Method for measuring furosemide by high performance liquid chromatography and genetic toxic impurity in its preparation
A high-performance liquid chromatography and furosemide technology, applied in the field of analytical chemistry, can solve the problems of weak ultraviolet response and neglected control, and achieve the effect of ensuring effectiveness and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Instrument
[0031] High performance liquid chromatography: Shimadzu, LC-2030C, UV detector.
[0032] Column: Ultimate AQ-C 18 (4.6mm × 250mm, 5μm).
[0033] 2. Chromatographic conditions.
[0034] Mobile phase: the aqueous phase is phosphoric acid aqueous solution; the organic phase is acetonitrile.
[0035] Elution gradient:
[0036]
[0037] Flow rate: 1.0mL / min;
[0038] Column temperature: 35°C;
[0039] Detection wavelength: 270nm;
[0040] Injection volume: 20μL;
[0041] The diluent is a mixed solution of acetonitrile and water.
[0042] 3. Experimental steps.
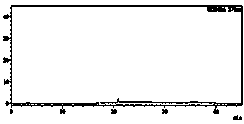
[0043] Weigh 20mg of the impurity C reference substance, put it in a 10mL volumetric flask, add diluent to dissolve and dilute to the mark, and then quantitatively dilute to 1μg / mL solution successively to obtain the system suitability solution.
[0044] Weigh 100mg of furosemide respectively, put it in a 10mL volumetric flask, add diluent to dilute to the mark, and use it as the test sol...
Embodiment 2
[0049] 1. Instrument
[0050] With embodiment 1;
[0051] Column: Thermo BDS C 18 (4.6mm × 250mm, 5μm).
[0052] 2. Chromatographic conditions.
[0053] Mobile phase: the aqueous phase is phosphate buffered saline; the organic phase is acetonitrile;
[0054] Elution gradient: same as Example 1;
[0055] Flow rate: with embodiment 1;
[0056] Column temperature: with embodiment 1;
[0057] Detection wavelength: 220nm, 277nm;
[0058] Injection volume: 10μL;
[0059] Diluent is with embodiment 1.
[0060] 3. Experimental steps.
[0061] System suitability solution: same as Example 1;
[0062] Weigh an appropriate amount of furosemide injection respectively, add diluent to dilute properly, and use it as the test solution.
[0063] Detection was carried out according to the chromatographic conditions mentioned above.
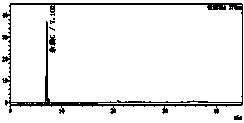
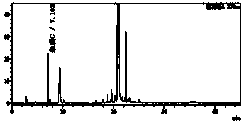
[0064] Under the above detection conditions, prepare impurity B and C reference substance solutions of different concentrations, and detect them respect...
Embodiment 3
[0069] 1. Instrument
[0070] With embodiment 1;
[0071] Chromatographic column: with embodiment 1.
[0072] 2. Chromatographic conditions
[0073] With embodiment 2;
[0074] Diluent is with embodiment 1.
[0075] 3. Experimental steps.
[0076] System suitability solution: same as embodiment 1
[0077] Get the furosemide tablet and grind it, accurately weigh an appropriate amount, add a diluent to dilute properly, and use it as the test solution.
[0078] Detection was carried out according to the chromatographic conditions mentioned above.
[0079] Six parts of the test solution were prepared in parallel, and the average impurity C content was determined to be 37ppm, and the RSD value of the measurement result was 1.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com