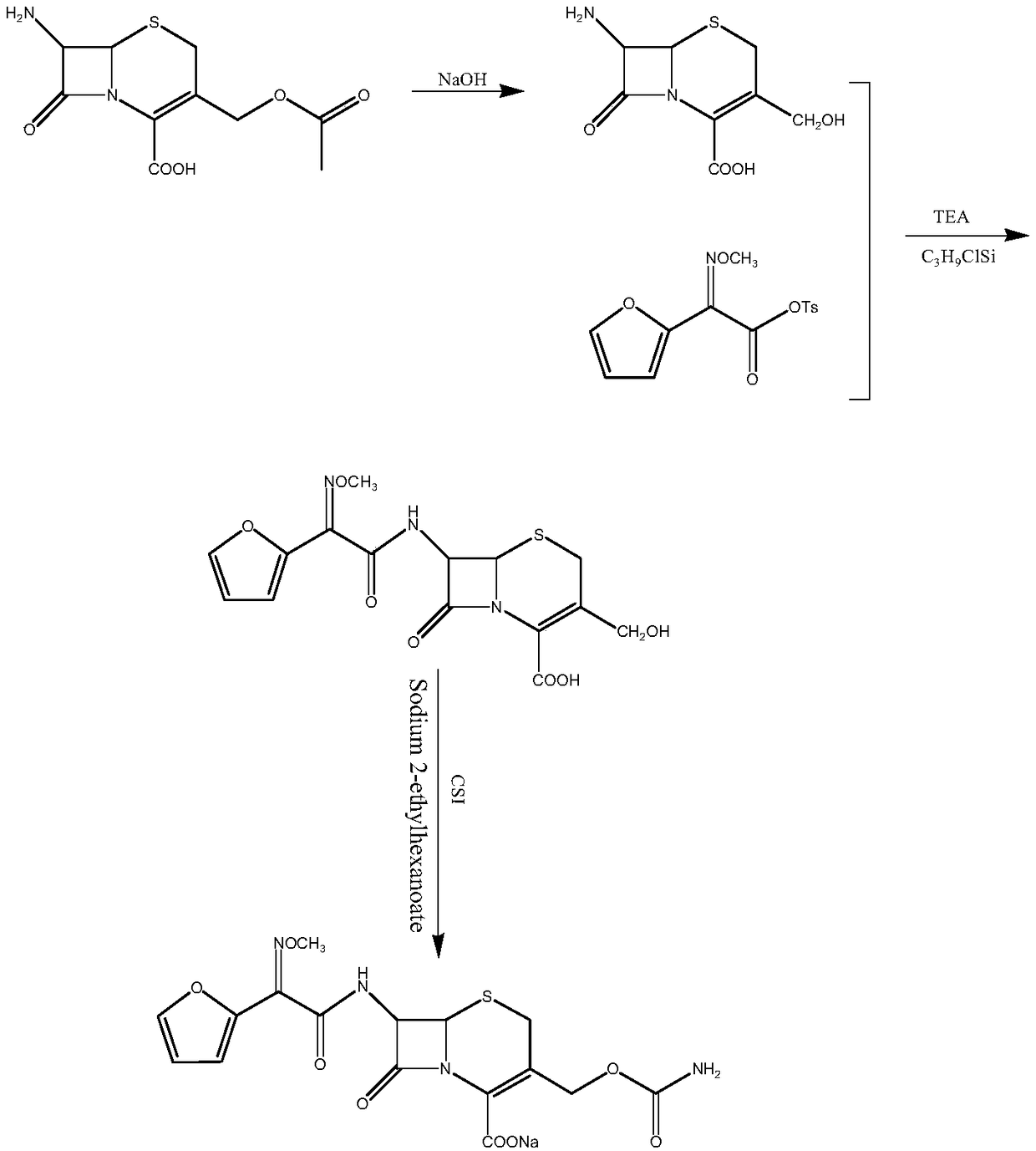
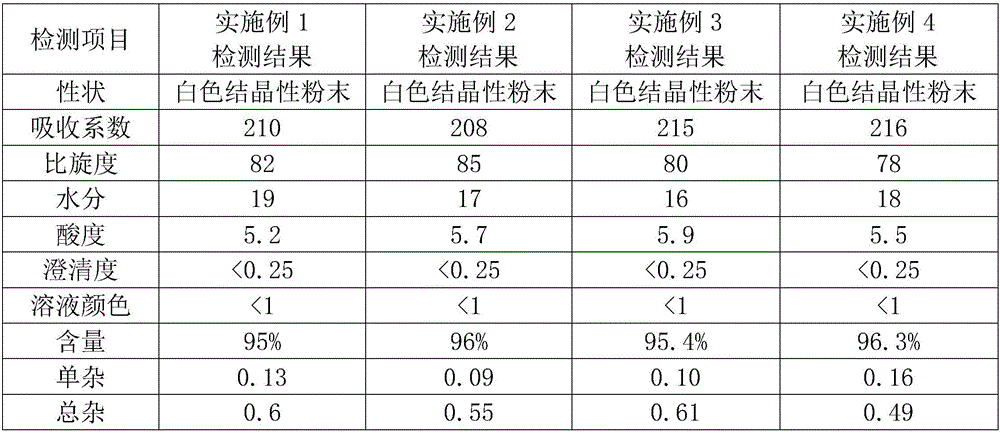
Patents
Literature
105 results about "Cefuroxime Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
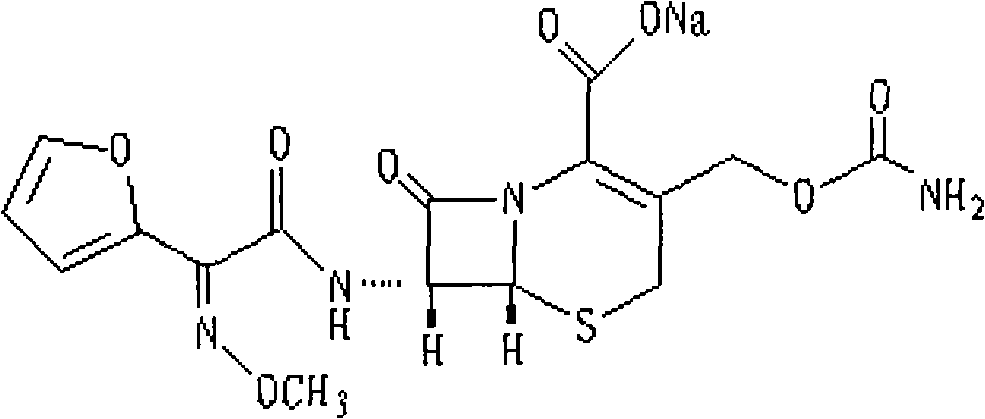
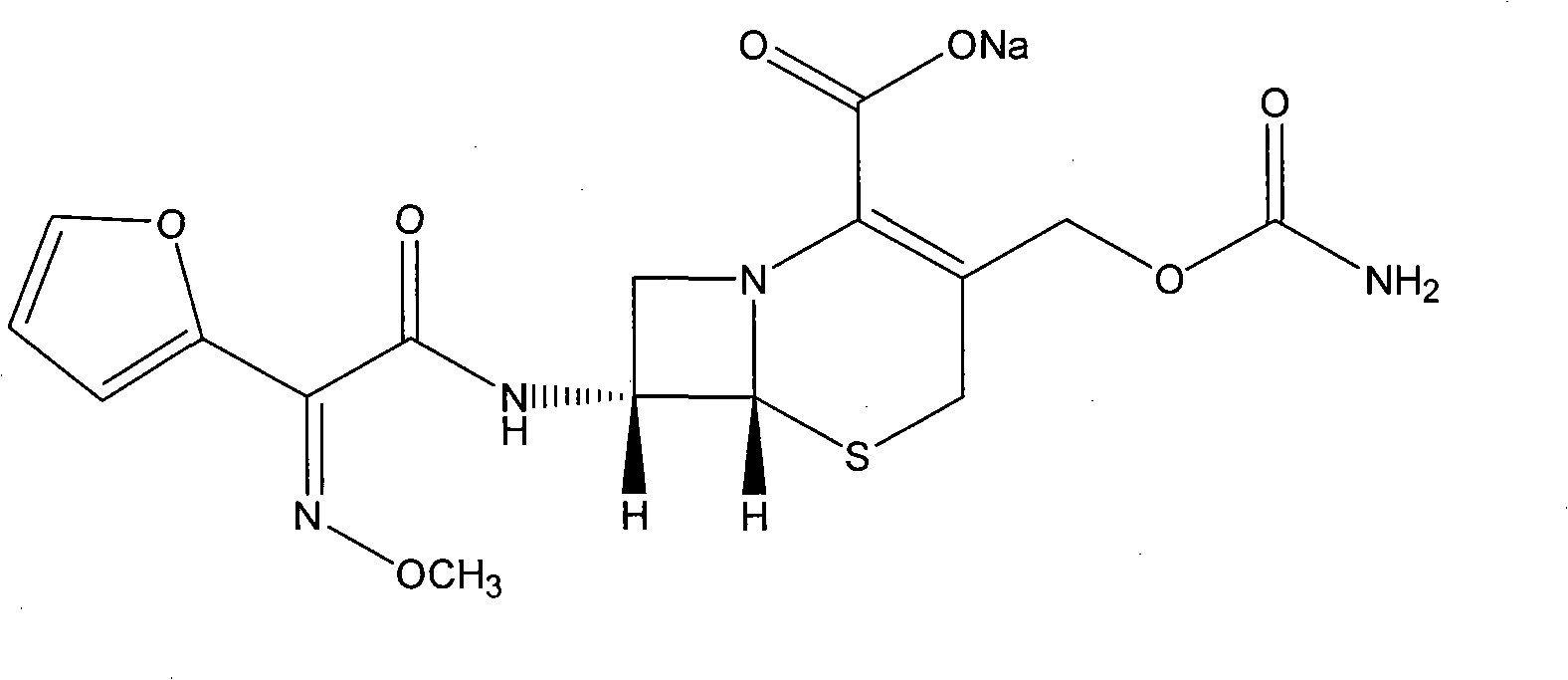
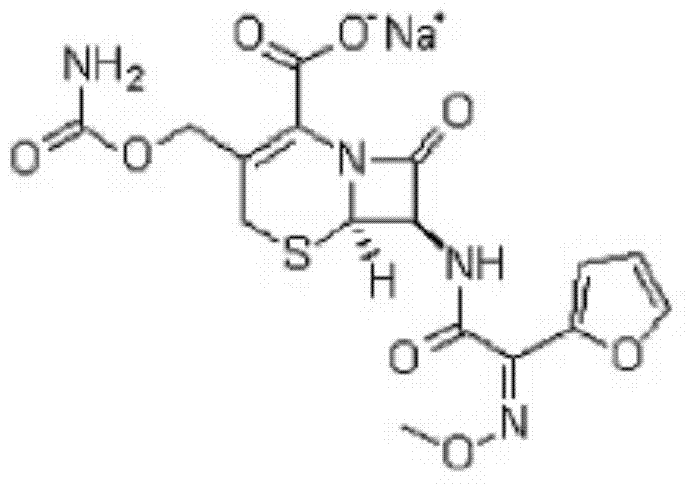
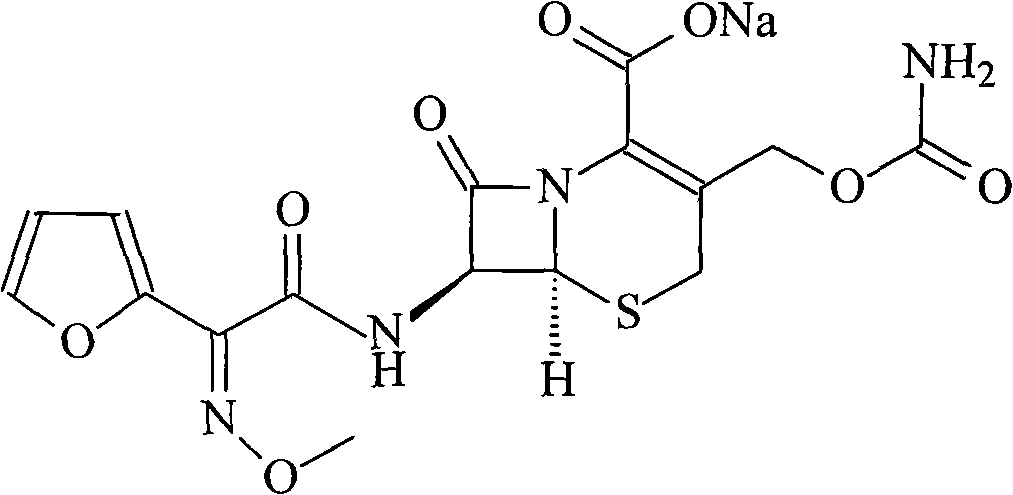
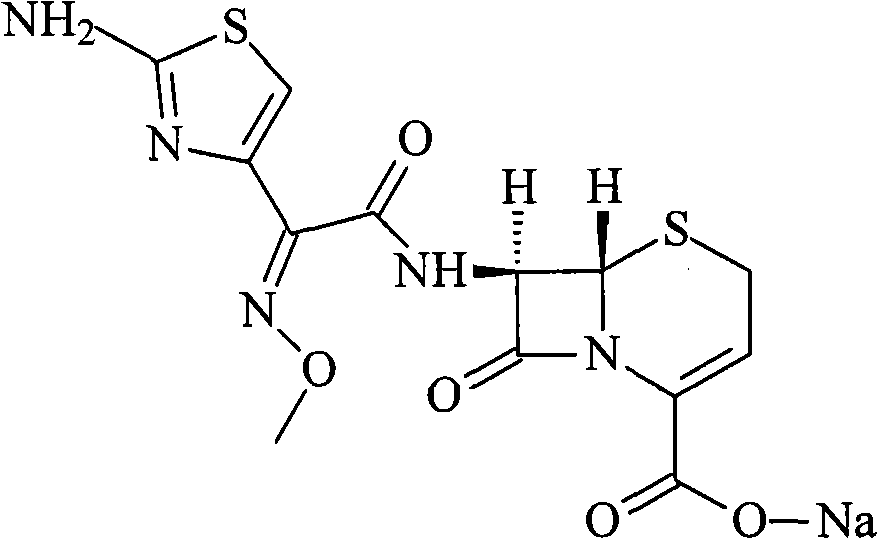
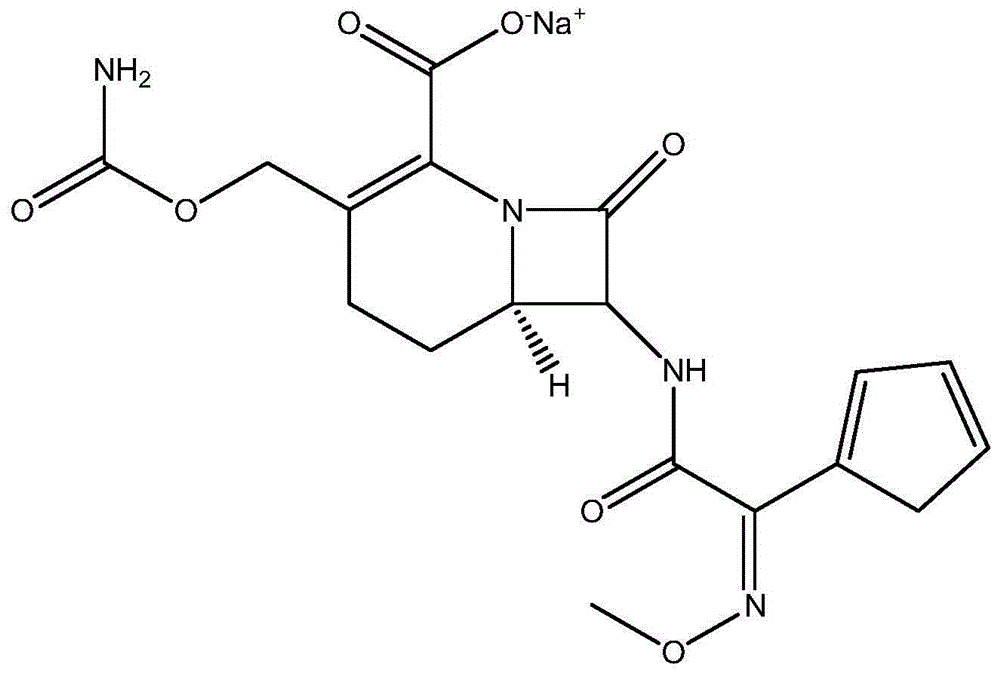
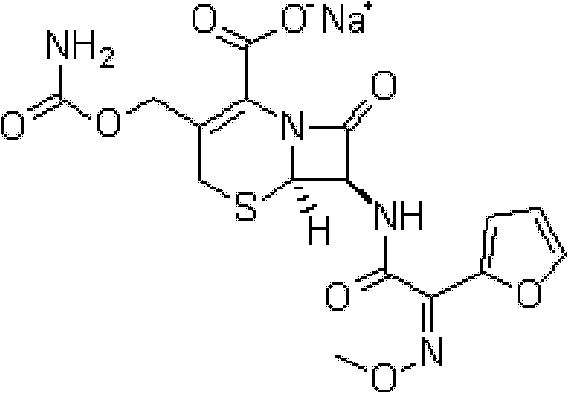
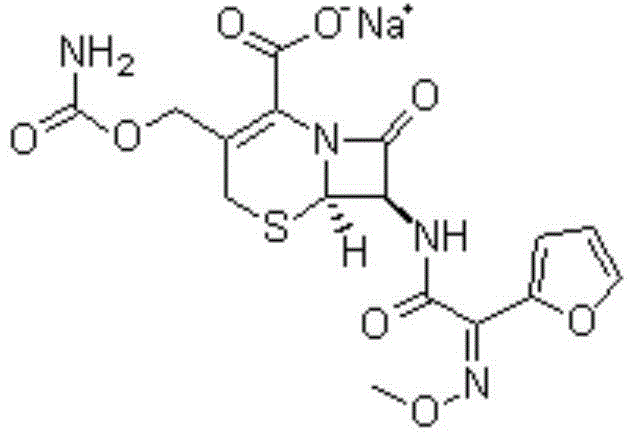
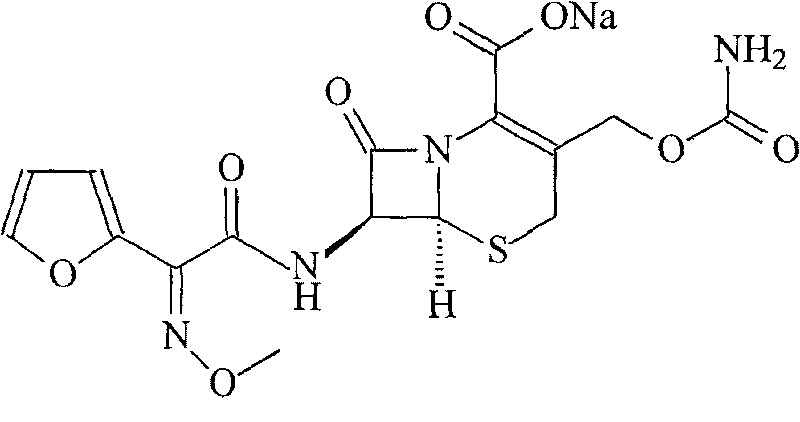
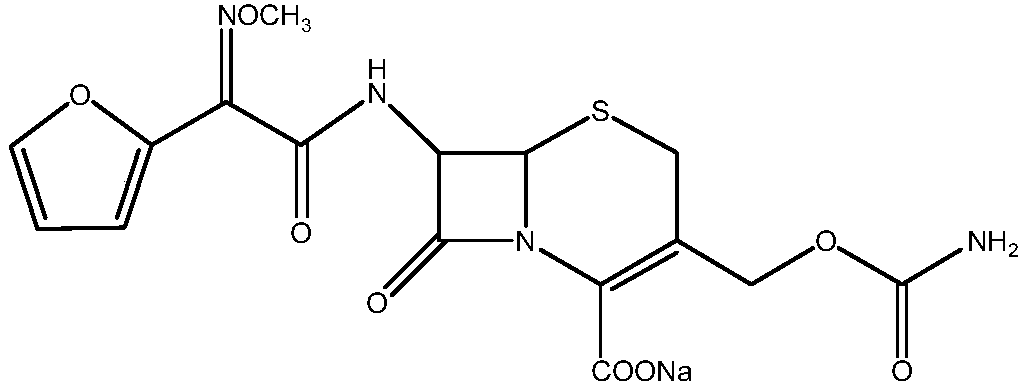
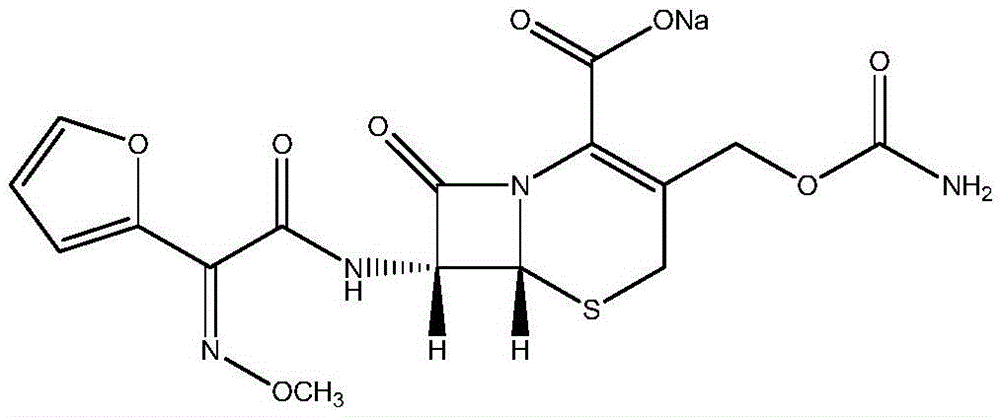
The sodium salt form of cefuroxime and a semi-synthetic, broad-spectrum, beta-lactamase resistant, second-generation cephalosporin antibiotic with bactericidal activity. Cefuroxime sodium inhibits bacterial cell wall synthesis by inactivating penicillin binding proteins (PBPs) thereby