Double specificity oligopeptide-cefuroxime sodium strengthened fusion protein Ec-LDP-Hr-AE
A fusion protein, lidamycin technology, applied in the direction of peptide/protein composition, specific peptide, recombinant DNA technology, etc., to achieve the effect of small molecular weight, good stability and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
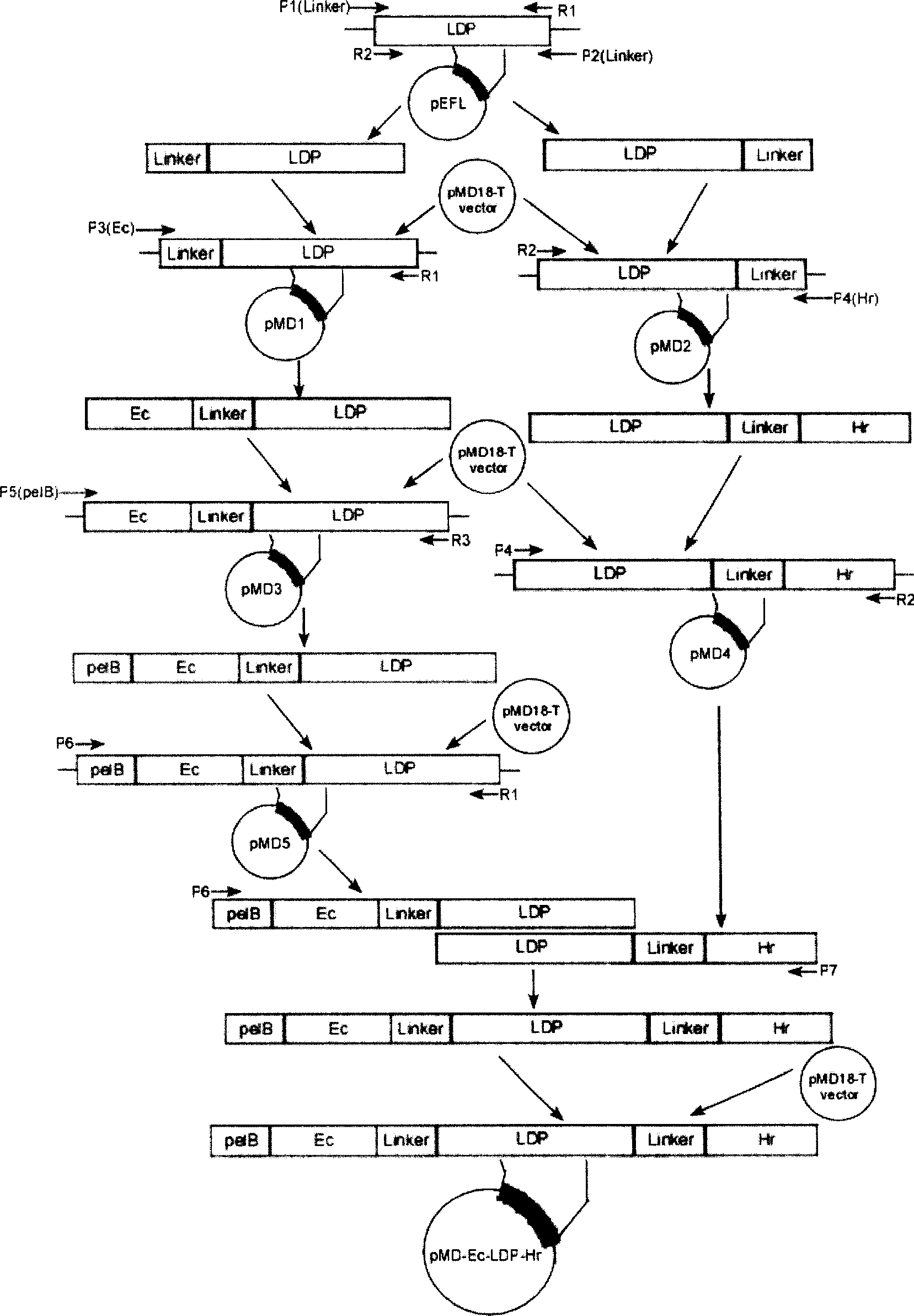
[0067] Construction of recombinant expression plasmid pET-Ec-LDP-Hr:
[0068] PCR reaction primers:
[0069] P1: 5'ggt gga ggc ggt tca ggt gga ggt tca gcg ccc gcc ttc tcc gtc agt 3'
[0070] (1-27bp is (G 4 S) 2 Connecting peptide sequence, 28-48bp paired with lidamycin prosthetic protein 1-21bp)
[0071] P2: 5’tga acc gcc tcc acc tga acc gcc tcc acc gcc gaa ggt cag agc cac gtg 3’
[0072] (1-30bp for (G4S) 2 Connecting peptide sequence, 31-51bp paired with lidamycin prosthetic protein 639-660bp)
[0073] P3: 5’aac tgt gtg gtg ggc tat att ggc gaa cgc tgt cag tat cgc gat ctg aaa tgg tgg gaa ctgcgc ggt gga ggc ggt tca ggt 3’
[0074] (1-66bp is the oligopeptide Ec fragment sequence, 67-84bp and (G 4 S) 2 connecting peptide 1-18bp pairing)
[0075] P4: 5' gc ctc gag cac gcc cag cca ttc cgg cca ttt cgc aca gct gcg atc ggt aca ata gcc cacatc atg tga acc gcc tcc acc tga 3’
[0076] (The underlined part is the XhoI restriction site, 9-60bp is the oligopeptide Hr sequenc...
