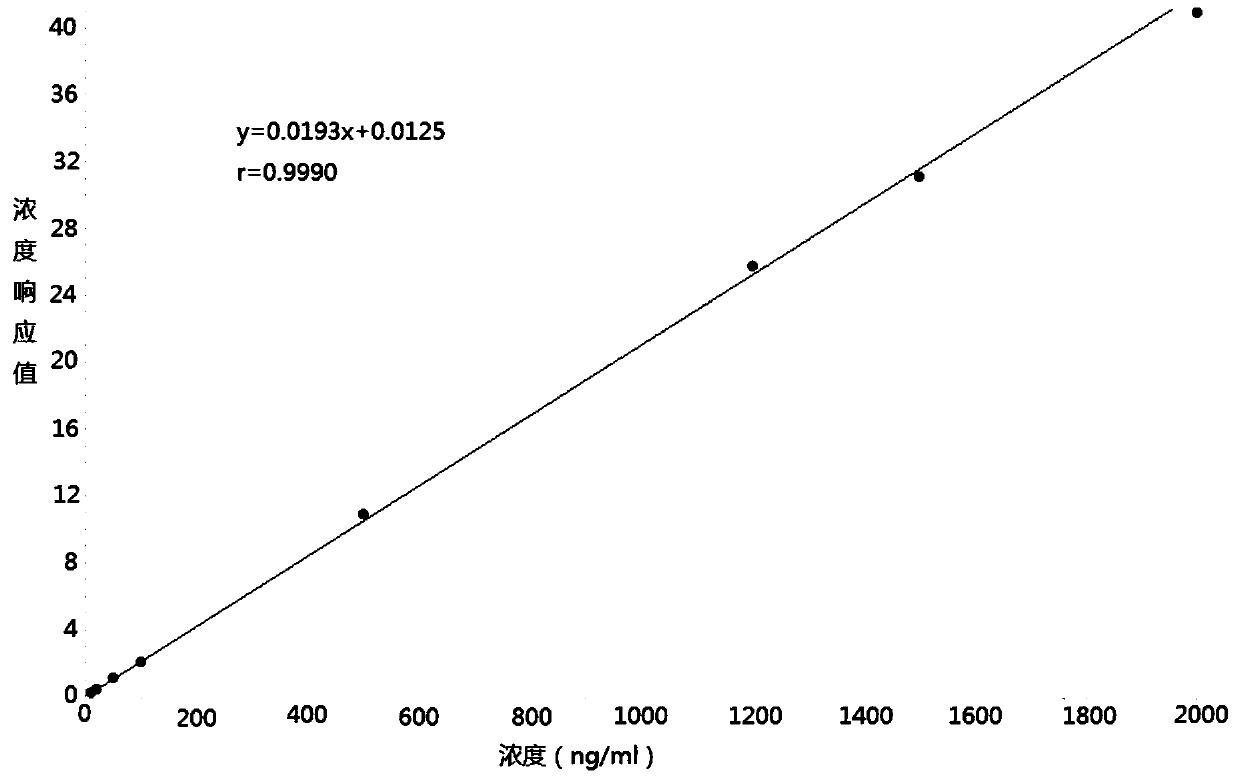
The invention discloses a method for determining the concentration of furosemide in plasma by using liquid chromatography-mass spectrometry. The concentration of furosemide in the plasma is determinedby using a liquid chromatography-mass spectrometry system; taking a sample to be tested first, and adding a certain amount of mixed organic solvent for extraction twice; after pretreatment, performing chromatographic column separation, and then detecting by using a mass spectrometry detector. The method for determining the concentration of furosemide in the plasma by using the liquid chromatography-mass spectrometry disclosed by the invention has the advantages of rapid, accurate, high sensitivity and simple operation, and provides a basis for determining the blood concentration of furosemide; and the plasma standard curve linear range of the method is 10 to 2000 ng / mL, and the intra-assay and inter-assay precisions RSDs are less than + / -15%, which is suitable for determining the concentration of furosemide in the plasma.