interfering with the final transpeptidation step required for cross-linking of peptidoglycan units which are a component of the cell wall. Lack of cross-linking results in a reduction of cell wall stability and leads to cell lysis.
Preparation method of high-purity cefuroxime acid
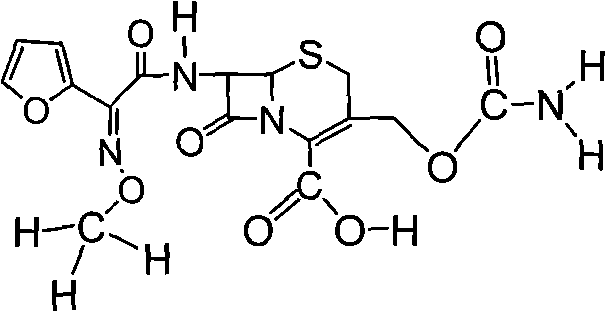
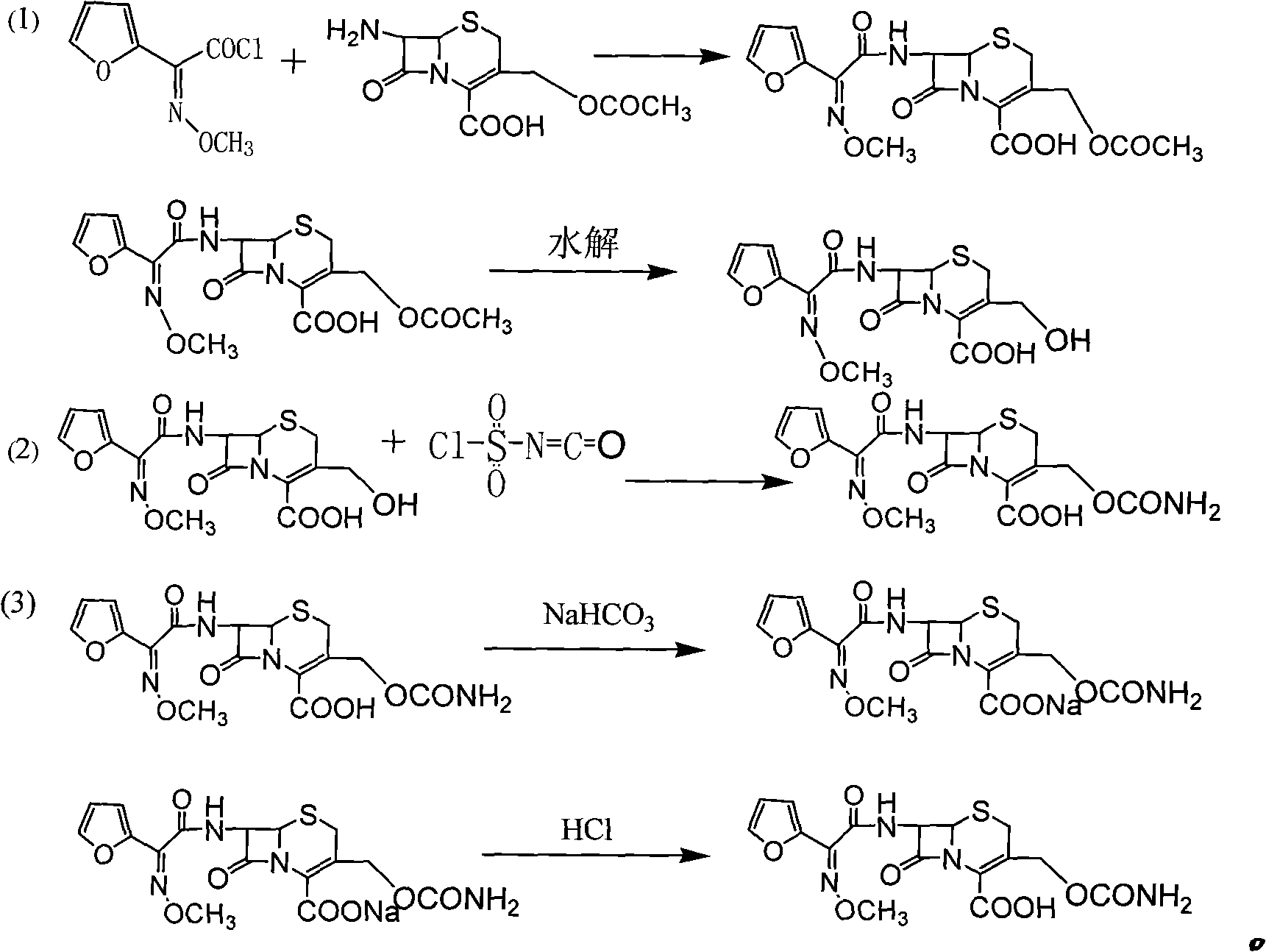
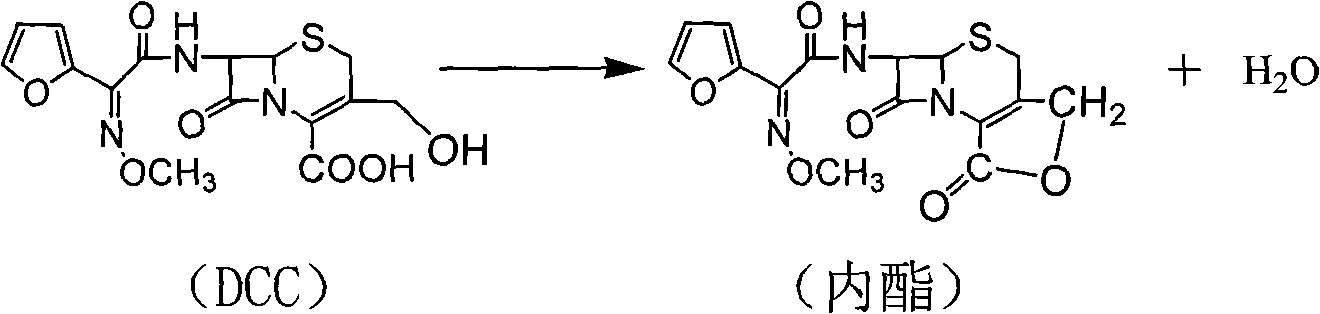
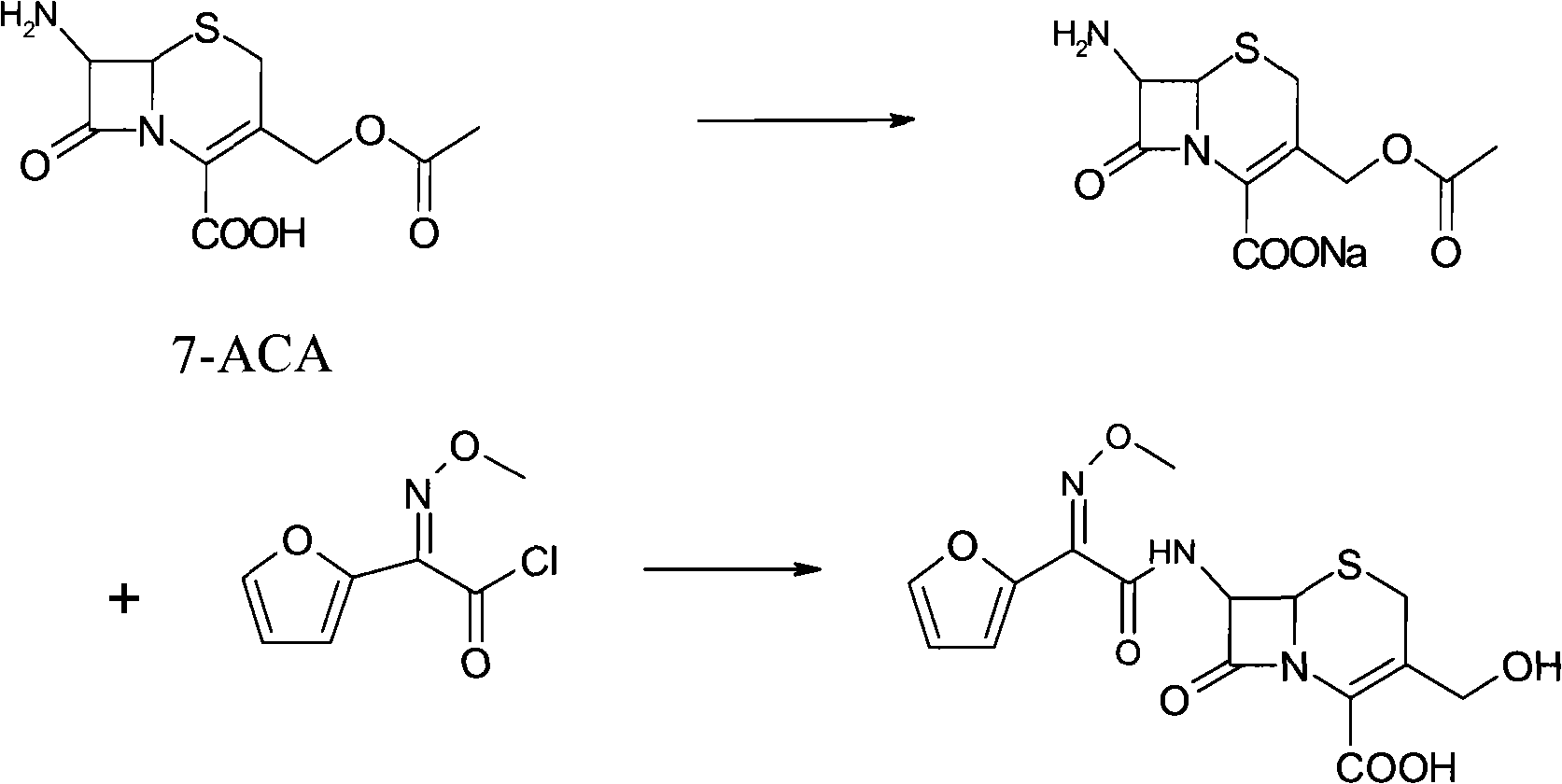
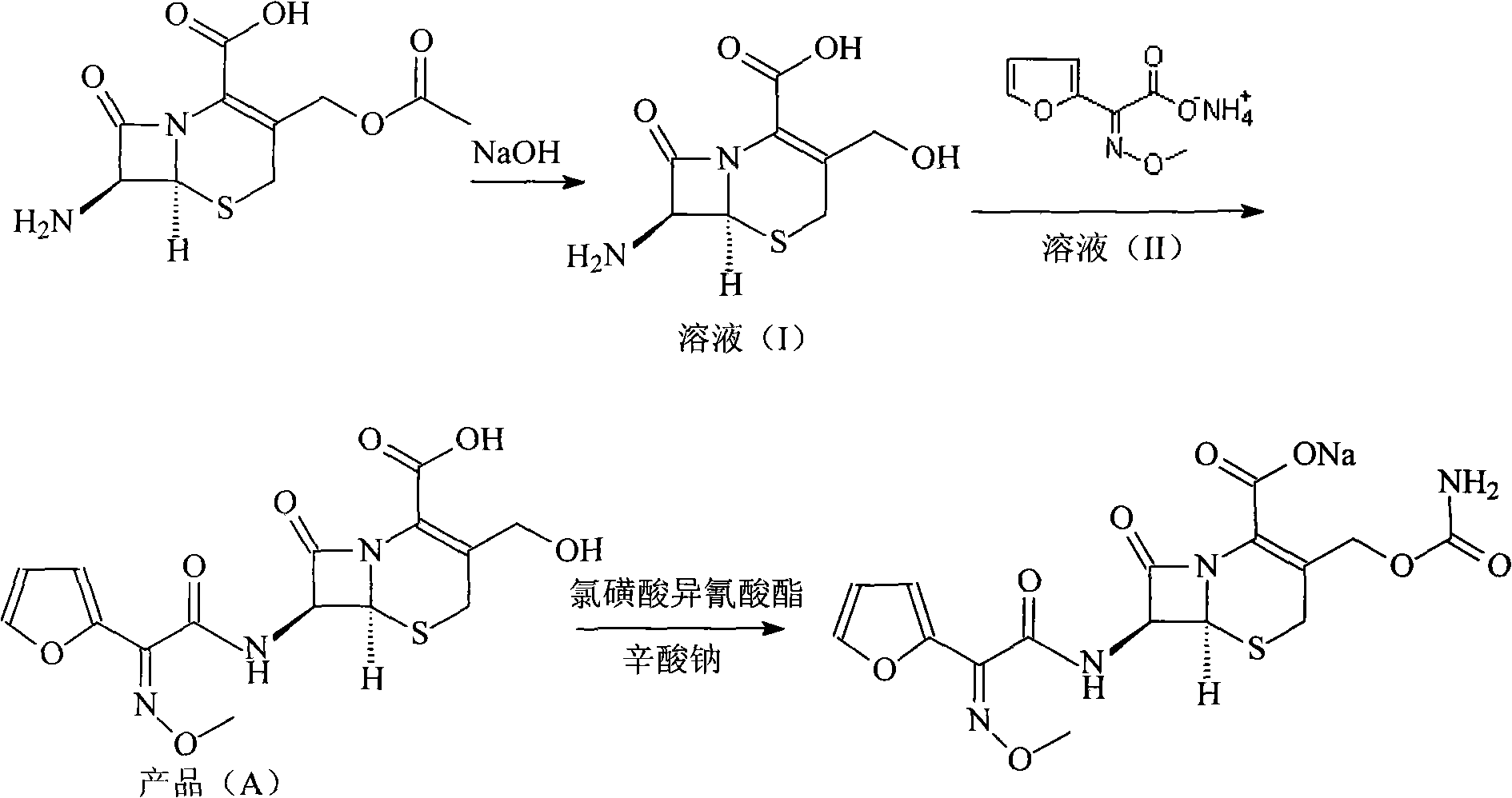
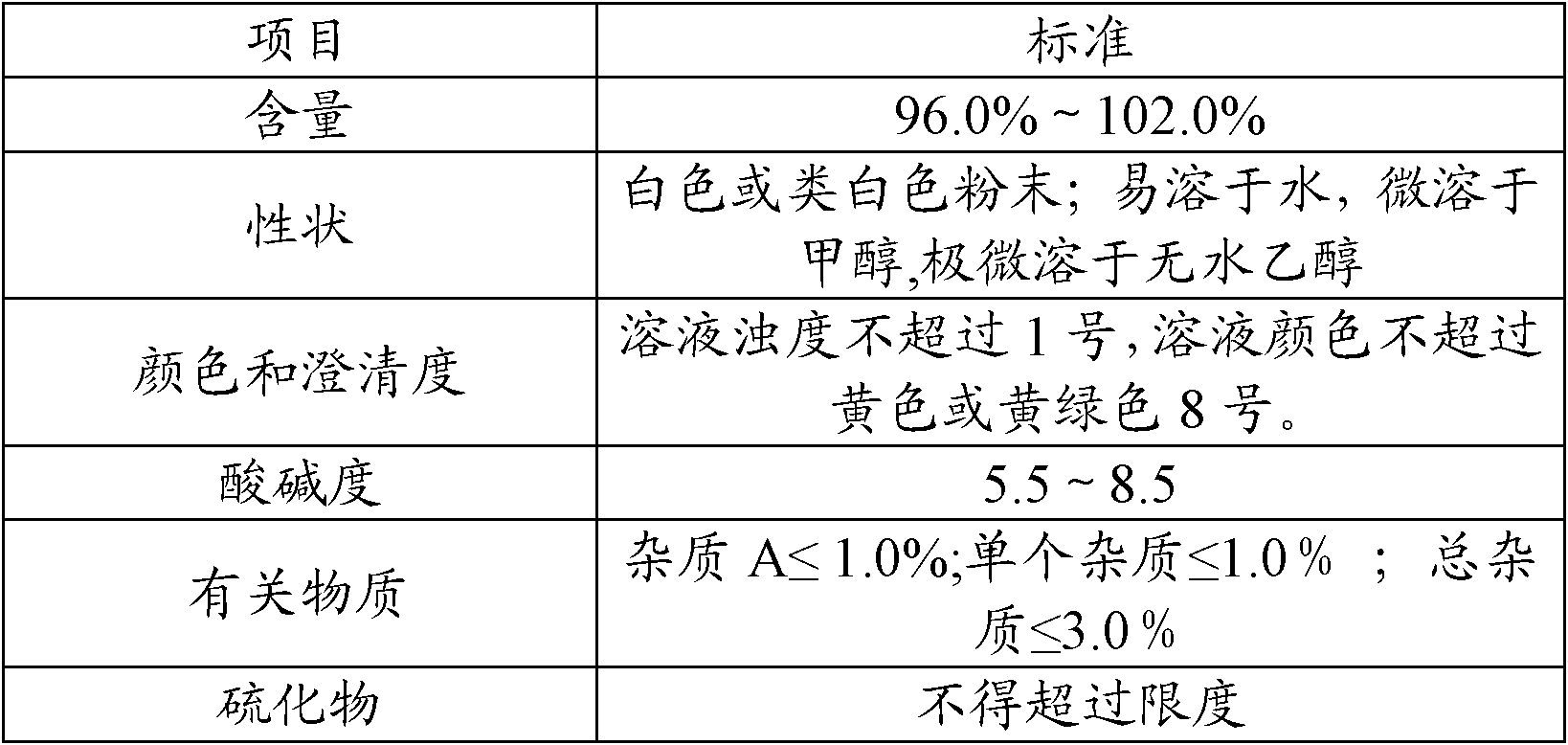
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
Method for synthesizing cefuroxime sodium
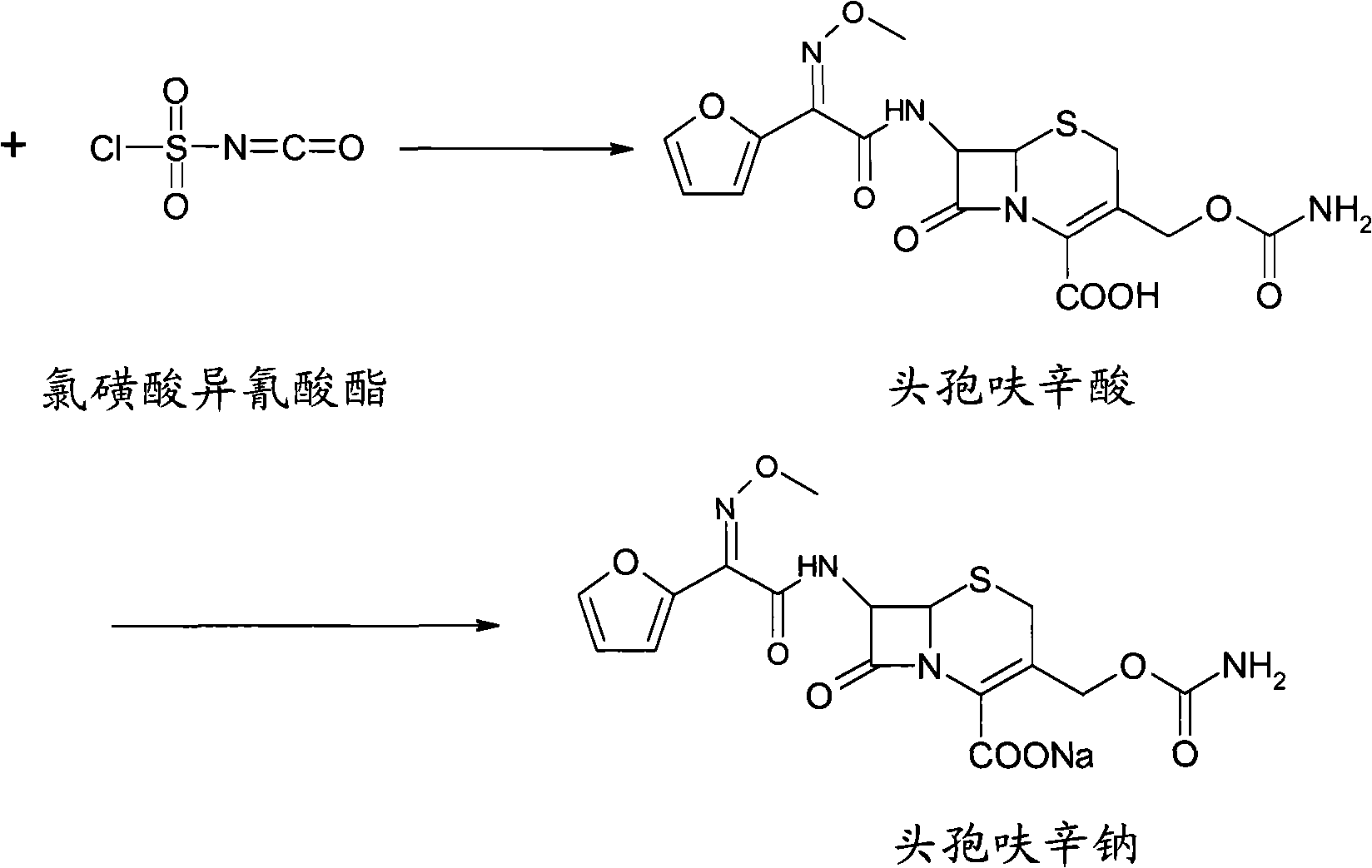
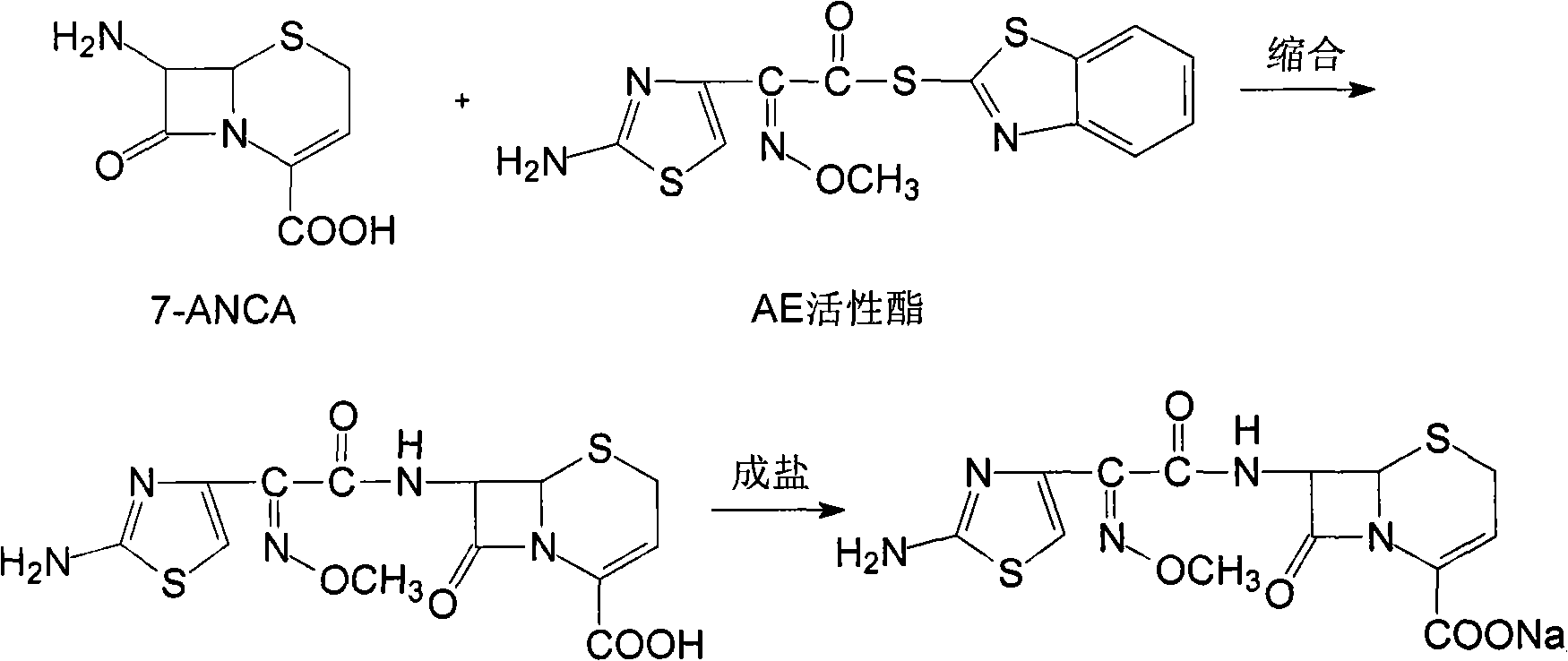
The invention relates to a method for synthesizing cefuroxime sodium, which comprises the following steps: 1, performing the N- acylation reaction of 3-deacetyl-7aminocephalosporanic acid and methoxyaminofuranyl ammonium salt which serve as raw materials and adjusting the pH value with hydrochloric acid to less than 7 in a mixed solvent phase to precipitate crystals to obtain 3-deoxyformyl cefuroxime acid; 2, performing the addition reaction of the 3-deoxyformyl cefuroxime acid and chlorosulfonyl isocyanate serving as a strong ammonia formylating agent in an organic solvent to obtain chlorosulfonyl cefuroxime acid, dehydrating the chlorosulfonyl cefuroxime acid to obtain the cefuroxime acid, decarburizing and concentrating the cefuroxime acid, crystallizing the cefuroxime acid in a solvent phase, and drying the crystals under vacuum to obtain a solid product of cefuroxime acid; and 3, dissolving the cefuroxime acid in alkaline solution, decarburizing the resulting product, crystallizing the resulting product in a mixed solvent phase, filtering crystals, and drying the crystals under vacuum to obtain the cefuroxime sodium.
