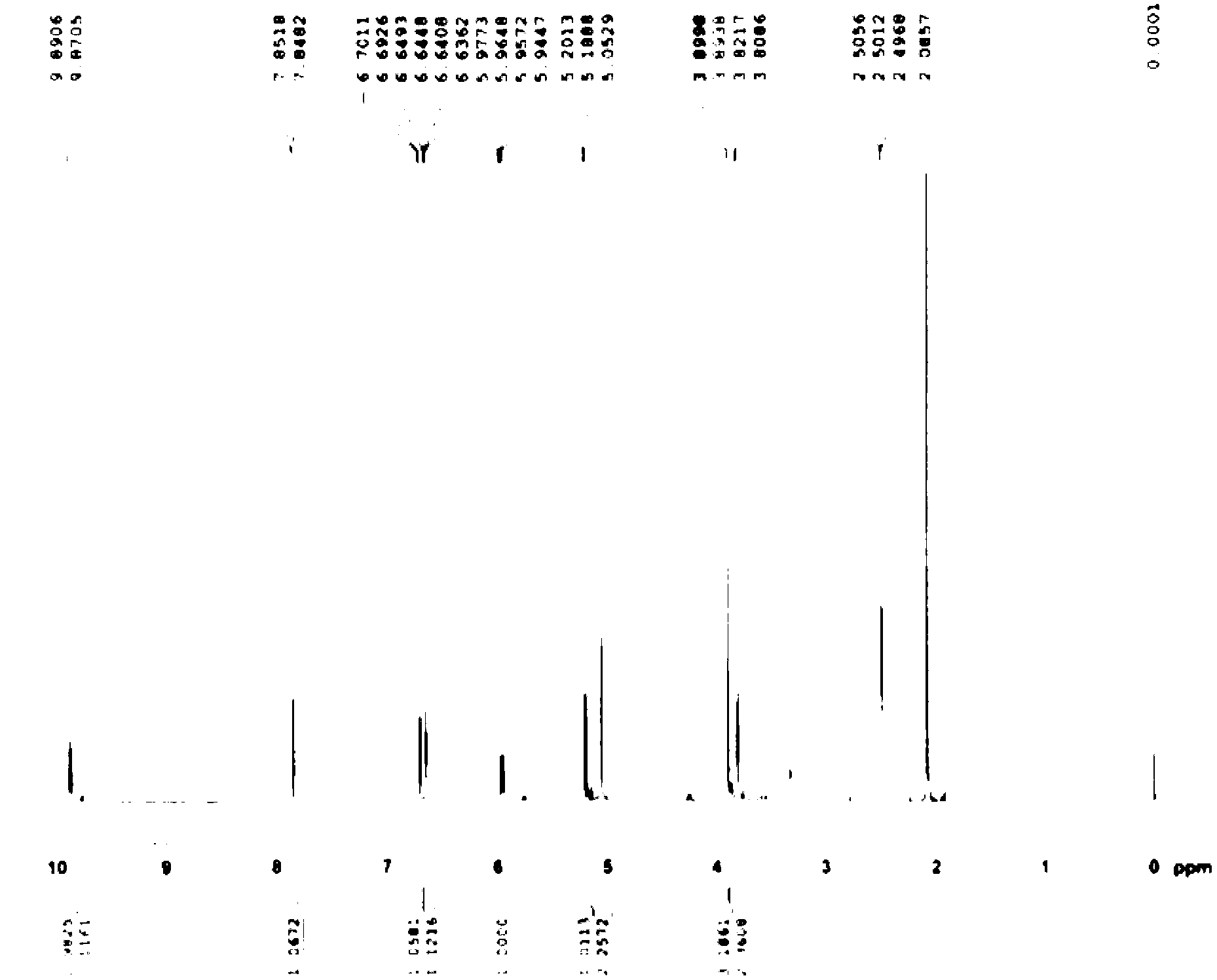
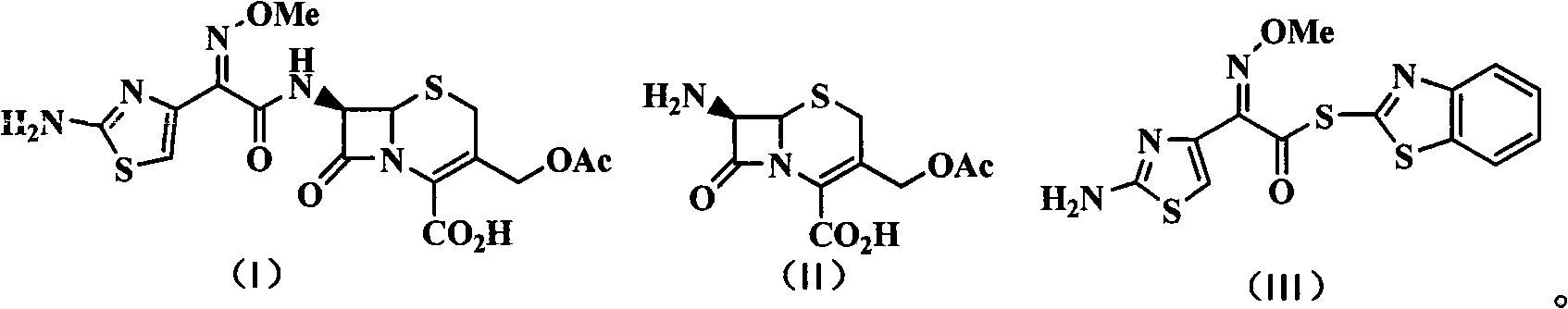
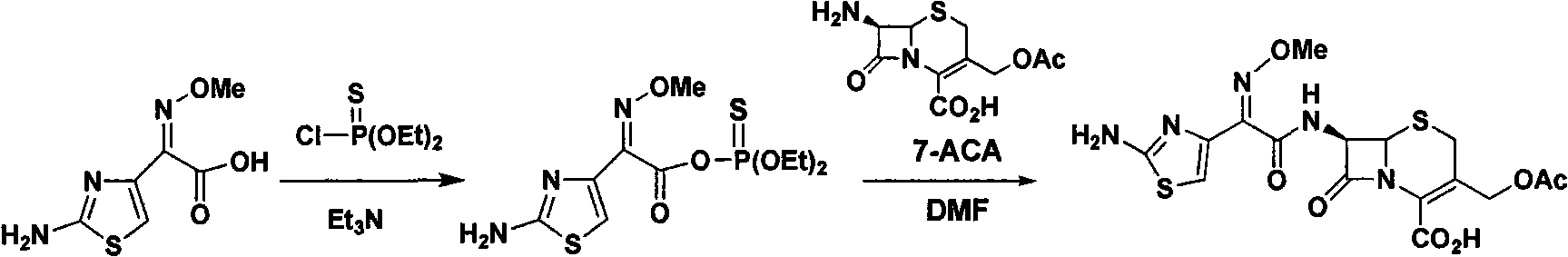