Owner:徐州立顺康达医药科技有限公司
Compound injection for preventing and treating edema disease of piglet and preparation method of compound injection
InactiveCN104224805AImprove survival rateReduce morbidityAntibacterial agentsOrganic active ingredientsSodium hydroxideEthanol
The invention discloses a compound injection for preventing and treating an edema disease of a piglet and a preparation method of the compound injection. The compound injection comprises the following components in parts by weight: 5-20 parts of enrofloxacin, 0.25-5 parts of furosemide, 50-300 parts of ethanol, 50-300 parts of propylene glycol and 400-900 parts of injection water. The preparation method comprises the following steps: gradually dissolving the components successively; adjusting the pH to be 9.5-10.5 by using hydrochloric acid or sodium hydroxide; and adding injection water to 1,000 parts, thereby preparing the compound injection. The preparation prepared by the preparation method disclosed by the invention is stable, wide in antibacterial spectrum, fast and significant in curative effect, and high in bioavailability. The experiment proves that the curative effect can be enhanced by joint use of enrofloxacin and furosemide, the compound injection is wide in clinical application, can be used as a choice drug for treating the edema disease of the piglet, the survival rate of the piglet is increased, and the morbidity is reduced.
Owner:TIANJIN JUNHEXIN TECH DEV
Frusemide oral solution and preparation method thereof
InactiveCN109303765AReduce usageEasy accessOrganic active ingredientsDispersion deliveryMedicineAromatic agent
The invention discloses a frusemide oral solution and a preparation method thereof. The oral solution is prepared from frusemide, meglumine, a corrigent, an aromatic agent, a coloring agent and purified water, wherein the meglumine is a co-solvent as well as a stabilizer. The oral solution disclosed by the invention has good stability under high temperature conditions, convenient storage, convenient administration, excellent mouthfeel and high compliance.
Owner:南京泽恒医药技术开发有限公司
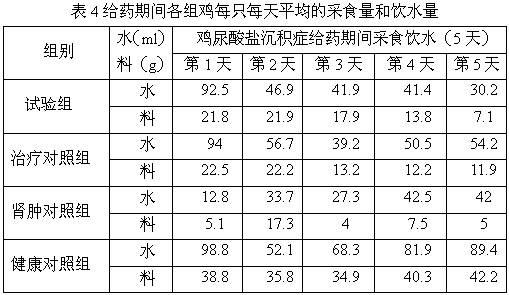
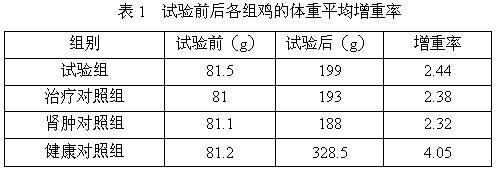
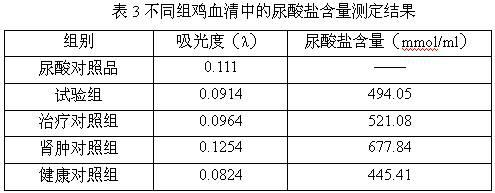
Medicine composition for treating nephrosis of chicken
InactiveCN102198154AThe ratio is scientific and reasonableHydroxy compound active ingredientsInorganic phosphorous active ingredientsTreatment effectExcipient
The invention relates to a medicine composition for treating nephroses of chicken, which is characterized by comprising the13661304957 13240789042 13240119209 raw materials according to the following ratio: 8-12 g of furosemide, 10-15 g of disodium hydrogen phosphate, 2-10 g of vitamin A and 60-80 g of excipient, wherein the excipient is water-soluble starch. The medicine composition in the invention has scientific and reasonable proportion, and is mainly used for treating renal cyst, urate aggradation, spotted kidney, and the like caused by a plurality of reasons, such as fowl kidney type infectious bronchitis, bursa of fabricius, poisoning, gout, ascites, and the like, and has a better treating effect for treating the chicken's nephroses caused by high protein feed and high calcium drinking water. The treating effect of the medicine composition is better than that of the common medicine for treating renal cyst.