Owner:哈药集团股份有限公司 +1
Preparation method of cefuroxime sodium
InactiveCN101955492AHigh purityFast dissolutionAntibacterial agentsOrganic chemistrySodium lactateAlcohol
The invention discloses a preparation method of cefuroxime sodium, comprising the following steps of: 1, adding anhydrous methanol or alcohol, an aqueous solution of sodium lactate and sodium iso-octoate to a vessel to obtain sodium liquid; 2, adding acetone, water and cefuroxime acid to the other vessel, and then adding active carbon for stirring, decoloring and filtering to obtain filtrate of cefuroxime acid; 3, adding the filtrate of cefuroxime acid to the sodium liquid, wherein the amount of the sodium liquid is not less than that of the cefuroxime acid, stirring the mixture of the filtrate and the sodium liquid to generate sedimentation, and filtering to obtain a wet product of cefuroxime sodium; 4, washing the wet product with a methanol / acetone mixed liquid or anhydrous alcohol / acetone mixed liquid, and then washing the wet product with a solution of acetone, and drying to obtain the product of the cefuroxime sodium. In the invention, the prepared product of the cefuroxime sodium has high purity; the operation is simple and convenient; the reaction condition is gentle; in addition, the invention is suitable for industrial production, and can be carried out under the aseptic condition and can be used for preparing the aseptic product of the cefuroxime sodium.
Owner:上海新先锋药业有限公司 +1
Cefuroxime sodium and preparation method thereof
The invention provides cefuroxime sodium and a preparation method thereof. The preparation method comprises the following steps that: (1) 7-aminocephalosporanic acid reacts with 2-(furan-2-base)-2-(methoxyimino) acetyl chloride to produce 3-deacety-7-aminocephalosporanic acid; (2) crystallization is conducted after the 3-deacety-7-aminocephalosporanic acid reacts with chlorosulfonyl isocyanate to produce cefuroxime acid; and (3) the cefuroxime acid is salified to obtain the cefuroxime sodium, wherein solvent for crystallization is selected from one or more of petroleum ether, normal hexane, cyclohexane, solvent oil and tetrahydrofuran. Since the preparation method adopts solvents such as petroleum ether for crystallization in the process of the preparation of the cefuroxime acid, the invention has the advantages that the yield of the product is effectively improved, the product purity is further improved, the impurity content is reduced, the product quality is compliant with Chinese Pharmacopoeia of version 2005, the operation of the method is simple, the raw materials can be easily obtained, the cost is relatively low and the industrial production can be realized easily.
Owner:LIVZON PHARM GRP INC
Method for recrystallizing cefuroxime sodium
InactiveCN101967156AImprove solubilityHigh recrystallization efficiencyOrganic chemistryCefuroxime SodiumDark color
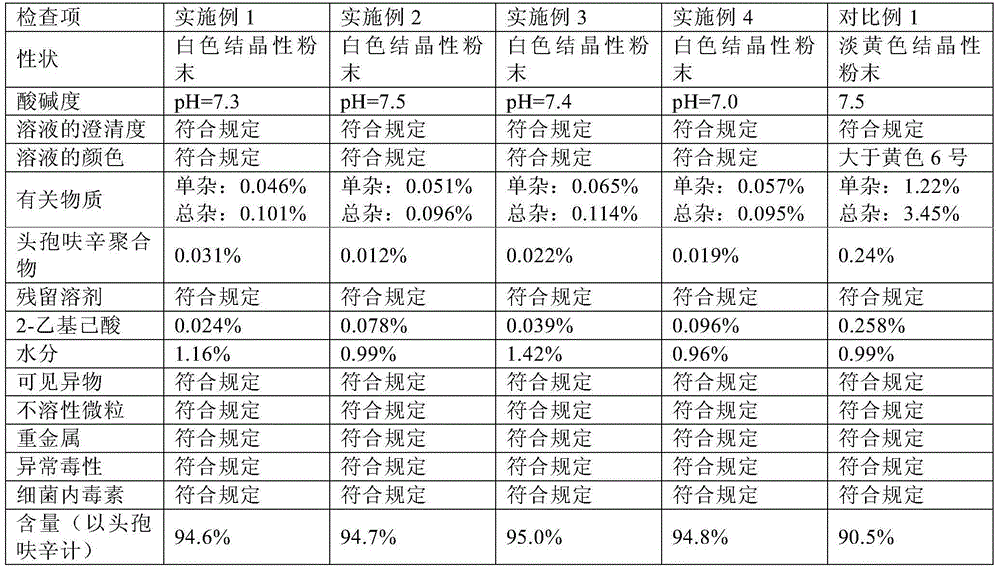
The invention relates to a method for recrystallizing cefuroxime sodium, which is used for solving the problem of the refining of cefuroxime sodium raw material medicaments with darker colors. In the technical scheme, white crystalline powder recrystallized by the cefuroxime sodium is prepared by the following four steps of: a, preparing crude product solution; b, preparing a crystallization solvent; c, crystallizing; and d, discharging. By the method, the yield is over 83 percent, and the color level and the pH value of final products meet the pharmacopoeial standard; and the method is suitable for recrystallizing the raw material medicaments with unqualified color levels.
Owner:石药集团中诺药业(石家庄)有限公司
Method for preparing cefuroxime sodium
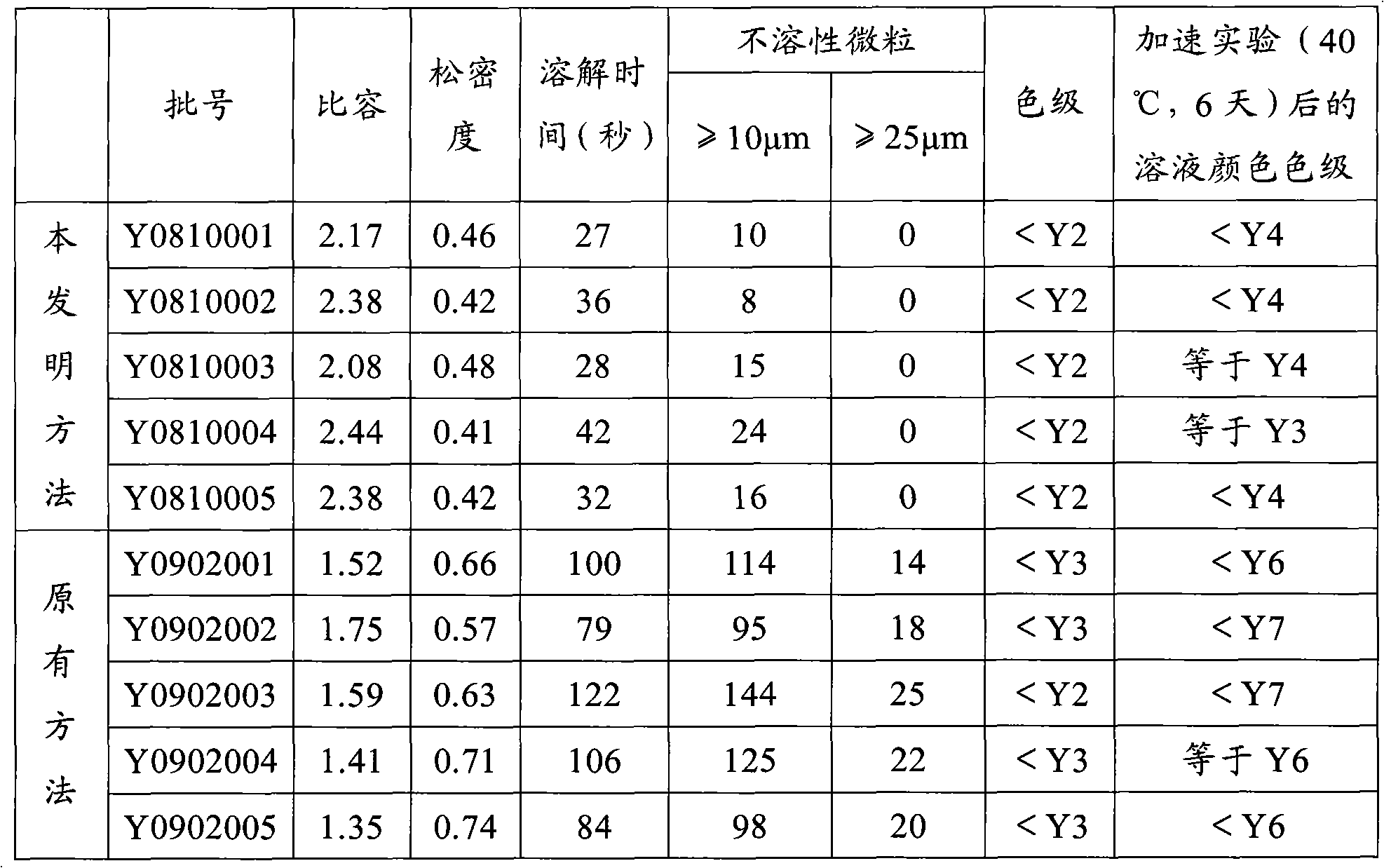
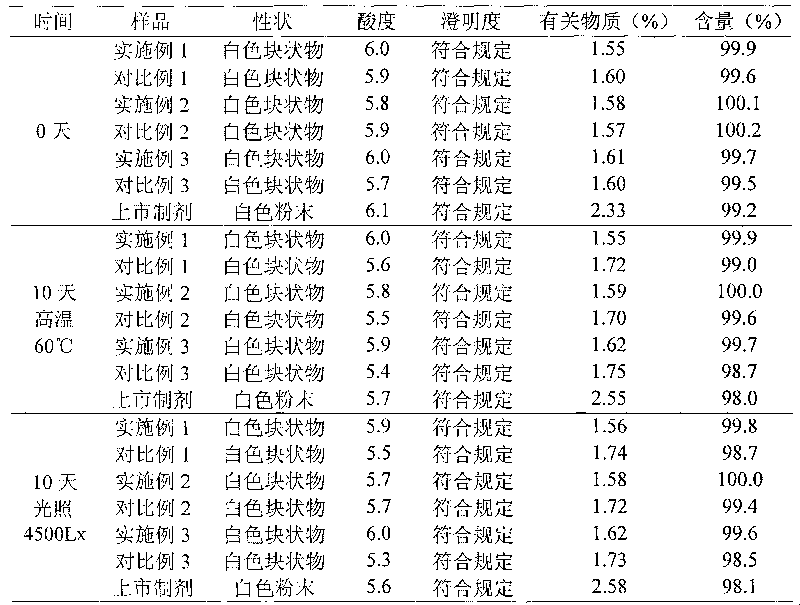
The invention provides a method for preparing cefuroxime sodium. The method comprises a step of reacting cefuroxime acid with mixed sodium salt to produce the cefuroxime sodium, wherein the mixed sodium salt comprises two or three of sodium acetate, sodium lactate and sodium iso-octoate. The product prepared by the method has the advantages of uniform crystal dispersion, greatly improved fluidity and easy packaging; the solubility of the product is greatly improved, and compared with similar products prepared by the conventional methods, the dissolution time is the shortest; the method greatly shortens the time for washing, filtering and drying the product, reduces the opportunities of powder exposure and human contact, more easily controls visible foreign matters in the product and effectively reduces the number of insoluble particles in the product; because the method improves the crystal form of the product, the crystal is easy to wash and dry, the time of the product at a high temperature is shortened and the stability of the product is improved effectively. The color grade of the product is further reduced, and the product is more stable and uniform and has better performance on indexes such as the color grade, content, impurities and the like.
Owner:LIVZON PHARM GRP INC +1
Cefuroxime sodium and sulbactam sodium composition for injection
InactiveCN1729987AImprove antibacterial propertiesImprove the bactericidal effectAntibacterial agentsHeterocyclic compound active ingredientsCefuroxime SodiumSulbactam Sodium
The invention provides a composition of cefuroxime sodium and sulbactam sodium for injection, which comprises cefuroxime sodium and sulbactam sodium by the weight ratio of 15:1. The composition has good antibiotic action and low cost of production.
Owner:刘全胜
Preparation method of stable cefuroxime sodium
InactiveCN102838622ALess impuritiesImprove stabilityOrganic chemistryCefuroxime SodiumCrystallization
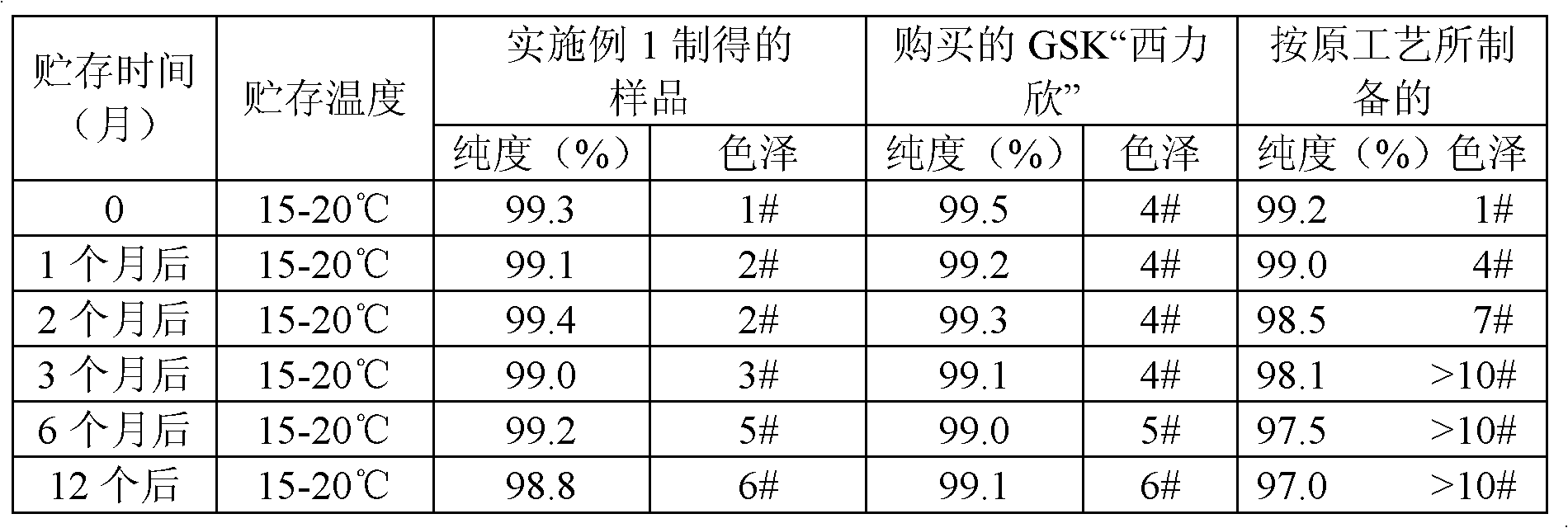
The invention belongs to the technical field of medicine, relates to a preparation method of cephalosporin, and more specifically relates to a preparation method of a stable cefuroxime sodium. According to the invention, a process control technique and a multiscale simulation technology are utilized to conduct on-line crystallization control of cefuroxime sodium, so as to obtain a cefuroxime sodium product with stable quality and crystal form, and moderate particles. The quality and especially the stability of the product are improved greatly, and product quality achieves a same level as that of ''Xilixin'' from an original researching factory.