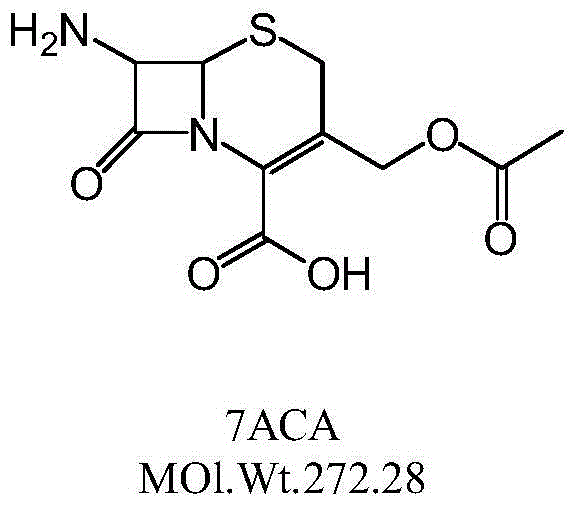
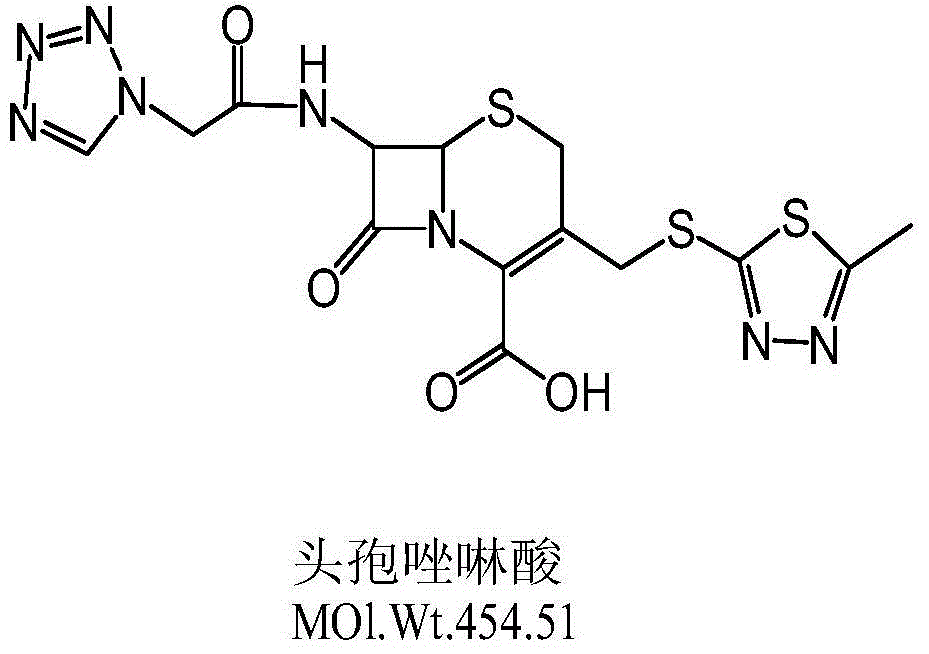
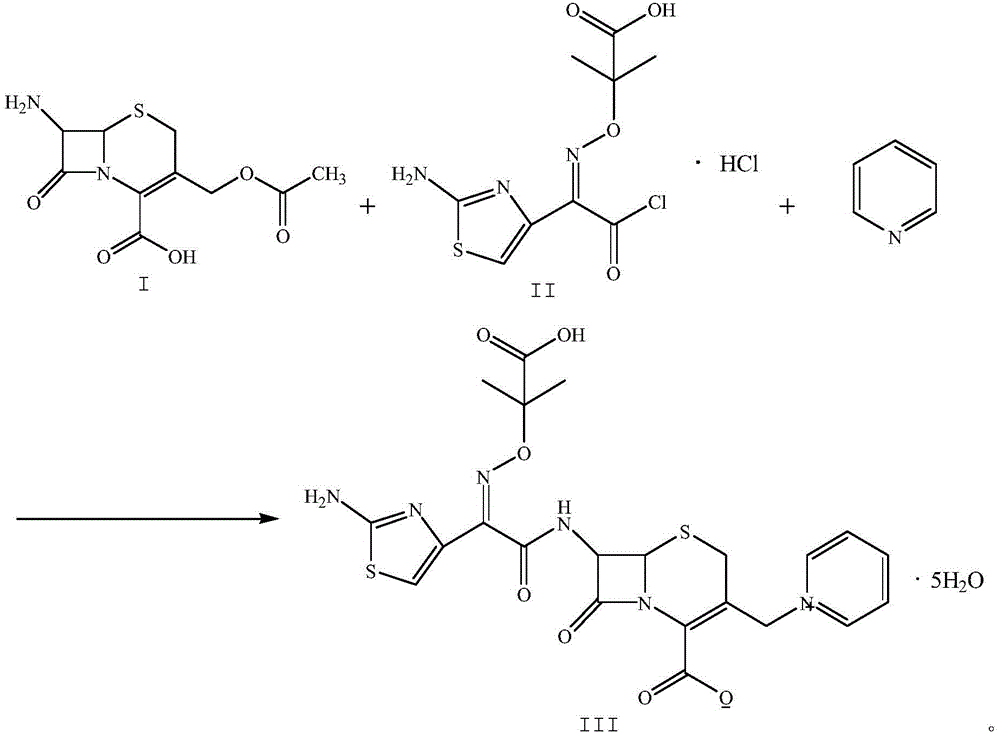