Owner:HENAN SOAR VETERINARY PHARMA
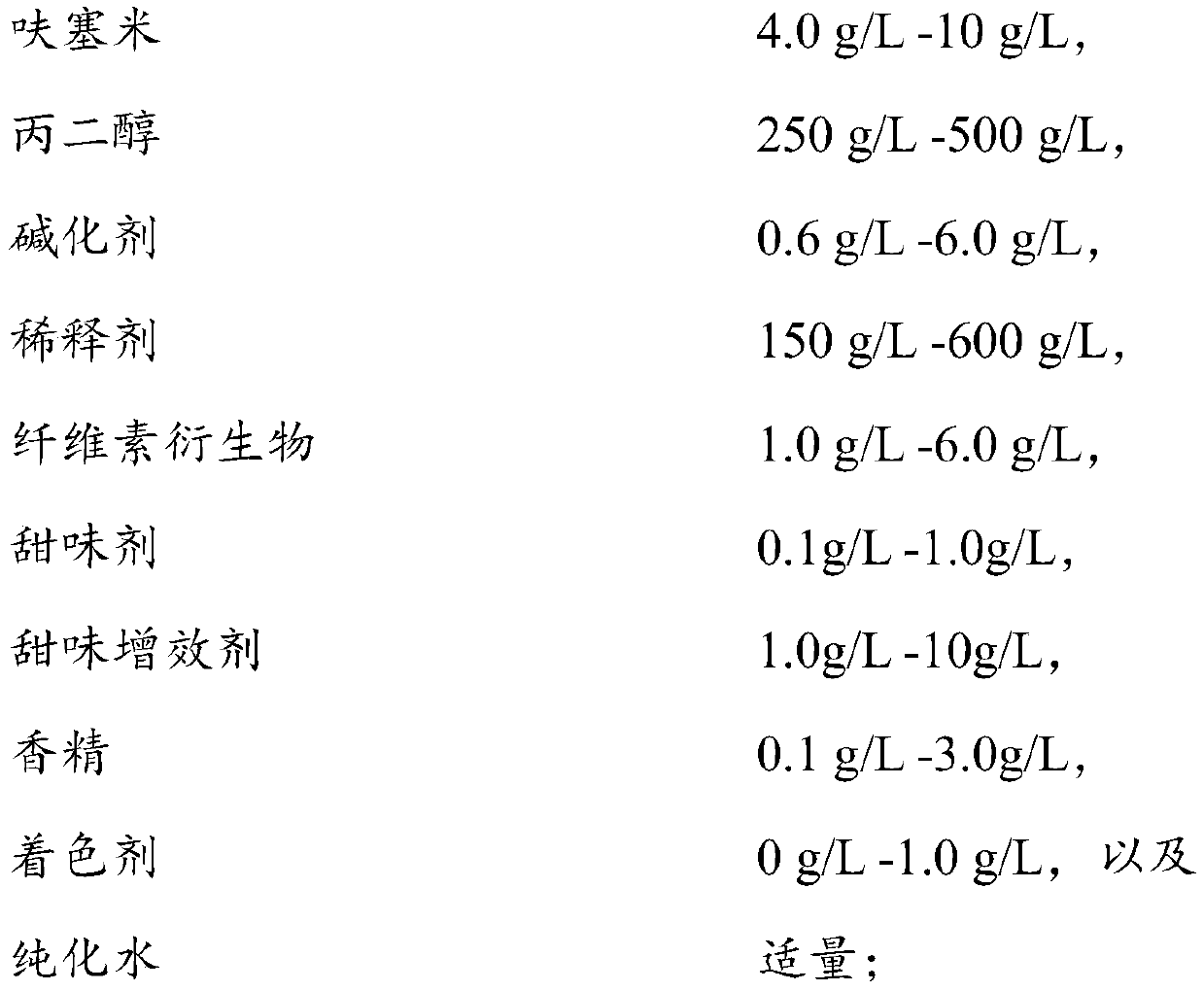
Furosemide oral solution and preparation method thereof
PendingCN111467305AImprove long-term stabilityImprove astringencyOrganic active ingredientsDispersion deliverySweetnessPerylene derivatives
The invention relates to a furosemide oral solution and a preparation method thereof. The furosemide oral solution contains a cellulose derivative and a sweet synergist with specific contents, and iscompounded with furosemide, propylene glycol, a diluent, a sweetening agent and essence; and on the premise of ensuring long-term stability of a preparation, the astringent taste of the conventional furosemide oral solution can be improved into a smooth, fragrant, sweet and rich taste on the whole.