Owner:SHANXI WEIQIDA PHARMA IND
Double specificity oligopeptide-cefuroxime sodium strengthened fusion protein Ec-LDP-Hr-AE
ActiveCN101497666APenetratingStrong lethalityPeptide/protein ingredientsHybrid peptidesTherapeutic effectOligopeptide
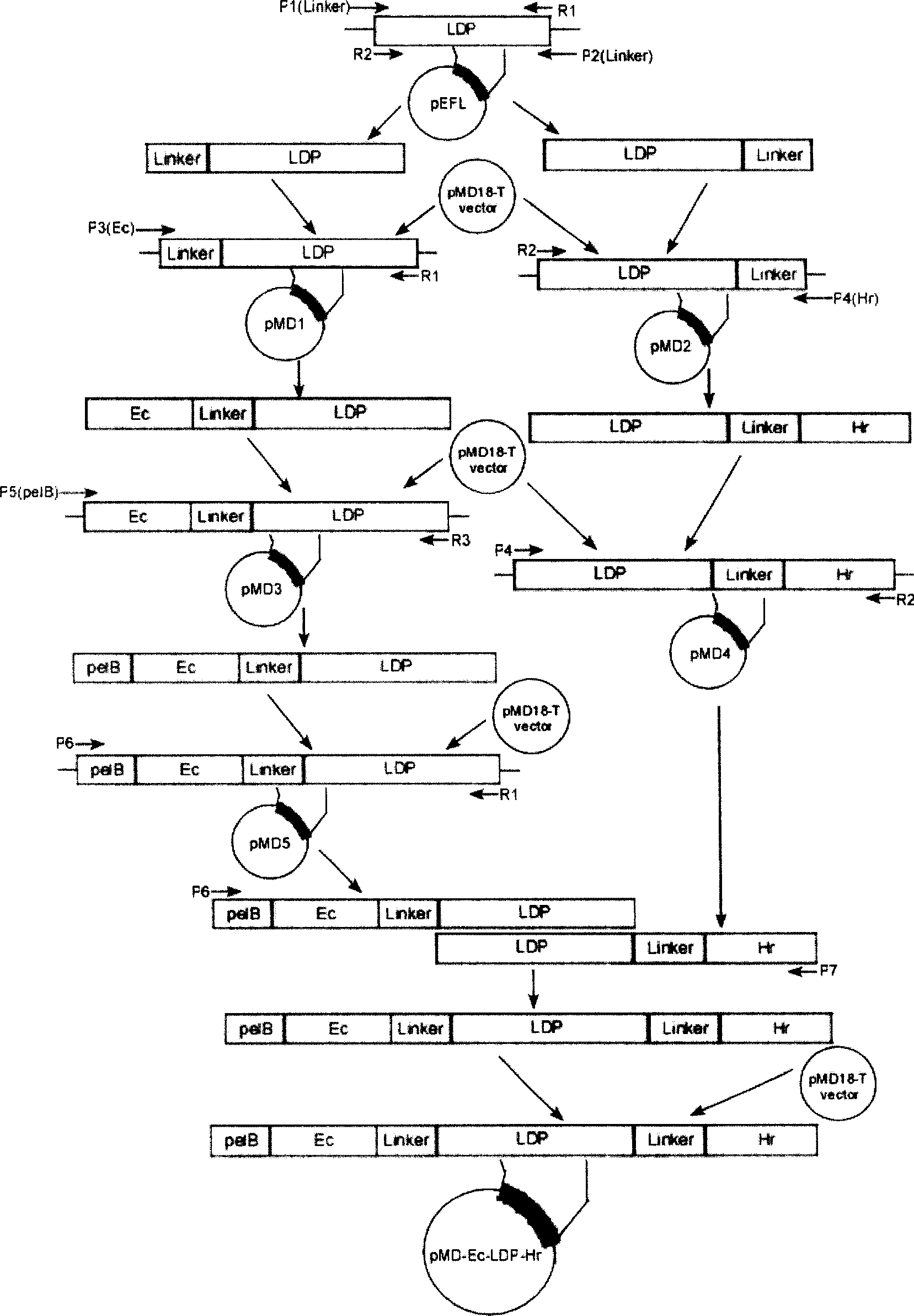
The invention relates to a bispecific oligopeptide-lidamycin energized fusion protein Ec-LDP-Hr-AE. The fusion protein comprises two oligopeptides of targeted epidermal growth factor receptors EGFR and HER2, a lidamycin apoprotein and a lidamycin activity chromophore, and researches show that the fusion protein in vitro can be combined with the epidermal growth factor receptors EGFR and HER2 for expressing the specificities of the tumor cells, has strong damaging effect for the tumor cells and remarkable therapeutic effect for the transplanted tumor of nude mice for the ovarian cancer SK-OV-3 after the in vivo test, displays the characteristics of miniaturization and high efficiency of targeting drugs and has good application prospect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
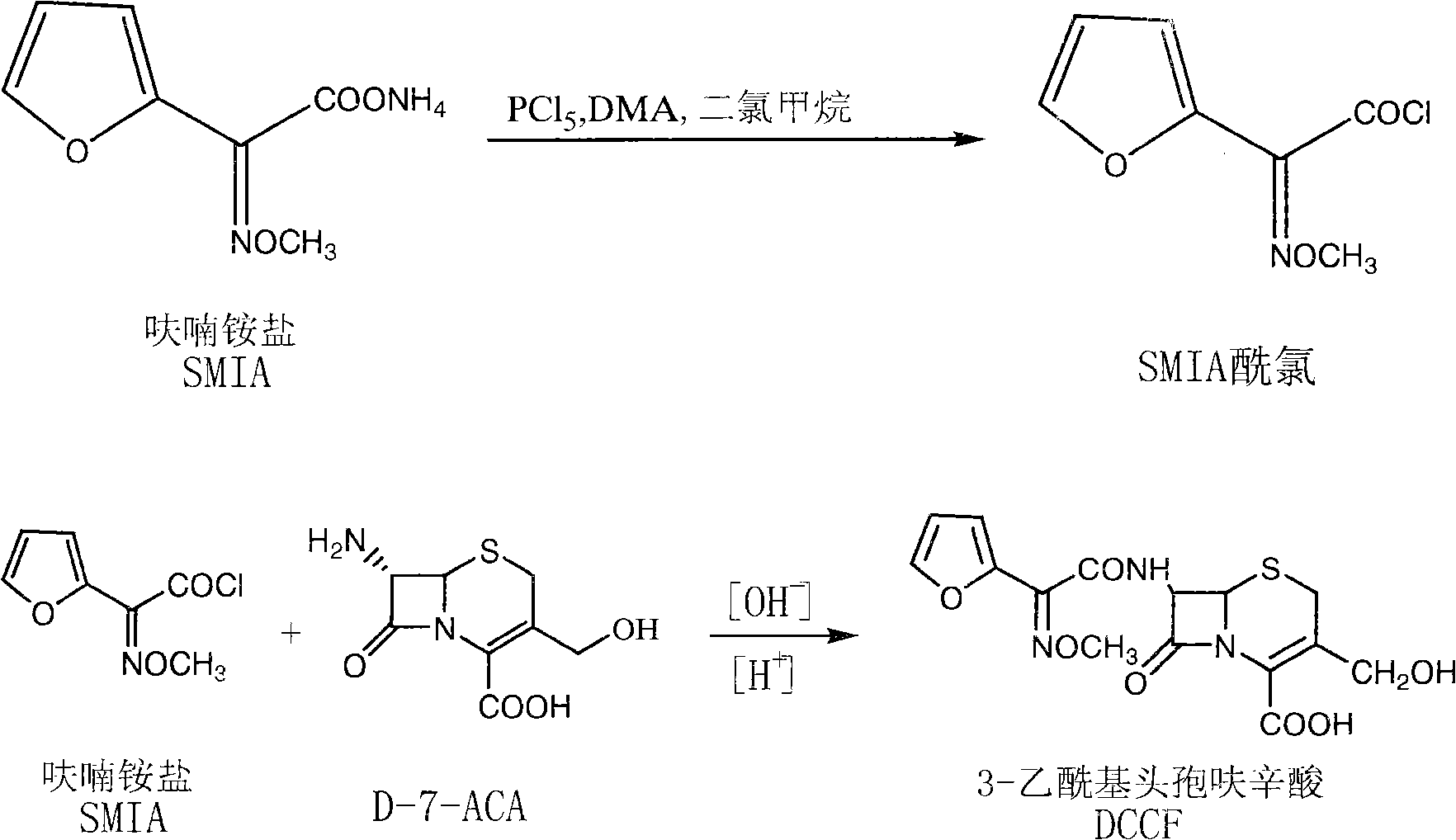
Novel process for synthesizing 3-deacetyl cefuroxime sodium (DCCF)
InactiveCN101289457AReduce typesEasy to recycleAntibacterial agentsOrganic chemistry7-ACAChemical reaction
The invention relates to a synthesis art of a chemical product, in particular to a new synthesis art for 3-deacetyl cefuroxime acid (DCCF). The art of the invention uses SMIA acyl chloride and 3-deacetyl cephalosporin acid (D-7-ACA) as the main synthesis ingredients to replace the SMIA acyl chloride and the 7-ACA, which reduces the chemical reaction process needed in the synthesis, shortens the production cycle, reduces the using solvent species, facilitates the solvent recovery and improves the product synthesis rate.
Owner:河源市制药工程技术研究开发中心
Cefuroxime sodium powder preparation for injection
ActiveCN104771372ALess impuritiesImprove stabilityAntibacterial agentsOrganic active ingredientsSodium acetateFiltration
The present invention discloses a cefuroxime sodium powder preparation for injection. The preparation is prepared by the following steps: (1) preparing a sodium acetate solution by dissolving sodium acetate with a solvent for standby; (2) adding the solvent and water, and adding cefuroxime acid with stirring until complete dissolution; adding activated carbon, decolorizing, filtering, mixing with the solvent, washing the residue and a filter bottle, and filling a crystallization tank with the filtrate; (3) applying the particle process morphology and molecular assembly process crystal product molecular assembly and shape-state optimization technology by Hebei Huamin Pharmaceutical Co. Ltd. of North China Pharmaceutical Co. Ltd., controlling the temperature and stirring speed, and dropwise adding sodium acetate solution; and adding a solvent agent according to the flow rate; (4) carrying out pumping filtration, washing, vacuum drying, weighing, and packing; and 5) packing the preparations in different specifications to obtain the cefuroxime sodium for injection. The cefuroxime sodium powder prepared by the method has the advantages of excellent hydrodynamic performance, perfect crystal shape, uniform particle size distribution, and greatly improved color grade, clarification, purity and stability.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
New method for preparing cefuroxime sodium compound
ActiveCN101671349AImprove responseSuitable for large-scale industrial productionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCephalosporanic AcidsTriphenylphosphine oxide