Embodiment 2
[0094] Induced expression of fusion protein Ec-LDP-Hr in Escherichia coli
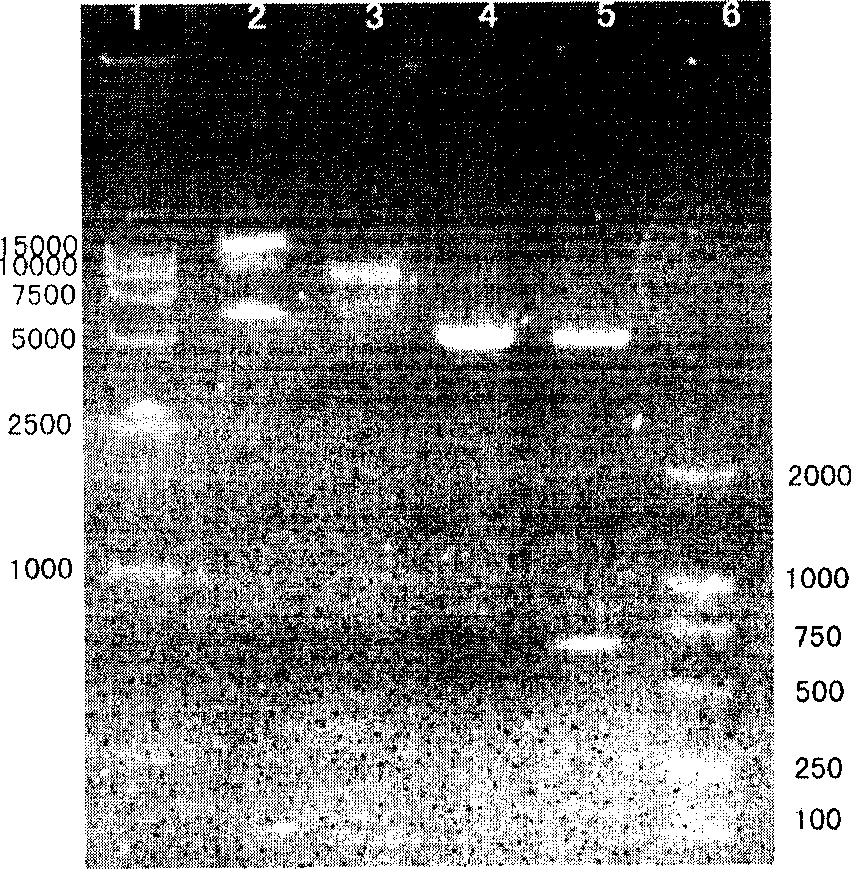
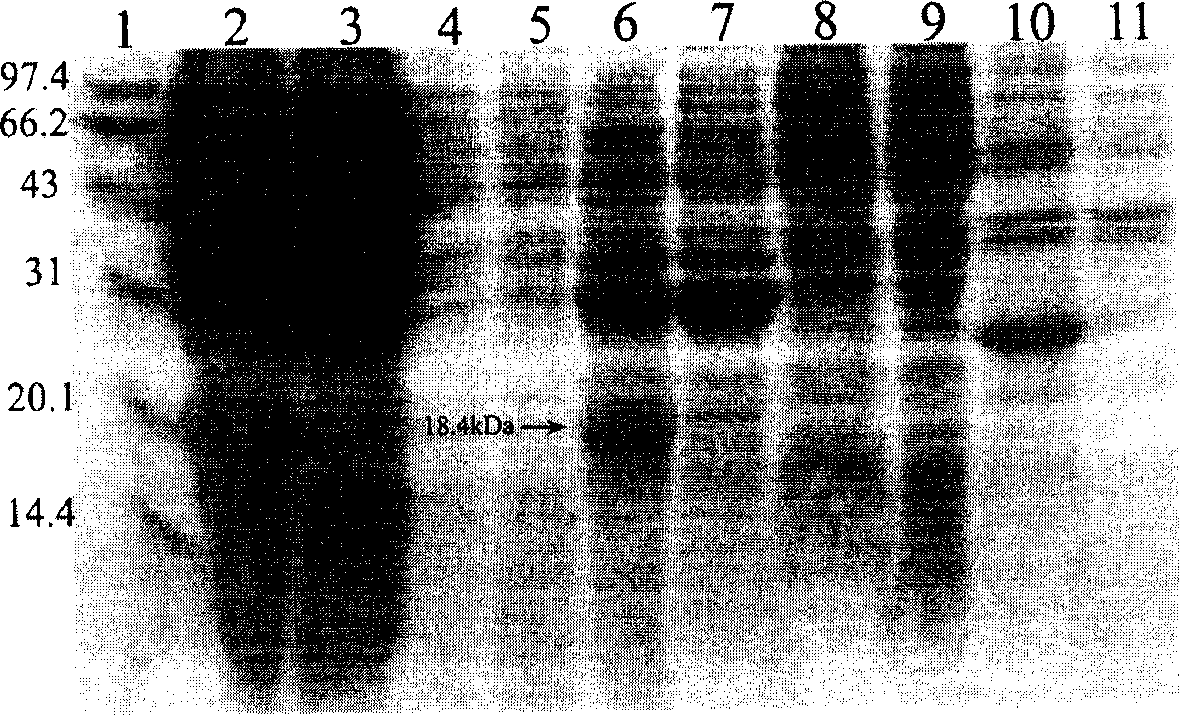
[0095] Transform the expression vector pET-Ec-LDP-Hr into Escherichia coli BL21star TM (DE3) Competent cells. Pick a single clone from the plate and inoculate it into 10 ml of LB medium (containing 50 μg / ml of kanamycin), and cultivate overnight at 37° C. on a shaker. On the next day, 10 ml of the bacterial liquid was transferred to 1 L of LB medium (containing 50 μg / ml of kanamycin), and cultured on a shaker at 37°C until OD600=2. The bacterial solution was centrifuged at 4°C and 5,000 g for 10 minutes under sterile conditions, and the bacterial pellet was resuspended in 1 L of fresh LB medium (containing 50 μg / ml kanamycin), and IPTG was added to a final concentration of 0.1 mM. 37 Cultivate on a shaker for 8 hours. According to the steps in the pET system operation manual, the whole cell fraction, culture supernatant fraction, periplasmic cavity fraction, cytoplasmic soluble fraction and inclusi...
Embodiment 3
[0096] Separation and purification of fusion protein Ec-LDP-Hr
[0097] (1) Centrifuge the induced bacterial liquid at 4°C and 10,000g for 10 minutes to collect the bacterial cells. The fusion protein is mainly located in the periplasmic cavity of E. coli, and the periplasmic cavity protein is extracted using the osmotic shock method;
[0098] (2) Resuspend the bacteria in 100ml hypertonic solution (30mM Tris-Cl, 1mM EDTA, 20% sucrose, pH8.0), and stir slowly at room temperature for 10 minutes with a magnetic stirrer;
[0099] (3) Centrifuge at 4°C and 10,000g for 10 minutes, collect the bacteria, and discard the supernatant;
[0100] (4) Resuspend the bacteria with 90ml pre-cooled distilled water (hypotonic solution), and stir slowly at 4°C for 10 minutes with a magnetic stirrer;
[0101] (5) Centrifuge at 4°C, 10,000g for 10 minutes, and collect the supernatant;
[0102] (6) Add 10ml of 10×binding buffer (200mM phosphate buffer, 5M NaCl, 200mM imidazole) to the supernatan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com