Owner:JIANMIN PHARMA GRP CO LTD
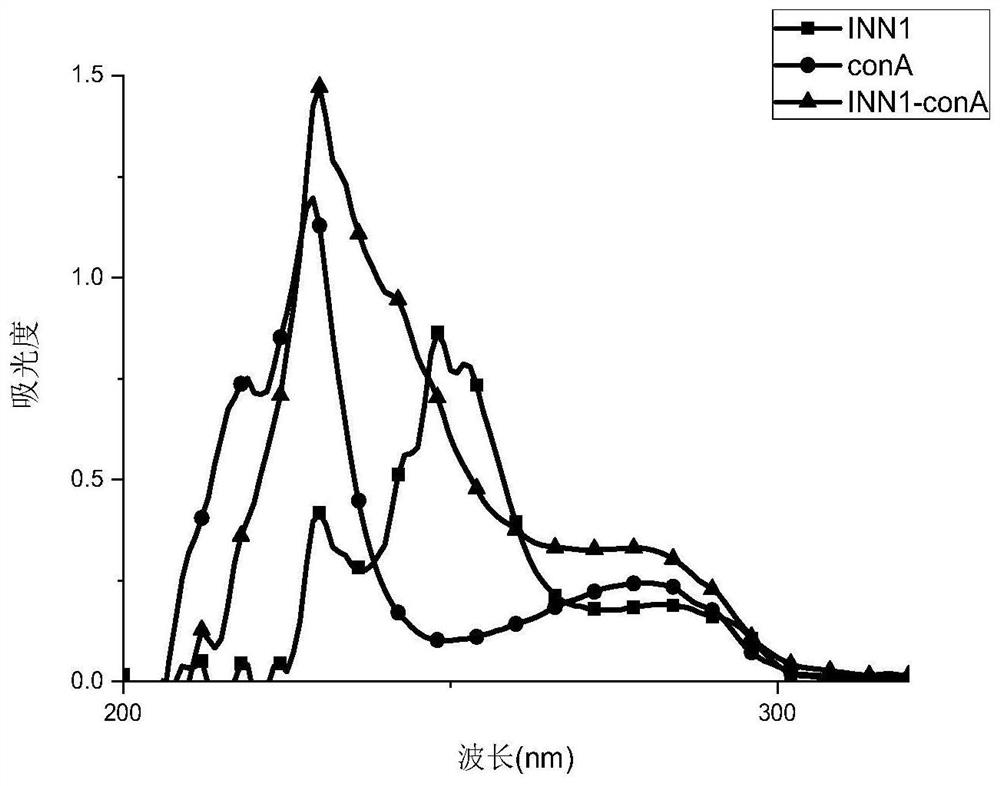
Furosemide artificial antigen, antibody and application of antibody in detection of furosemide
The invention discloses a furosemide artificial antigen, an antibody and application of the antibody in detection of furosemide. The furosemide artificial antigen is obtained by coupling carrier protein to carboxyl of 2, 4-dichloro-5-sulfamoylbenzoic acid; the antibody is obtained by immunizing an animal with the furosemide artificial antigen. The artificial antigen is prepared by taking the 2, 4-dichloro-5-sulfamoylbenzoic acid as a specific hapten structure to replace an analysis object furosemide; the animal is immunized to obtain the high-specificity furosemide antibody, the lowest detection limit of the antibody to the furosemide is 0.58ng / mL, and the half-inhibition concentration is 12.6ng / mL; and an immune rapid detection method for the furosemide in health food is established, thebreakthrough of rapidly detecting the furosemide in the health food is achieved, and the furosemide artificial antigen has great significance in safety supervision of illegally added drugs in weight-losing health food.
Owner:SOUTH CHINA AGRI UNIV
Furosemide chewable tablets for dog or cat
InactiveCN101190192ABreak through the shortcomings of poor palatabilityHigh cure rateOrganic active ingredientsPill deliveryDiseaseOral glucose
The invention discloses a furosemide chewable tablet used for dogs and cats. The invention overcomes the poor palatability defect of the prior tablet to promote the thorough chewing absorption by dogs and cats, so as to effectively guarantee the medicine-supply dose, to enhance the cure rate of diseases of dogs and cats and to reduce the medicine waste. The tablet of the invention comprises the following components represented by weight-percentage: 1 to 5 percent of aspartame; 20 to 30 percent of a mixture of oral dextrose and degreased milk powder; the ratio of 1:1 to 1:4 between the oral dextrose and degreased milk powder in the mixture; 40 to 60 percent of excipient; 0.5 to 1 percent of glidant and 10 to 30 percent of furosemide.