The invention relates to a new method for preparing a cefuroxime sodium compound. A target product is prepared by using triphosgene and triphenylphosphine oxide as catalysts, reacting 7-amino-cephalosporanic acid with (Z)-methoxyl imido furylacetic acid ammonium salt and sequentially adding chlorosulfonyl isocyanate and sodium iso-octoate for reaction. The cefuroxime sodium compound prepared by the method is greatly enhanced in purity and yield coefficient and has advantages of inexpensive using materials, simple synthesizing technology and equipment and easy production separation and purification.
Owner:灵康药业集团股份有限公司
Pharmaceutical packaging composition for injection and preparation method thereof
InactiveCN102670400AGuaranteed sterilityTotal quality stabilityPharmaceutical containersMedical packagingPolyesterCephazolin sodium
The invention provides a pharmaceutical packaging composition for injection, which comprises the combination of an aluminum-plastic composition cover containing a coating plastic plug, a sterile antibiotic glass bottle and pharmaceutical sterile powder for injection, wherein the coating material of the coating plastic plug is polydimethylsiloxane coating, polyparaxylene coating, polytetrafluoroethylene coating, ethylene-tetrafluoroethylene copolymer coating, polyester coating, polyethylene coating or polypropylene coating; and the pharmaceutical sterile powder for injection is cephalo-type pharmaceutical sterile powder, such as cefuroxime sodium, cefoxitin sodium, cephazolin sodium and ceftriaxone sodium. The pharmaceutical packaging composition for injection is compatible with the pharmaceutical sterile powder for injection, so that the transition incidence rate is lower, and the quality risks of unqualified solution clarity due to compatibility and addition of related substances are reduced. The invention further provides a preparation method for the pharmaceutical packaging composition for injection, so that the obtained pharmaceutical packaging composition for injection can solve the compatibility problem very well and guarantee the stability of pharmaceutical quality.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
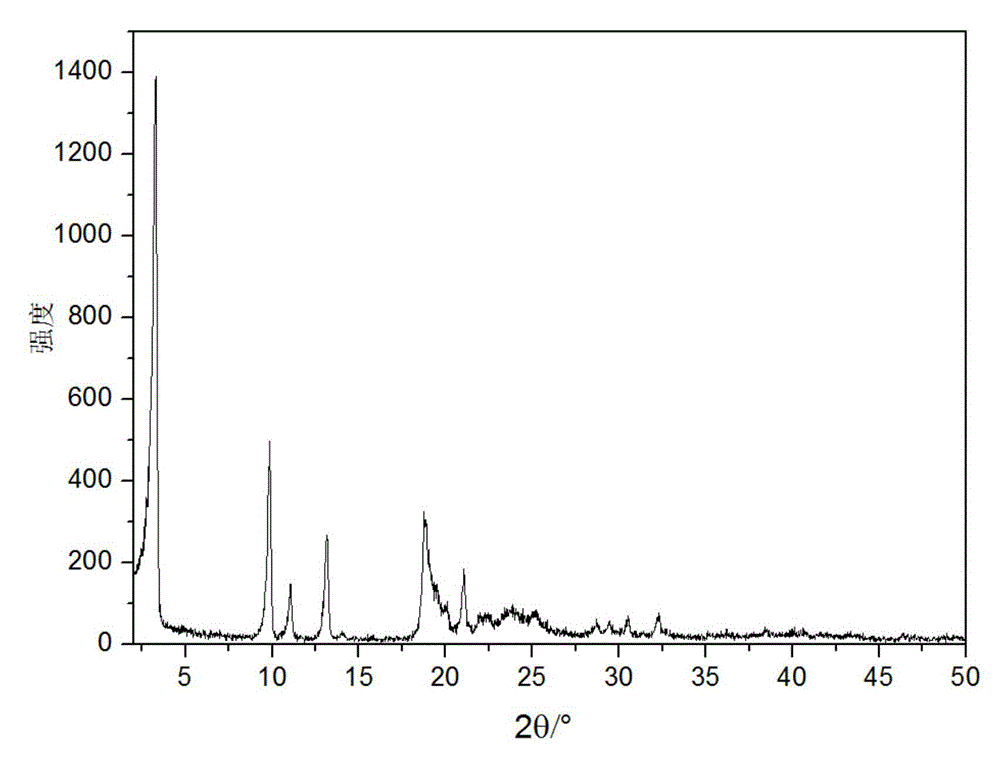
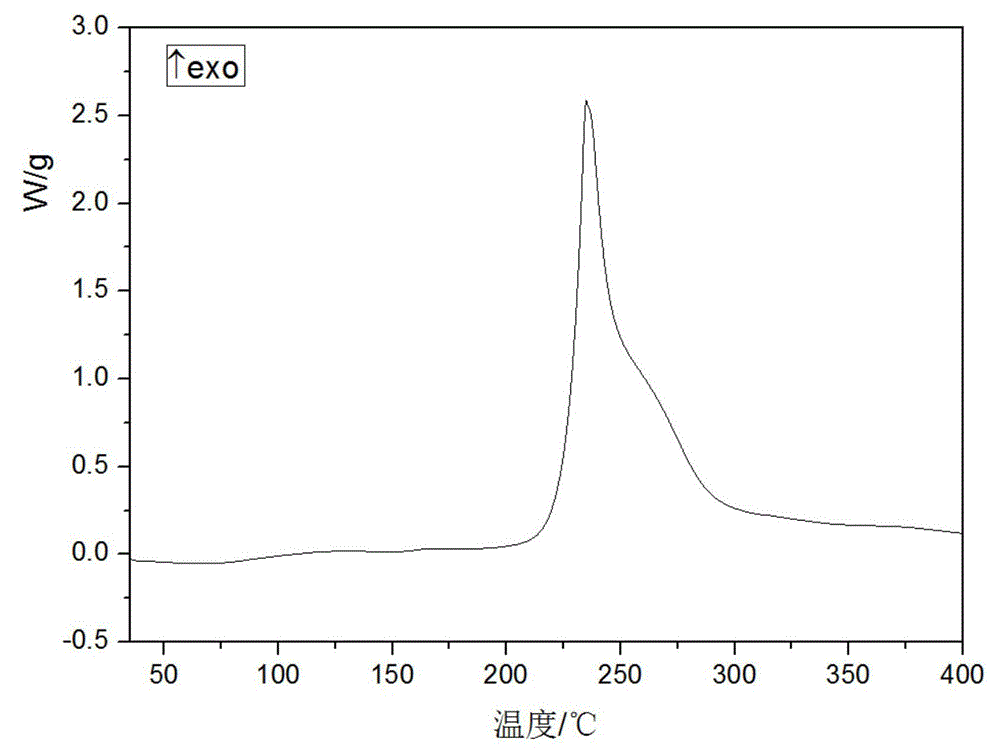
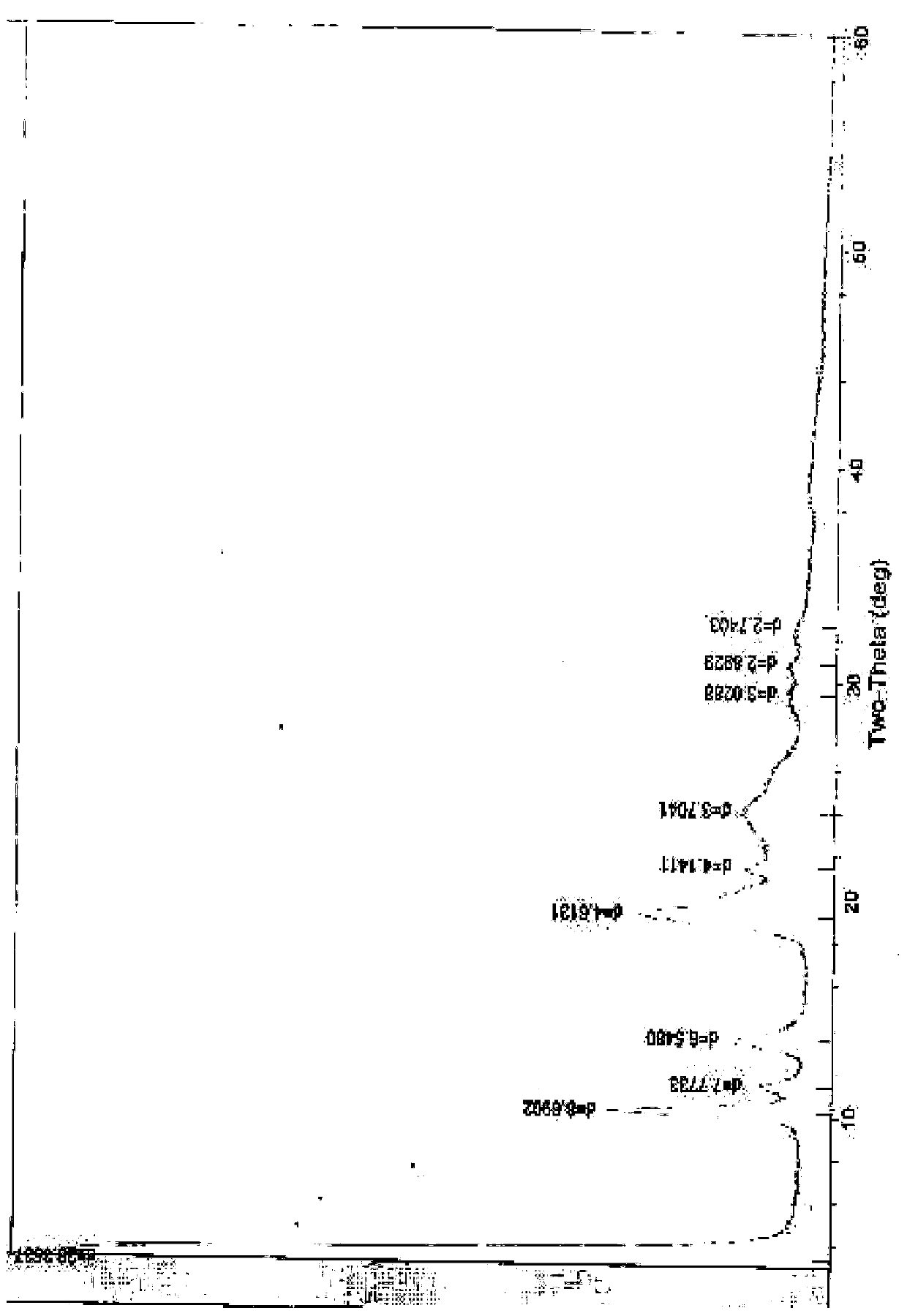
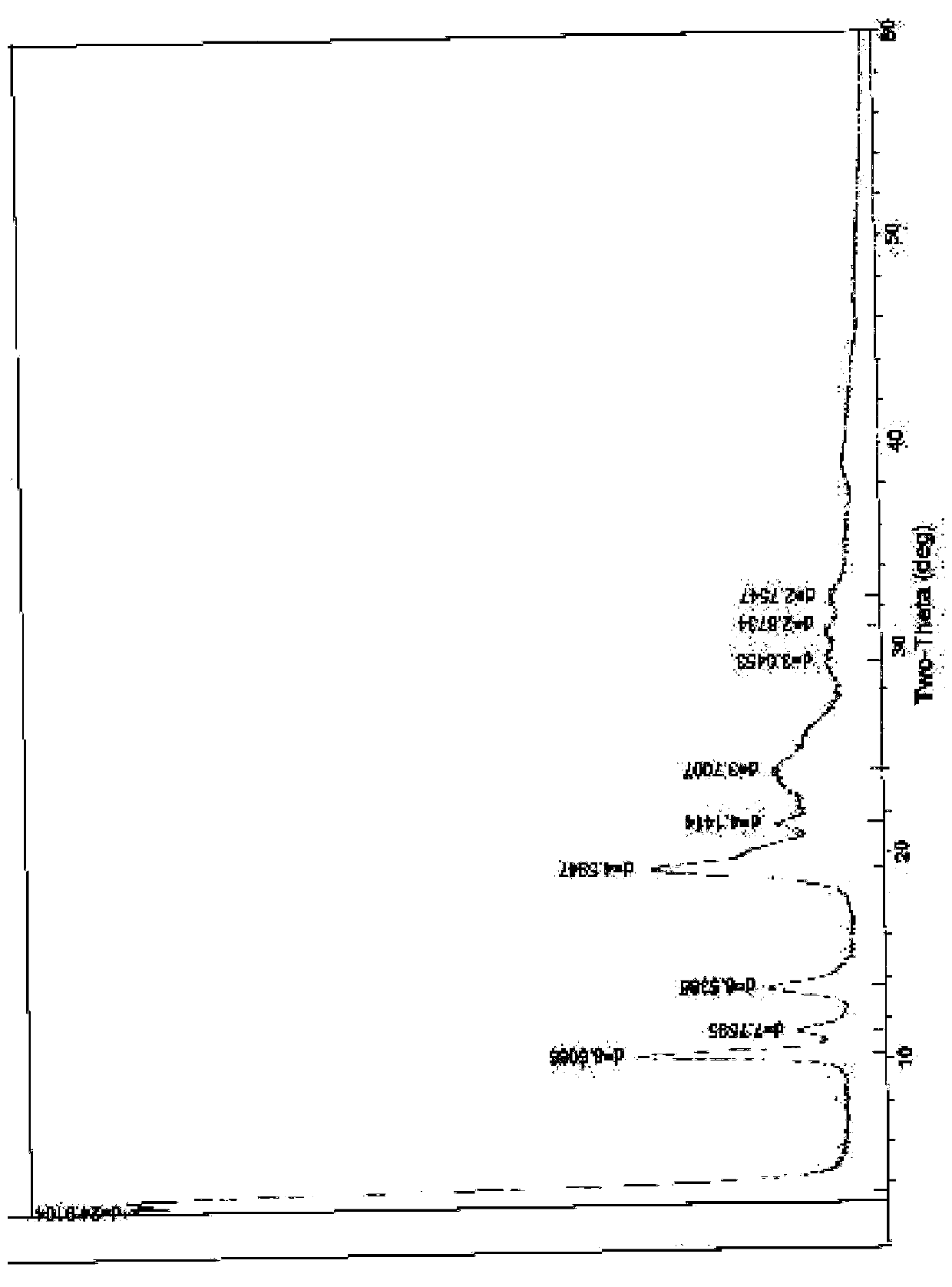
Novel crystal form of cefuroxime sodium and preparation method of cefuroxime sodium crystal
ActiveCN104530084AAvoid degradationReduce decolorization filtration processOrganic chemistrySodium lactateCefuroxime Sodium
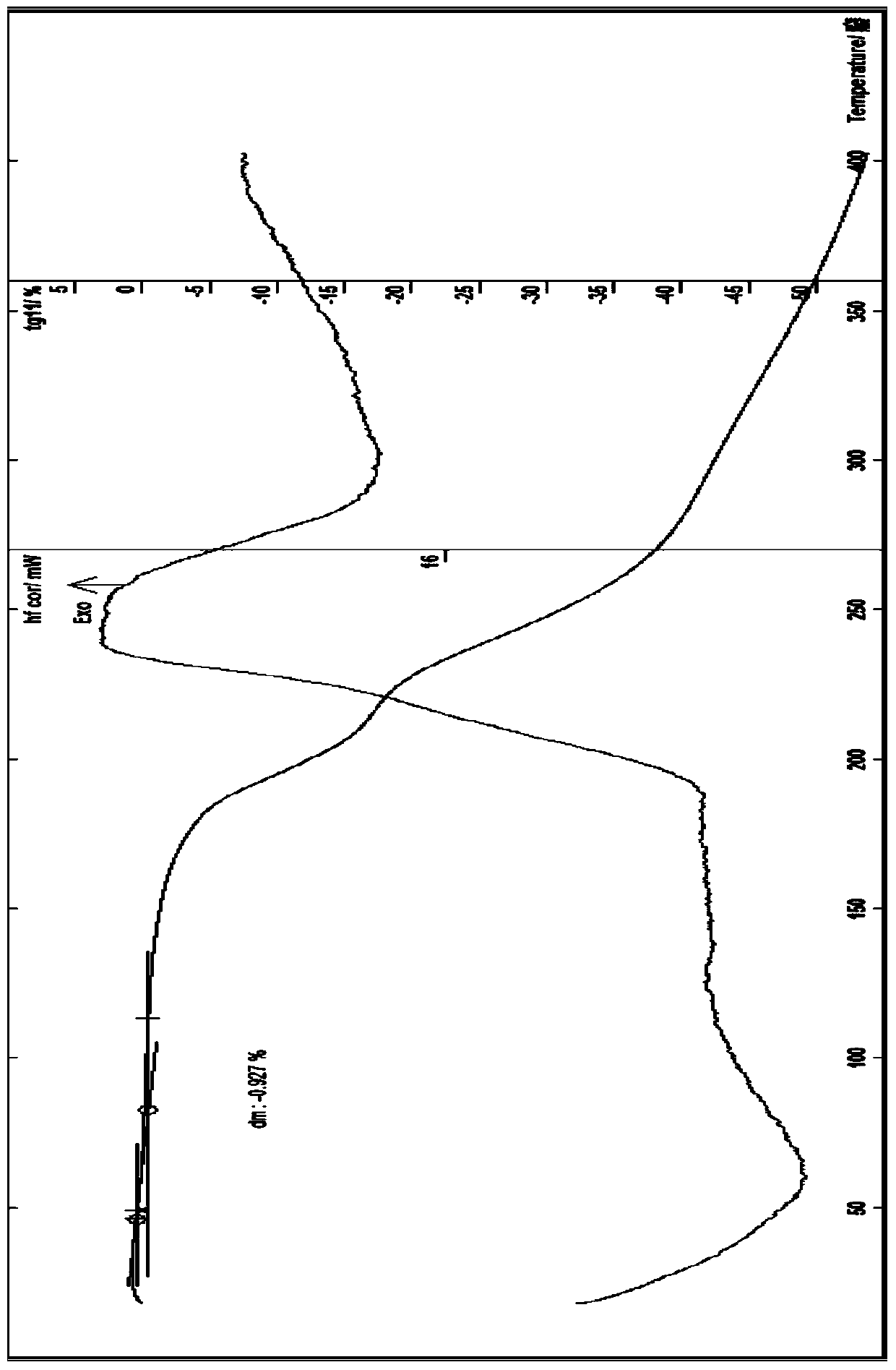
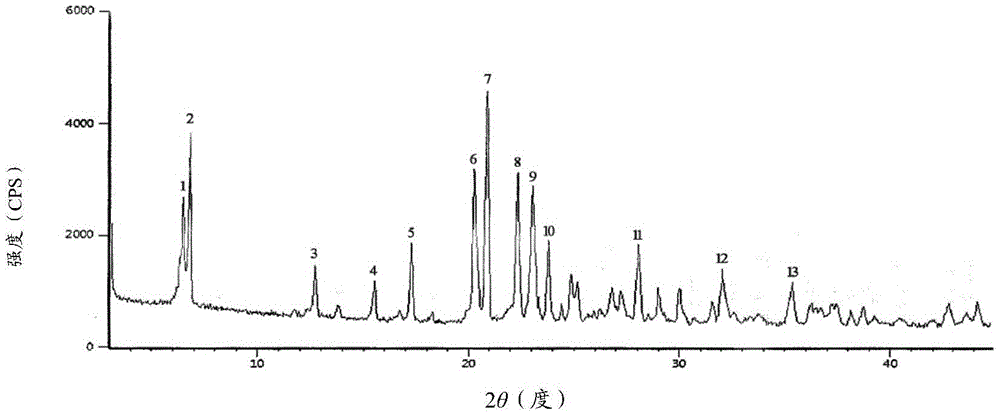
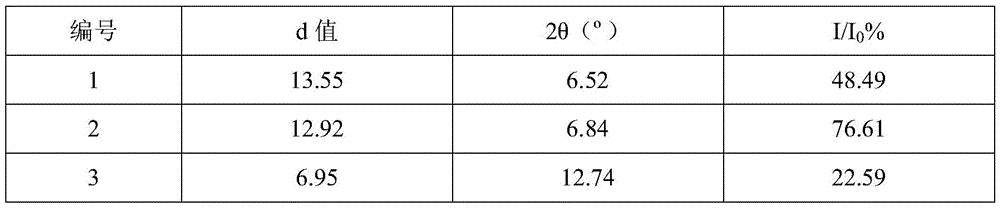
The invention provides a novel crystal form of cefuroxime sodium and a preparation method of cefuroxime sodium crystal. According to the novel crystal form crystal, the X-ray powder diffracting at the diffraction angles 2theta of 3.5+ / -0.2, 10.6+ / -0.2, 12.7+ / -0.2, 18.6+ / -0.2, 19.5+ / -0.2 and 22.4+ / -0.2 has characteristic peaks. The DSC graph has a characteristic peak at the angle of 235+ / -2 degrees. The method for preparing the novel cefuroxime sodium crystal comprises the following steps: adding 2.5-5g of cefuroxime acid with the purity of 99 percent into 100mL of a solvent for stirring at the temperature of 20-25 DEG C, drilling 0.2-0.4g / mL of sodium lactate-methanol solution for reacting until the pH value of the solution is 6.0-6.5; adding a solventing-out agent for performing solvent-out crystallization; and filtering, washing and drying the crystal mush, thereby obtaining the cefuroxime sodium crystallization product with the crystal form.
