Furosemide artificial antigen, antibody and application of antibody in detection of furosemide
An artificial antigen, furosemide technology, applied in the direction of biological testing, measuring devices, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis and identification of embodiment 1 furosemide immunogen
[0042] 1, the synthetic method of furosemide immunogen
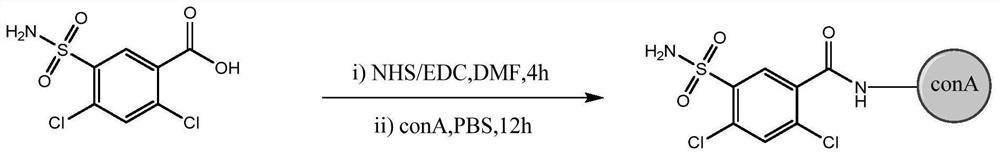
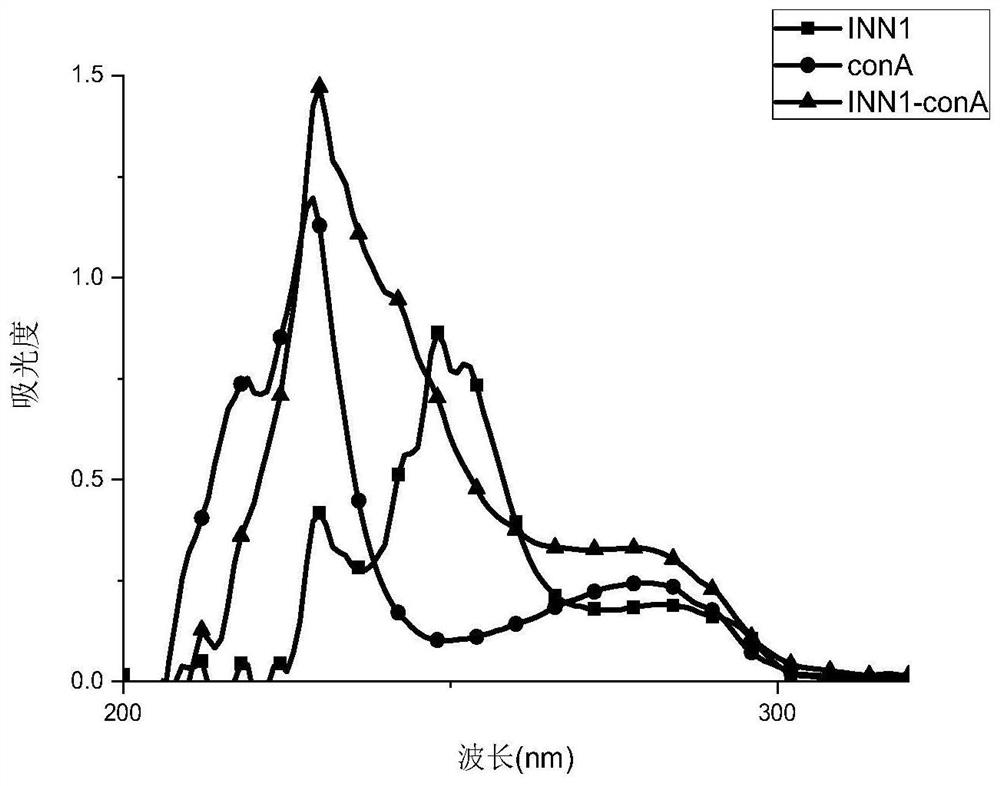
[0043] Using 2,4-dichloro-5-sulfonamidobenzoic acid as a hapten, INN1-conA was synthesized by coupling soybean protein (conA) through the active ester method. The synthetic route is as follows figure 1 shown.
[0044](1) Accurately weigh 2 mg of 2,4-dichloro-5-sulfonamidobenzoic acid, dissolve 1 mg of NHS and 2 mg of EDC in 150 μL DMF, and stir for 4 hours at room temperature in the dark to obtain INN1 activation solution;
[0045] (2) Add 10mg of soybean protein (conA) to 1mL of PBS buffer (0.01moL / L, pH=7.4);
[0046] (3) Slowly add the INN1 activation solution to the conA solution drop by drop, and react at 4°C for 12 hours;
[0047] (4) Dialyze with PBS buffer for two days, 4 times a day. After the dialysis, dilute the protein solution to 2ml to obtain a 5mg / ml protein conjugate, namely furosemide immunogen INN1-conA, which is packed in a c...
Embodiment 2
[0052] Synthesis and identification of embodiment 2 furosemide coating former
[0053] 1. Cationicization of carrier protein
[0054] (1) Weigh 0.675g bovine serum albumin BSA (0.015mmol) and 56mg ethyl-[3-(dimethylamino)propyl]carbodiimide (EDC) (0.3mmol) and dissolve in 20mL PBS buffer (0.01moL / L, pH 7.4), placed on a magnetic stirrer and stirred at room temperature for 30min to fully dissolve;
[0055] (2) Slowly add the above-prepared buffer into 20 mL of PBS buffer (0.01 moL / L, pH 7.4) dissolved with 18 mg of ethylenediamine (0.3 mmol), and place on a magnetic stirrer and stir at room temperature for 120 min;
[0056] (3) Transfer the prepared mixed solution to a dialysis bag, use PBS buffer solution (0.01moL / L, pH 7.4) as the dialysate, place it at 4°C for 3 days with stirring and dialyze, and replace the dialysate every 6 hours, Centrifuge the dialyzed solution at 4000r / min, 4°C for 30min to remove excess ethylenediamine, collect the supernatant and lyophilize, the wh...
Embodiment 3
[0066] Example 3 Preparation of Furosemide Polyclonal Antibody
[0067] The prepared immunogen INN1-CONA was evenly emulsified with an equal amount of immune adjuvant (incomplete Freund's adjuvant was used for the first immunization, and incomplete Freund's adjuvant was used for subsequent booster immunizations), and animals were immunized. The 2.5-3kg New Zealand white rabbits were immunized by subcutaneous injections on the back, subcutaneous injections in various parts, leg muscles and ear veins respectively. The second immunization was done after 4 weeks, and the booster immunization was performed every 3 weeks thereafter. One week after the third booster immunization, blood was collected from the ear vein, and the serum titer was determined by indirect competitive ELISA. When the titer no longer rises, the ear vein is used to boost the immunization. One week later, blood was collected from the heart, bathed in water for 0.5-1 hour, centrifuged at 4°C and 10,000 rpm for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com