Owner:TIANJIN RINGPU BIO TECH
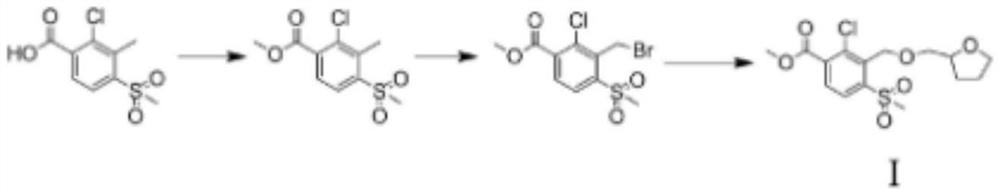
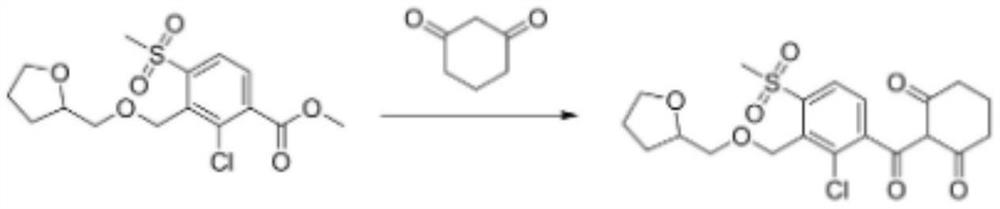
A kind of preparation method of furansulcotrione
The invention provides a preparation method for tefuryltrione, and belongs to the technical field of compound preparation. The preparation method comprises the following steps that esterification reaction is carried out on 2-chloro-3-methyl-4-methylsulfonylbenzoic acid serving as a raw material and methanol so as to generate methyl 2-chloro-3-methyl-4- methanesulfonylbenzoate; then reaction is carried out with N-bromosuccinimide to replace one hydrogen in a methyl group on a benzene ring so as to generate methyl 2-chloro-3-methyl bromide-4-methanesulfonylbenzoate; then a product of methyl 2-chloro-4-(methylsulfonyl)-3-(((tetrahydrofuran-2-yl)methoxy)methyl) benzoate is synthesized by using a Williamson synthesis method; and then condensation reaction is carried out with cyclohexanedione under the action of ethylene diamine so as to generate enol ester, and finally, acetonitrile is added for rearrangement catalysis under the action of the ethylene diamine so as to obtain a target product of the tefuryltrione. The preparation method has the advantages of being mild in reaction conditions and simple in synthesis steps.
Owner:安徽工大化工科技有限公司
Furosemide micro-tablet and preparation method thereof
PendingCN107519139AEasy to swallowGuaranteed curative effectOrganic active ingredientsNervous disorderMedicineFluidized bed
The invention discloses a furosemide micro-tablet preparation and a method for preparing the furosemide micro-tablet. The method comprises the following steps: taking furosemide, an adhesive, a diluent and other raw materials, performing top-spraying fluidized bed pelletization and other preparation methods, thereby obtaining the furosemide micro-tablet with excellent compressibility. The furosemide micro-tablet disclosed by the invention can be repeatedly administrated at a small dose, particularly for crowds with great individual metabolic differences, such as the old and infants, the administration can be counted so as to achieve accurate administration, and for crowds with difficulty of taking the conventional preparation, the compliance with medication can be improved. Moreover, the method and raw materials used in the invention are convenient for industrialized production.