Owner:TIANJIN UNIV
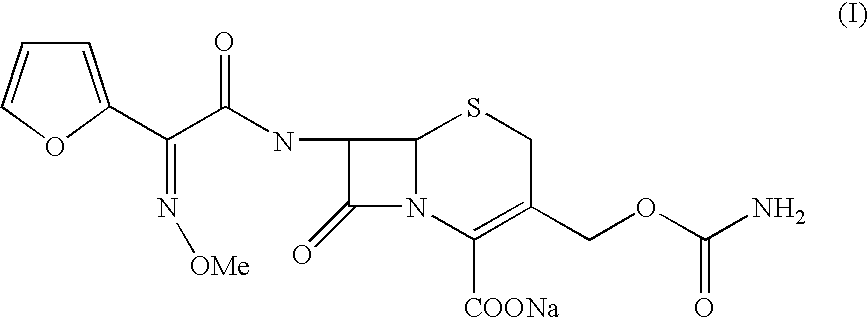
Process for the preparation of cefuroxime sodium
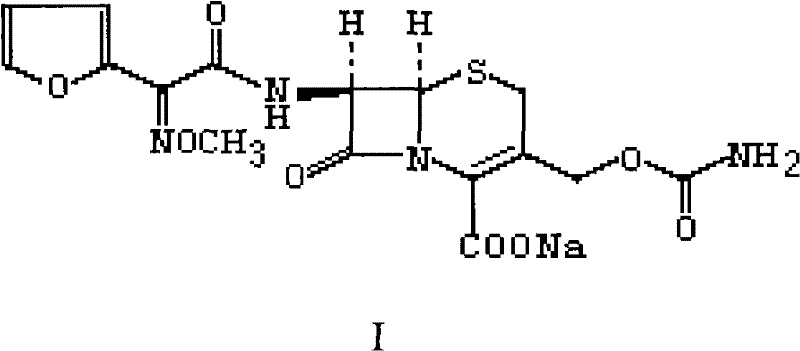
InactiveUS20040092735A1Quality improvementHigh yieldOrganic chemistryCefuroxime SodiumPhotochemistry
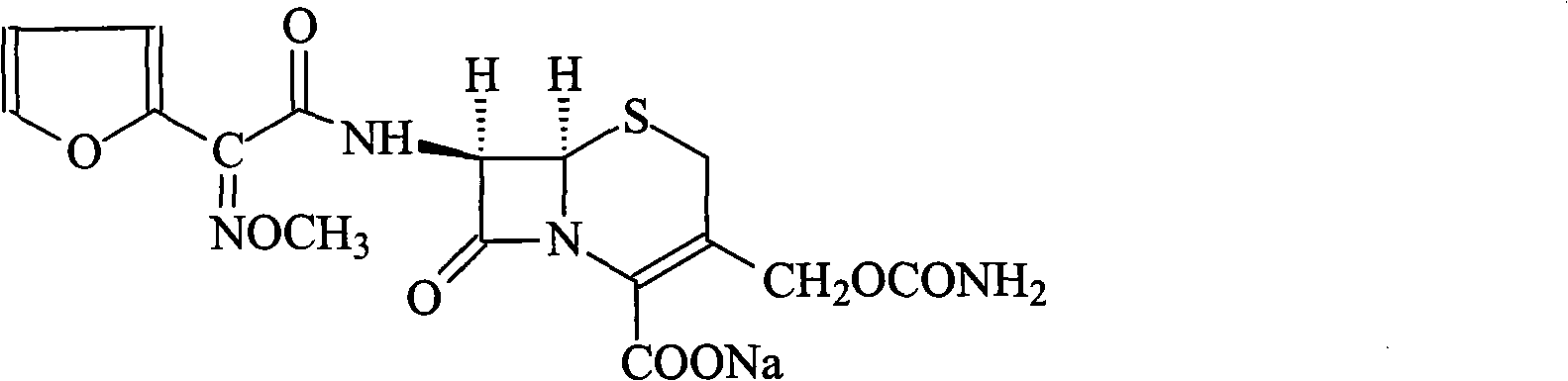
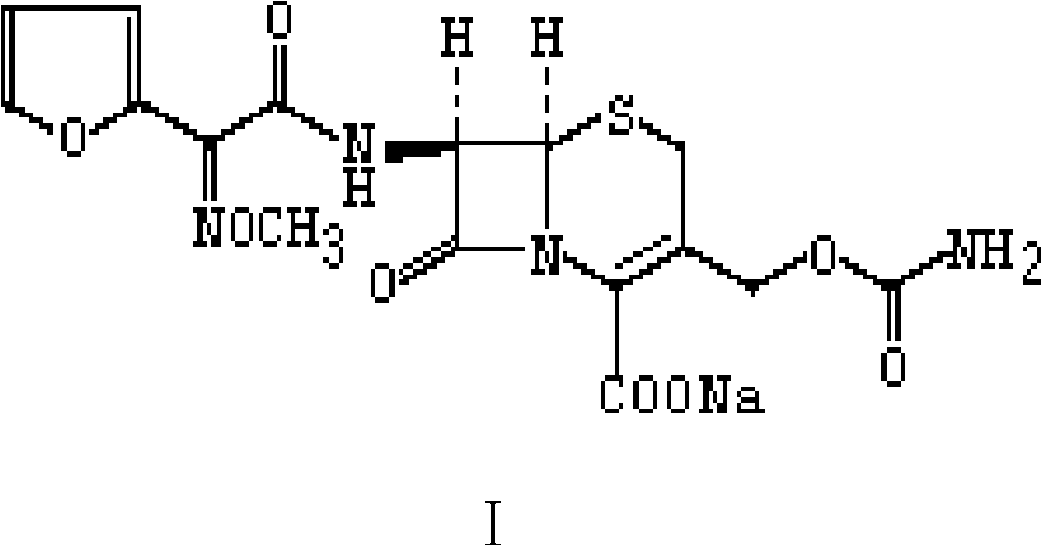
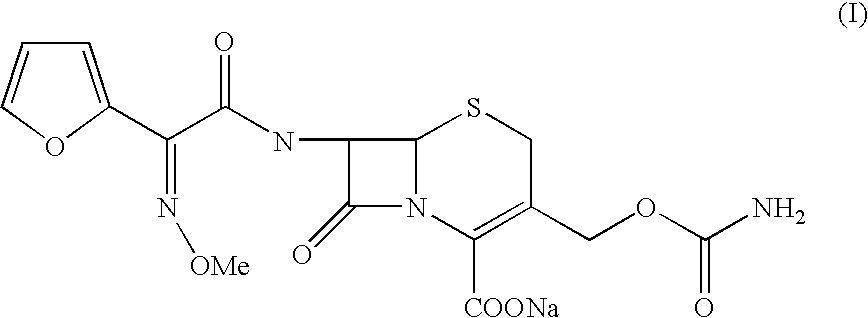
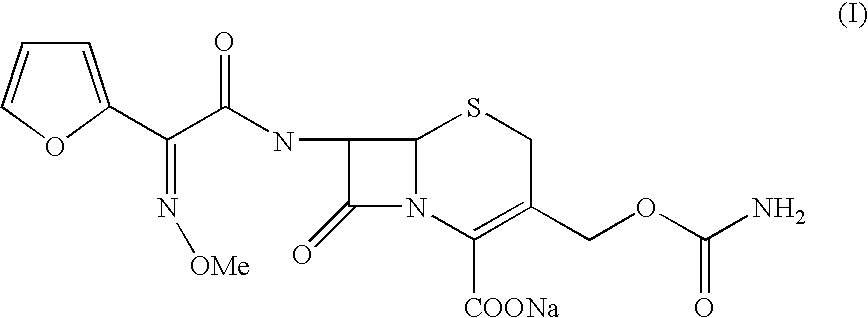
The present invention relates to an improved process for the preparation of the sterile cefuroxime sodium of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Cefuroxime sodium compound and new preparation method thereof
InactiveCN102079750AHigh purityIncrease contentAntibacterial agentsOrganic chemistryActivated carbonUpper urinary tract infection
The invention provides a cefuroxime sodium compound and a new preparation method thereof. The method comprises the following steps: performing acid-base reaction, absorbing with activated carbon, and separating and purifying with a chromatographic column. By adopting the method, the purity and content of cefuroxime sodium can be greatly increased, the product quality of the preparation can be increased, the toxic or side effects can be reduced and the safety of the preparations of the medicines for curing respiratory tract infection and urinary tract infection can be ensured; and compared with the prior art, the method has the advantages of simple and practical technology, mild reaction conditions, low cost, high yield and high product purity and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefuroxime sodium powder preparation for injection
ActiveCN106361706AHigh clarityImproved color level stabilityAntibacterial agentsPowder deliverySocial benefitsCefuroxime Sodium
The invention discloses a cefuroxime sodium powder preparation for injection, belonging to the technical field of medicine preparation. The cefuroxime sodium powder preparation for injection is prepared by the following steps: preparation of a sterile formed sodium agent solution, preparation of a sterile cefuroxime acid solution, crystallization and sub-packaging, wherein a two-time grain growing technology is adopted for the crystallization, so that the crystallization quality is improved. With good color and high stability, the product disclosed by the invention greatly improves the quality and safety of cefuroxime sodium, is favorable for improving the national health level, and realizes remarkable economic and social benefits.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
One-step recovery and preparation method of cefuroxime sodium
The invention relates to a one-step recovery and preparation method of cefuroxime sodium. The method comprises: dispersing cefuroxime sodium in a water-containing or non-aqueous mixed solvent of alcohol and ketone, adding hydrochloric acid or sulfuric acid at a temperature of 0-50°C, and reacting for 0.5-3 hours, and cefuroxime sodium is gradually converted into cefuroxime Fuuroctanoic acid is dissolved in the reaction system, and the by-product sodium chloride or sodium sulfate generated simultaneously is insoluble in the reaction system; Then, activated carbon is added for decolorization, filtered, and the by-product and activated carbon are filtered to obtain the cefuroxime acid solution; the obtained Cefuroxime sodium solution is mixed with sodium lactate or sodium isooctanoate aqueous or non-aqueous organic solvent solution to precipitate cefuroxime sodium crystals. The present invention adopts a one-step method for the recovery and preparation of cefuroxime sodium, without the need to separate and dry cefuroxime acid, thereby reducing the damage to cefuroxime acid, and the water content of the system is low during the recovery and preparation process, which is not suitable for unstable cefuroxime sodium Has a good protective effect.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Novel industrial crystallizing technology for cefuroxime sodium
ActiveCN104961749APromote enrichmentSimple crystallization processPowder deliverySolvent extractionPhysical chemistryCefuroxime Sodium
The invention discloses novel industrial crystallizing technology for cefuroxime sodium. Recrystallizing of cefuroxime sodium is realized by adopting a mode combining supercritical fluid extraction technology with conventional crystallizing technology. In a crystallizing system, the processes of extracting, adsorbing, crystallizing and drying are completed under specific temperature and pressure conditions and under joint action of supercritical fluid, solvent, an extraction pool and a crystallization pool to realize recrystallizing of cefuroxime sodium. The novel industrial crystallizing technology is high in separation efficiency and few in impurity, and quality of cefuroxime sodium is improved greatly.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Technology for preparing cefuroxime sodium for injection
InactiveCN106279209AMany solutions, deep colorFix stability issuesOrganic chemistry methodsBulk chemical productionSodium acetateCefuroxime Sodium
The invention discloses a technology for preparing cefuroxime sodium for injection. The technology includes the steps that a sodium acetate solution is dropwise added into a cefuroxime acid solution, and a cefuroxime sodium crude product is prepared with the crystallization technology; then the cefuroxime sodium crude product is extracted with the supercritical fluid extraction technology, recrystallization is carried out with the crystallization technology, and the cefuroxime sodium for injection is finally obtained. The cefuroxime sodium prepared with the technology is small in impurity number and good in stability, the problems that existing cefuroxime sodium is large in impurity number, deep in color, poor in stability and the like are solved, and the requirements for an injection can be completely met.
Owner:南昌立健药业有限公司
Compoiste preparation of cefuoxime sodium
InactiveCN1582942ALittle side effectsReduce dosageAntibacterial agentsOrganic active ingredientsCefuroxime SodiumChemistry
A composite cefuroxime sodium is prepared from cefuroxime sodium or its salt and beta-lactamase inhibitor in weight ratio of (4-8):1.
Owner:SHENYANG J & HEALTH PHARMA
Method utilizing coupling reaction crystallization to prepare cefuroxime sodium
InactiveCN102617604AReduce adsorption filtration processReduce degradationOrganic chemistryActivated carbonFiltration
The invention relates to a method utilizing coupling reaction crystallization to prepare cefuroxime sodium, which comprises dissolving cefuroxime acid in a mixed solvent at 20-30 DEG C to prepare a solution with the concentration to be 0.025g / ML-0.1g / Ml; adding an alkaline sodium salt water solution into the solution; mixing for 10-20min at constant temperature to enable a reaction to be complete, and adding cefuroxime sodium seed crystal; adding an elution agent after 10-20min; cooling the temperature of the solution to 0-5 DEG C, and keeping at the constant temperature for 0.5-2h; filtering, washing and drying the obtained suspension, and obtaining a cefuroxime sodium product. The method reduces the adsorption filtration process of activated carbon and avoids loss of yield. The method achieves coupling of reactions and crystallization, and the methods of elution crystallization and cooling crystallization are combined with each other in the crystallization process, the crystallization process is controlled easily, the particle size of the product is uniform, the liquidity is greatly improved, the purity is higher than 99.5%, and the yield is over 92%.
Owner:TIANJIN UNIV +1
Cefuroxime sodium compound entity and application thereof
InactiveCN104072519AReduce humidityEasy to makeAntibacterial agentsOrganic chemistryJoint infectionsBacilli
The invention discloses a cefuroxime sodium compound entity which is relatively poor in hygroscopicity, relatively good in storage stability and applicable to preparation of drugs for treating or preventing respiratory system infection, five-organ infection, urinary system infection, pelvic cavity infection, septicemia, skin soft-tissue infection, bone and joint infection, gonorrhea and meningitis of human or animals, caused by sensitive gram positive or negative bacteria.
