Furosemide preparation method
A technology of furosemide and tetrahydrofuran, which is applied in the preparation field of furosemide, can solve problems such as unfavorable industrialized production, complicated operation and the like, and achieve the effect of remarkable economic benefit and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
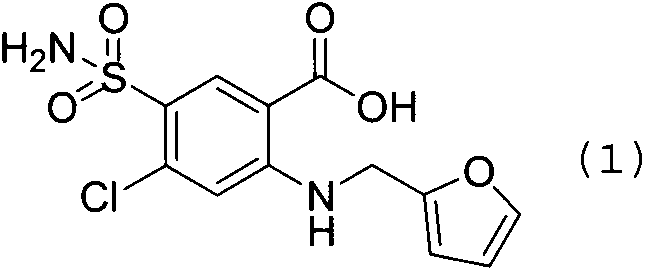
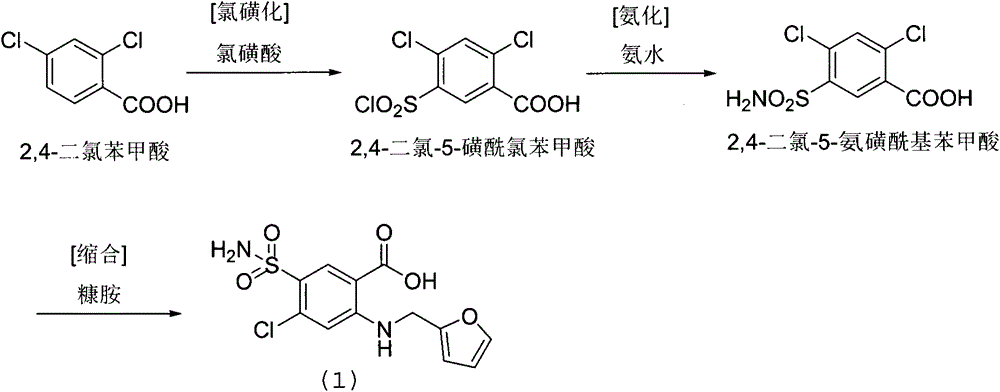
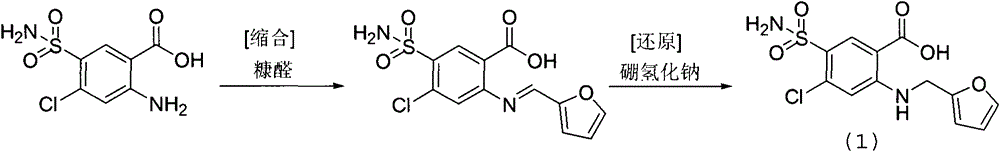
[0036] In the reaction flask, add DMF1000ml, 2-amino-4-chloro-5-sulfamoylbenzoic acid (250.7 grams, 1.0mol), 2-chloromethylfuran (186.4 grams, 1.6mol), potassium bromide (9.5 grams , 0.08mol), heated and stirred at 120 degrees Celsius for 24 hours, after cooling, add 5000ml of water, adjust pH=5 with hydrochloric acid, drop to room temperature, precipitate crystals, filter with suction, rinse the filter cake with ethyl acetate, and dry to obtain 2 -[(2-furylmethyl)amino]-5-(sulfamoyl)-4-chlorobenzoic acid 320.8 g, yield 97.0%, purity 99.8%.
[0037] The purity of furosemide was determined by high performance liquid chromatography (HPLC) under the following conditions:
[0038] Column: Megres TM C18 (Hanbang Technology)
[0039] Eluent: water / tetrahydrofuran / glacial acetic acid=70 / 30 / 1
[0040] Flow rate: 1.0mL / min
[0041] Detection wavelength: 272nmUV
Embodiment 2
[0043] In the reaction flask, add ethylene glycol 1200ml, 2-amino-4-chloro-5-sulfamoylbenzoic acid (250.7 grams, 1.0mol), 2-chloromethylfuran (139.9 grams, 1.2mol), copper iodide (31.7 grams, 0.1mol), 160 degrees centigrade heating and stirring reaction 24 hours, after cooling, add water 5000ml, adjust pH=5 with hydrochloric acid, drop to room temperature, precipitate crystal, suction filter, filter cake rinses with ethyl acetate, bakes 269.2 g of 2-[(2-furylmethyl)amino]-5-(sulfamoyl)-4-chlorobenzoic acid was obtained by drying, with a yield of 81.4% and a purity of 99.6%.
[0044] The purity of furosemide was determined by high performance liquid chromatography (HPLC) under the following conditions:
[0045] Column: Megres TM C18 (Hanbang Technology)
[0046] Eluent: water / tetrahydrofuran / glacial acetic acid=70 / 30 / 1
[0047] Flow rate: 1.0mL / min
[0048] Detection wavelength: 272nmUV
Embodiment 3
[0050]In the reaction flask, add DMF1000ml, 2-amino-4-chloro-5-sulfamoylbenzoic acid (250.7 grams, 1.0mol), 2-chloromethylfuran (256.3 grams, 2.2mol), sodium bromide (14.4 grams , 0.14mol), 100 degrees Celsius heating and stirring reaction for 24 hours, after cooling, add 5000ml of water, adjust pH=5 with hydrochloric acid, drop to room temperature, precipitate crystals, filter with suction, filter cake rinse with ethyl acetate, dry to obtain 2 -[(2-furylmethyl)amino]-5-(sulfamoyl)-4-chlorobenzoic acid 298.7 g, yield 90.3%, purity 99.8%.
[0051] The purity of furosemide was determined by high performance liquid chromatography (HPLC) under the following conditions:
[0052] Column: Megres TM C18 (Hanbang Technology)
[0053] Eluent: water / tetrahydrofuran / glacial acetic acid=70 / 30 / 1
[0054] Flow rate: 1.0mL / min
[0055] Detection wavelength: 272nmUV
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com