Owner:北京科信聚润医药科技有限公司
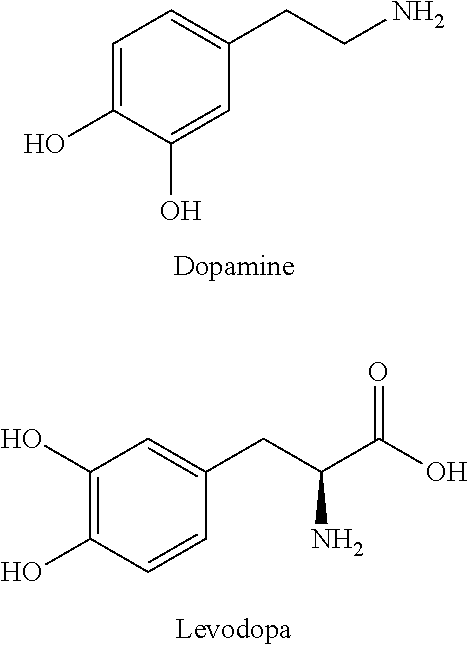
Methods of Treating Ascites
The invention relates to the treatment of ascites, and especially refractory ascites, with an orally bioavailable prodrug of dopamine. A preferred prodrug of dopamine is docarpamine. In one embodiment treated patients are, prior to treatment, on doses of furosemide >80 mg / day and / or spironolactone >100 mg / day or equivalent doses of an alternative loop-acting and / or distal-acting diuretic. The ascites treated by the invention are typically caused by liver cirrhosis due to alcohol or non-alcoholic fatty liver disease and generally not due to viral hepatitis or primary biliary cholangitis. Preferred dosing is greater that 2250 mg per day, with more preferred doses exceeding 3500 mg per day.
Owner:MACALLISTER THOMAS W +1
Pharmaceutical composition for preventing and treating poultry ascites
ActiveCN1732946APromote recoveryReduce lossesBlood disorderHeterocyclic compound active ingredientsAscitesFurosemide
Owner:TIANJIN RINGPU BIO TECH
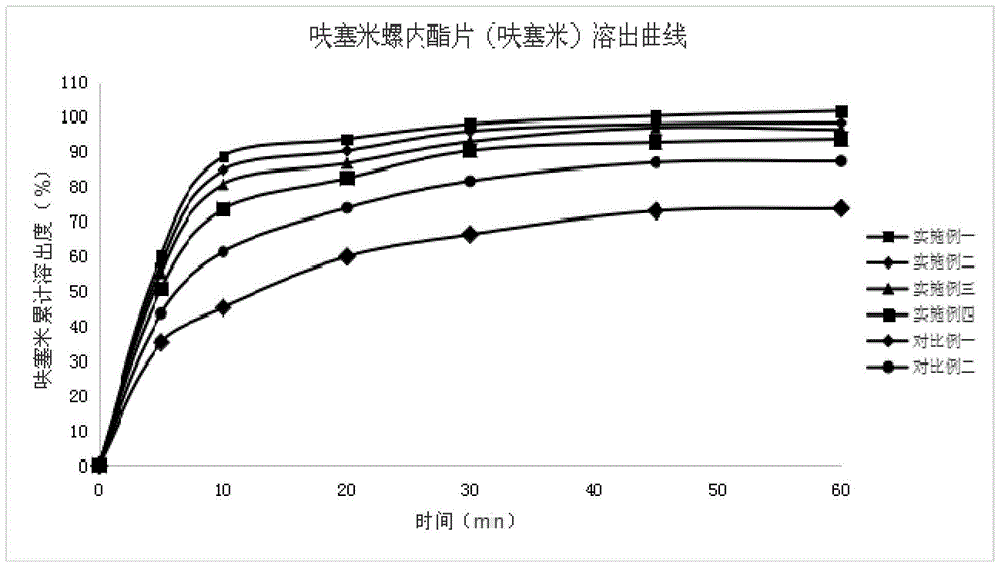
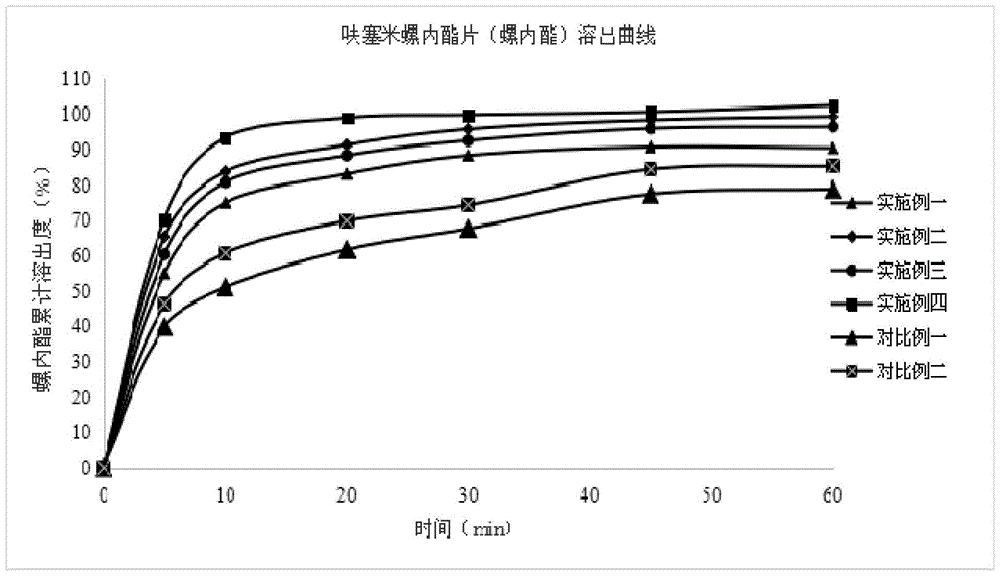