Owner:刘力
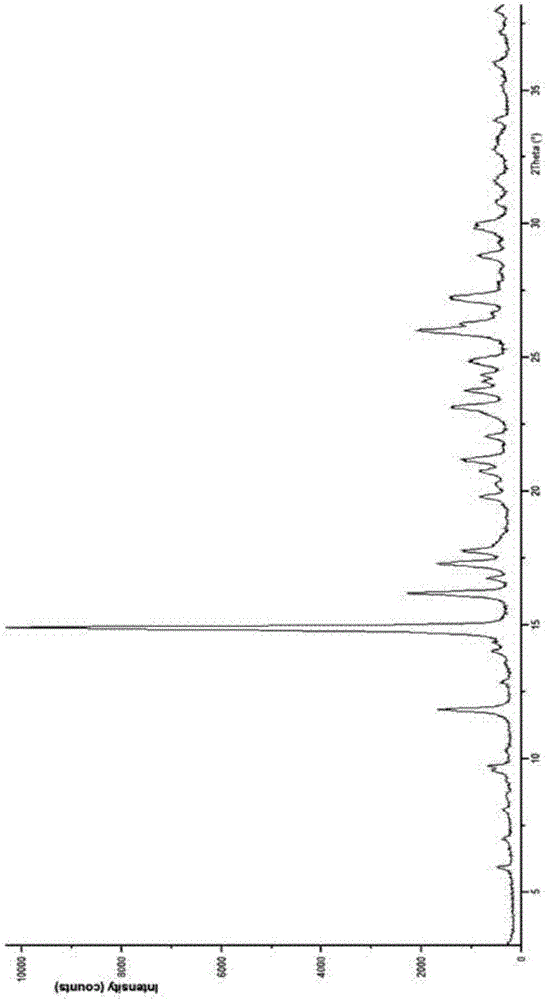
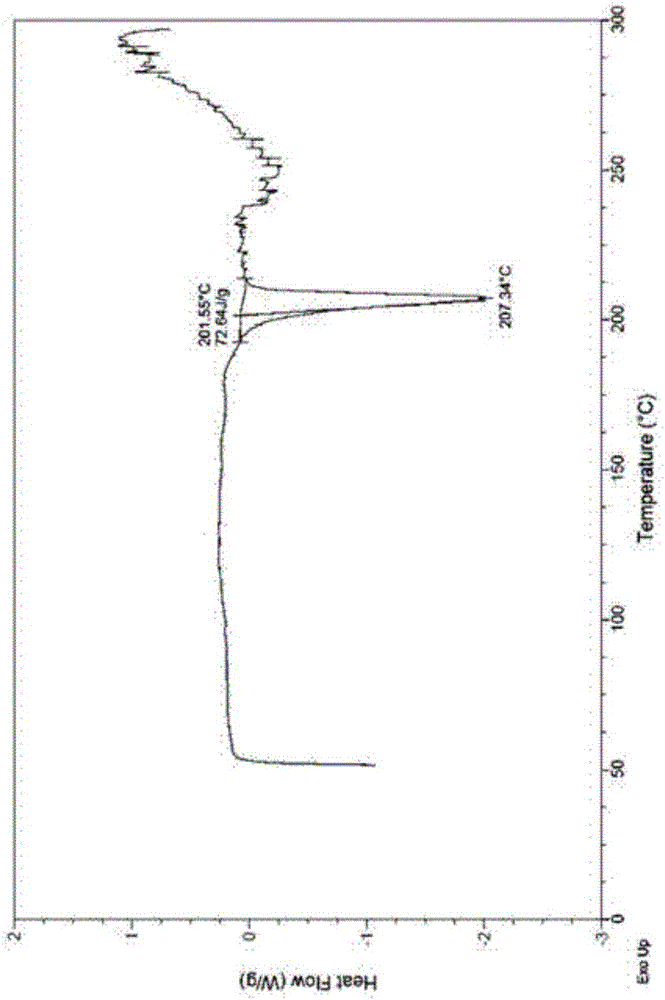
Novel cefuroxime sodium compound
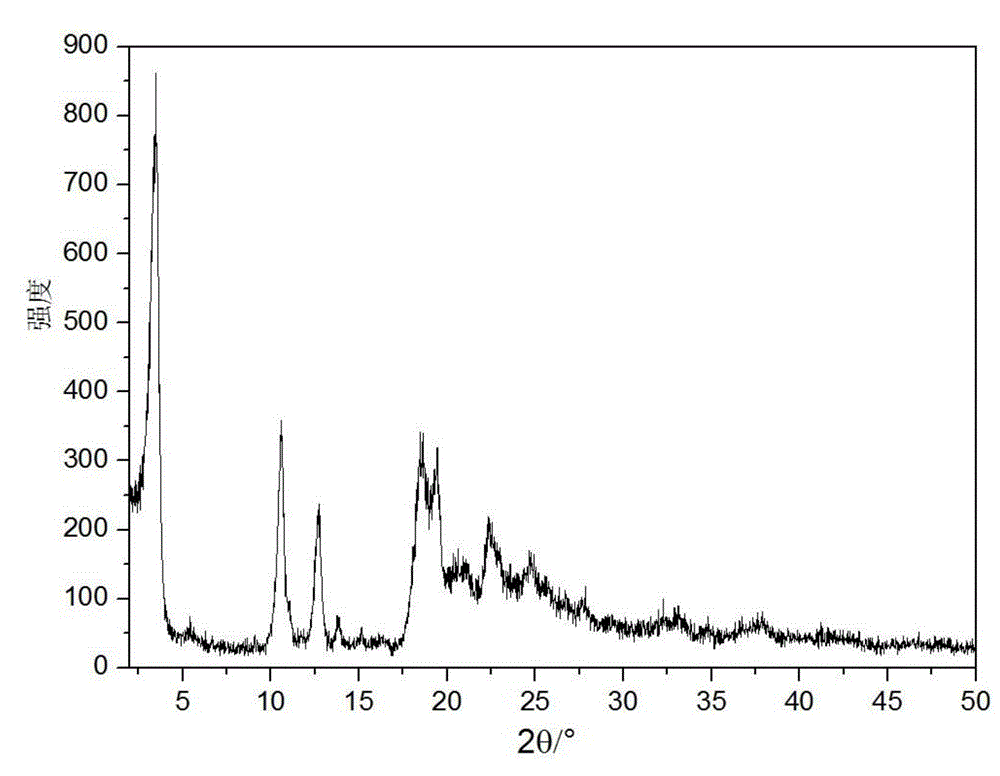
InactiveCN105884799AImprove liquidityImprove stabilityPowder deliveryOrganic active ingredientsX-rayCefuroxime Sodium
The invention relates to a novel cefuroxime sodium compound and a preparation method thereof and belongs to the technical field of medicine. According to the novel cefuroxime sodium compound, in an X-ray powder diffraction spectrum of the novel cefuroxime sodium compound, characteristic peaks are displayed at the positions where 2theta equals to 11.78 + / -0.1 degrees, 14.86 + / -0.1 degrees, 16.12 + / -0.1 degrees, 17.24 + / - 0.1 degrees, 19.74 + / -0.1 degrees, 23.07 + / -0.1 degrees, 25.96 + / -0.1 degrees and 27.16 + / - 0.1 degrees, and an endothermic peak exists at the position where the temperature is 200-210 DEG C in a differential scanning calorimetry spectrum. The novel cefuroxime sodium compound is good in mobility, more meets requirements of technology of pharmaceutics and is more suitable for preparing various pharmaceutic preparations. Due to the fact that powder-injection prepared from the novel cefuroxime sodium compound is good in stability, besides, stability can reach 72 hours or above after the powder-injection is prepared into an injection solution, storage time is greatly prolonged, therefore safety and efficiency of drug use are improved, and the occurrence rate of adverse reactions is reduced.
Owner:石药集团中诺药业(石家庄)有限公司
Cefuroxime sodium suspension powder injection
InactiveCN101721379AUnexpected effectImprove stabilityAntibacterial agentsOrganic active ingredientsCholesterolCefuroxime Sodium
The invention provides a cefuroxime sodium suspension powder injection. The cefuroxime sodium suspension powder injection comprises the following components by weight part: 1 part of cefuroxime sodium, 2 to 10 parts of surfactant and 3 to 18 parts of lyoprotectants. The cefuroxime sodium suspension powder injection is characterized in that the surfactant is a combination of cholesterol and tween-80 in a weight ratio of 6:1-4:1. The cefuroxime sodium suspension powder injection is prepared unexpectedly by using the emulsification and suspension technologies through specific surfactant after freezing and drying and solves the problem of poor stability of the cefuroxime sodium.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefuroxime sodium synthesizing method
The invention relates to the technical field of pharmaceutical and chemical industries and particularly discloses a cefuroxime sodium synthesizing method. The synthesizing method comprises the steps:dropwise adding alkali solution into 7-aminocephalosporanic acid aqueous solution for hydrolysis reaction, and then subjecting the mixture and (Z)-2-furyl-2-methoxyimino acetic acid p-toluene sulfonicanhydride to amidation to obtain 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid; dissolving the 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid into organic solvents to sequentially perform nucleophilic addition and hydrolysis reaction with chlorosulfonyl isocyanate, then adding sodium iso-octoate solution, and performing devitrification, filtering, washing and drying to obtain cefuroxime sodium. According to the synthesizing method, raw materials are wide and easy to obtain, the cost is low, the steps are simple, operation is simple, and side reaction is less.
Owner:湖北凌晟药业股份有限公司 +1
Cefuroxime sodium freeze dry power and preparation method thereof
InactiveCN101491502ASimple processReduce labor intensityAntibacterial agentsPowder deliveryActivated carbonPenicillin
The invention discloses a Cefuroxime sodium lyophilized powder and a method for preparing the same. The method is characterized in that a Cefuroxime sodium solution is added with active carbon, decolored, subjected to decarbonization and aseptic filtration and washed by purified water; a mixture of a filtrate and an eluant is pressed into a feed tray or a penicillin bottle of a freezedryer; a product with the moisture of Cefuroxime sodium of less than 2 percent is prepared by a lyophilization method; and the method simplifies a process, reduces labor intensity, aseptic risk, production cost and pollution and has rapid dissolution speed, good stability and high yield.
Owner:邢建荣
Cefuroxime sodium new crystal type compound and preparation adopting particle process crystal product molecular assembly and form optimizing technology
InactiveCN105669700AHigh purityImprove color gradeAntibacterial agentsPowder deliveryPhysical chemistryCefuroxime Sodium
The invention discloses a cefuroxime sodium new crystal type compound and a crystallizing preparation method thereof. The cefuroxime sodium new crystal type compound is prepared through a particle process crystal product molecular assembly and form optimizing technology. A stability test proves that the new crystal type compound has the advantages of being high in purity, low in impurity content, low in hygroscopicity and good in stability. Meanwhile, the invention discloses a preparation, cefuroxime sodium for injection. The preparation is prepared from cefuroxime sodium.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Preparation method for cefuroxime sodium and preparation thereof
ActiveCN106565748AImprove stabilityGood colorAntibacterial agentsOrganic active ingredientsCefuroxime SodiumImpurity
The invention discloses a preparation method for cefuroxime sodium and a preparation thereof, belonging to the technical field of medicine preparation. The preparation method comprises the following three steps: preparation of 3-deacetyl-7-aminocephalosporanic acid; preparation of cefuroxime acid; and preparation of cefuroxime sodium. According to the invention, charging and reaction manners are changed to improve reaction quality, and a crystallization system and a crystallization manner are changed at the same time; so prepared cefuroxime sodium and the preparation thereof have good colors, good stability, low impurity content and a purity increased by 0.2 to 0.3% compared with products produced by conventional processes, which is beneficial for improving the health level of people in China.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
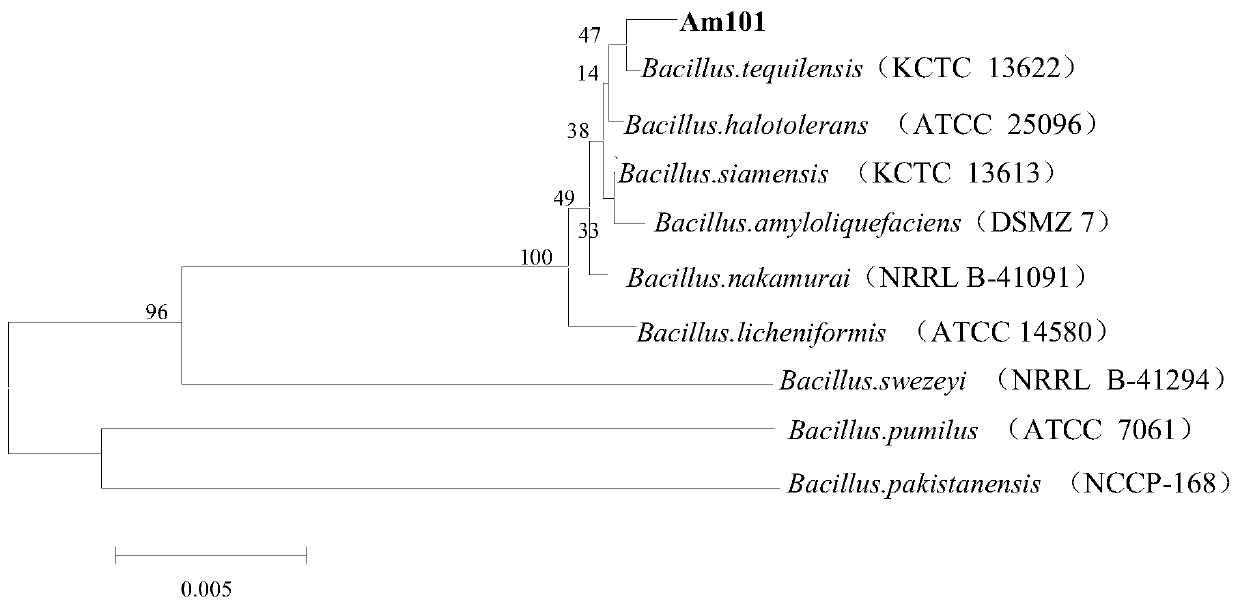
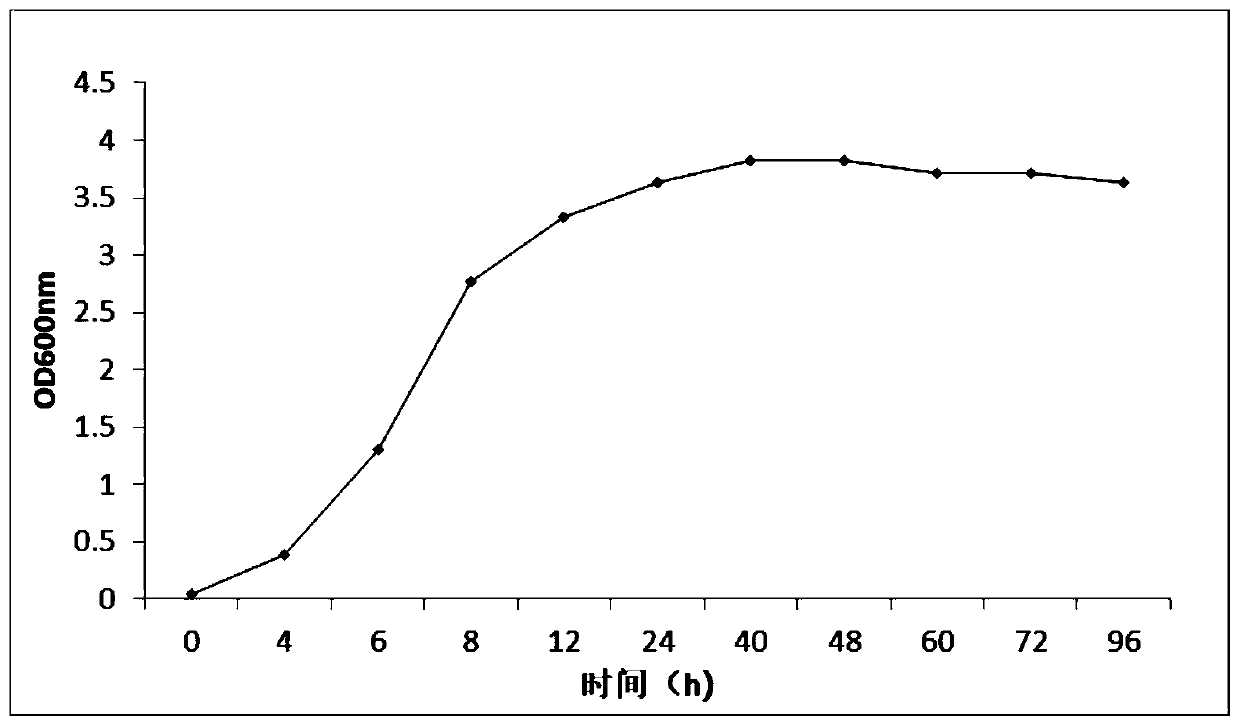
Strain Am101 capable of degrading various beta-lactam antibiotics and application of strain Am101
ActiveCN110791450ABroad degradation spectrumFast degradationBacteriaWater contaminantsBiotechnologyBenzylpenicillin potassium
The invention discloses bacillus tequilensis Am101 capable of degrading various beta-lactam antibiotics and an application of the bacillus tequilensis Am101. The strain is preserved in China General Microbiological Culture Collection Center on November 18, 2019, and a preservation number is CGMCC NO. 18965. The strain Am101 disclosed by the invention can be used for simultaneously degrading various beta-lactam antibiotics and can be used for efficiently degrading benzylpenicillin potassium, amoxicillin, cefuroxime sodium and ceftiofur sodium. The strain Am101 can be applied to degradation treatment of residual beta-lactam antibiotics in livestock and poultry manure, can also be applied to restoration of water and soil polluted by the beta-lactam antibiotics, and has the wide application prospect.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com