Preparation method for cefuroxime sodium and preparation thereof
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the problems of poor stability and color change of cefuroxime sodium that affect the quality and safety of drugs Speed and other issues, to achieve the effect of improving the national health level, improving the quality and stability of finished products, and improving the purity of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A preparation method of cefuroxime sodium preparation, comprising the following processing steps:
[0034] The preparation of step A, 3-deacetyl-7-aminocephalosporanic acid:
[0035] Step A1, preparation of 7-ACA solution: In the container, add purified water and 7-ACA, control the temperature at 0-2 degrees, add sodium hydroxide solution dropwise, adjust the pH value to 6.5-8.0, until 7-ACA is completely dissolved , to obtain 7-ACA solution;
[0036] Step A2, preparation of furanacetyl chloride dichloromethane solution: in another container, add dichloromethane, control the temperature at -20~-10°C, add phosphorus pentachloride, dimethylacetamide, furan ammonium salt, keep warm React for 1-2 hours, then add purified water, stir for 5-15 minutes, stand still and separate phases to obtain furoacetyl chloride dichloromethane solution;
[0037] Synthesis of step A3, 3-deacetyl-7-aminocephalosporanic acid:
[0038] Add purified water and dichloromethane into another cont...
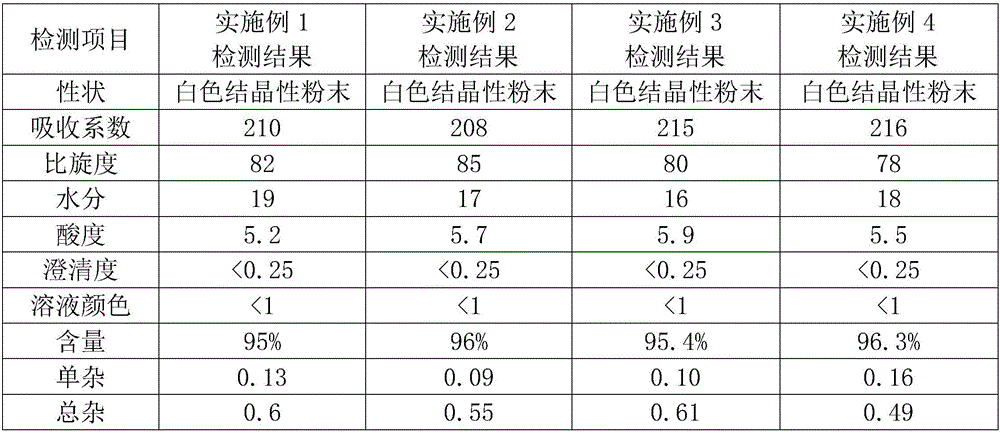
Embodiment 1
[0056] The preparation of step A, 3-deacetyl-7-aminocephalosporanic acid (7-DACA):
[0057] Step A1, 7 Preparation of ACA solution: In a four-necked bottle, add 230ml of purified water and 50g of 7-ACA, control the temperature at 0-2 degrees, add dropwise 15% by weight sodium hydroxide solution, adjust the pH value to 6.5-8.0,. Dissolve it completely for use;
[0058] Step A2, preparation of furoacetyl chloride: In another four-necked bottle, add 350ml of dichloromethane, control the temperature at -15°C, add 51.3g of phosphorus pentachloride, 75ml of dimethylacetamide (DMA), furan ammonium salt ( Here is 41g of methoxyimide ammonium furoacetate), heat preservation reaction for 1.5h, add 175ml of purified water, stir for 10min, stand still and separate phases to obtain furoacetyl chloride dichloromethane solution;
[0059] Synthesis of step A3, 3-deacetyl-7-aminocephalosporanic acid:
[0060] Add 25ml of purified water and 20ml of dichloromethane into another four-neck flask...
Embodiment 2
[0071] The difference between this example and Example 1 is that step A3 takes 1.5 hours to simultaneously add the 7ACA solution prepared in step A1, the furoacetyl chloride dichloromethane solution prepared in step A2 and the sodium hydroxide solution with a concentration of 15% by weight. The reaction temperature was controlled at 8°C, and the reaction pH was 7, and the reaction was maintained for 1.3 hours after the feed liquid was added, while the temperature and pH were controlled. After the reaction was completed, the phases were separated by standing still, and 350ml (a mixture of methanol and acetone with a volume mixing ratio of 5:5) was added to the water phase, and the temperature was lowered to 7°C for crystallization.
[0072] In step A4, add 50ml of methanol-acetone-water solution with a mixing volume ratio of 1:1:1, and add the synthetic solution of 3-deacetyl-7-aminocephalosporanic acid at a constant speed for 1h under rapid stirring (stirring speed is 500r / min)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com