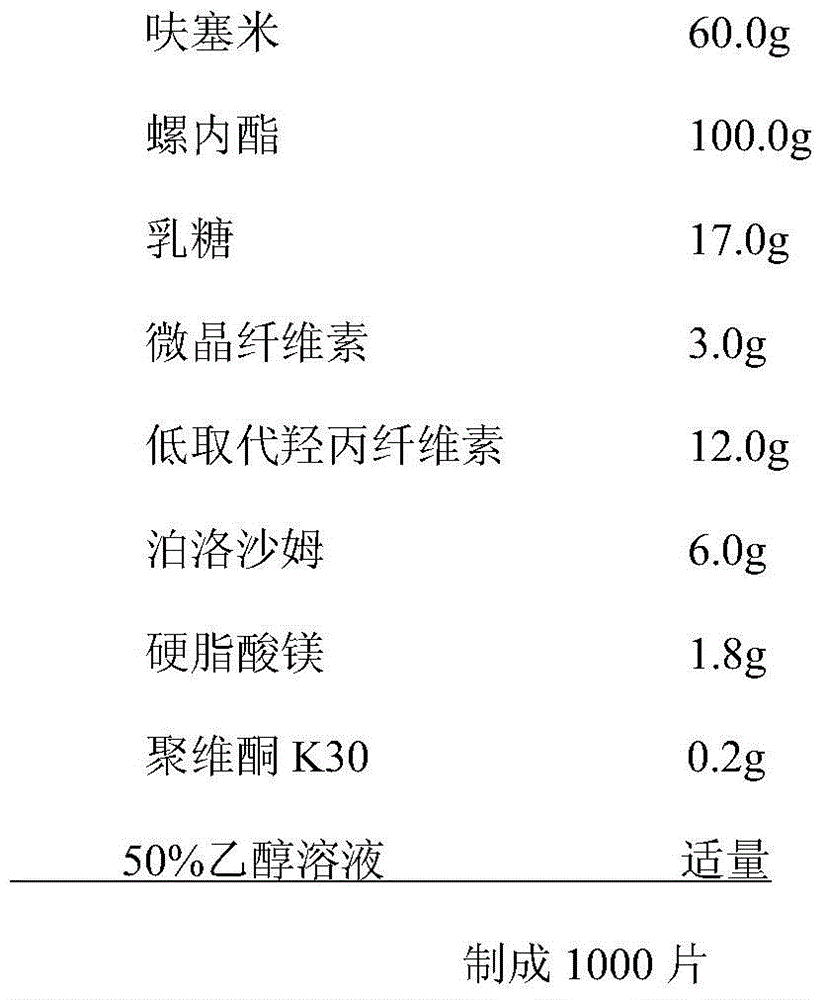
Compound antihypertensive drug combination preparation containing furosemide and spirolactone and preparation method of compound antihypertensive drug combination preparation
InactiveCN105687210AIncrease dissolution rateSmall particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsAntihypertensive drugLubricant
The invention discloses a compound antihypertensive drug combination preparation containing furosemide and spirolactone and a preparation method of the compound antihypertensive drug combination preparation. The compound antihypertensive drug combination preparation comprises a tablet core and a coating, wherein the tablet core is prepared from the following components counted according to the total weight of the table core: 10 to 40 percent of furosemide, 20 to 50 percent of spirolactone, 2 to 4 percent of solubilizer, 10 to 60 percent of filling agent, 4 to 8 percent of disintegrating agent, 0.5 to 1 percent of lubricating agent and 0.1 to 2 percent of binding agent.
Owner:KANGYA OF NINGXIA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com