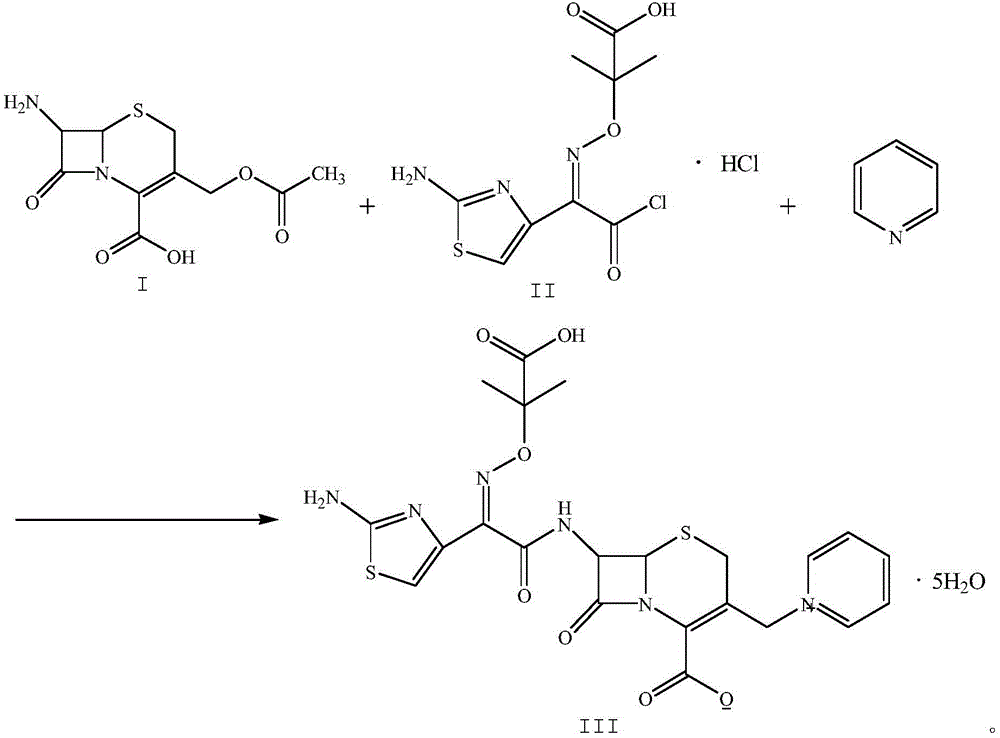
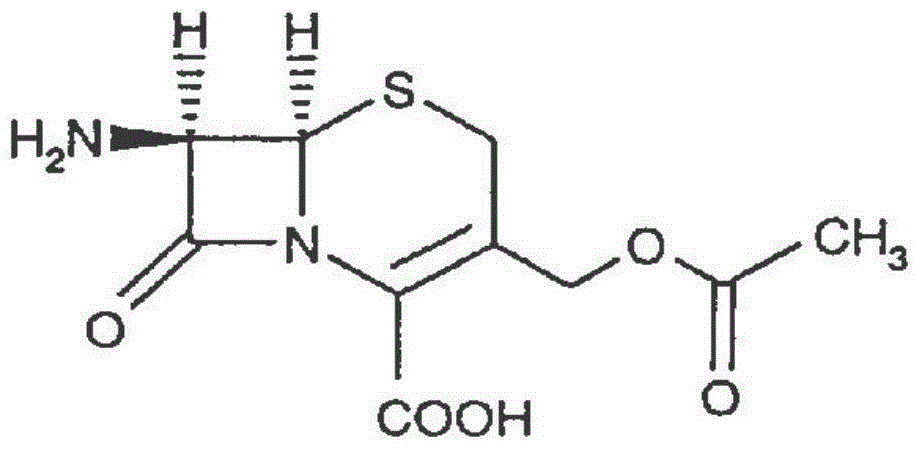
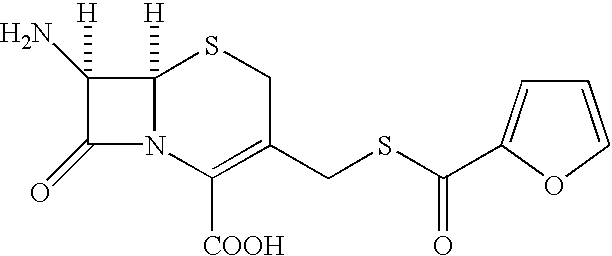
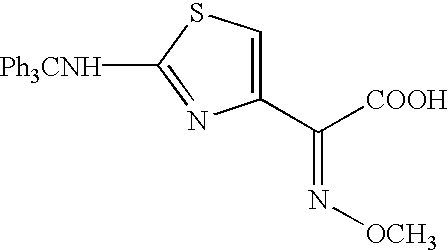
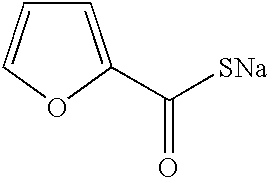
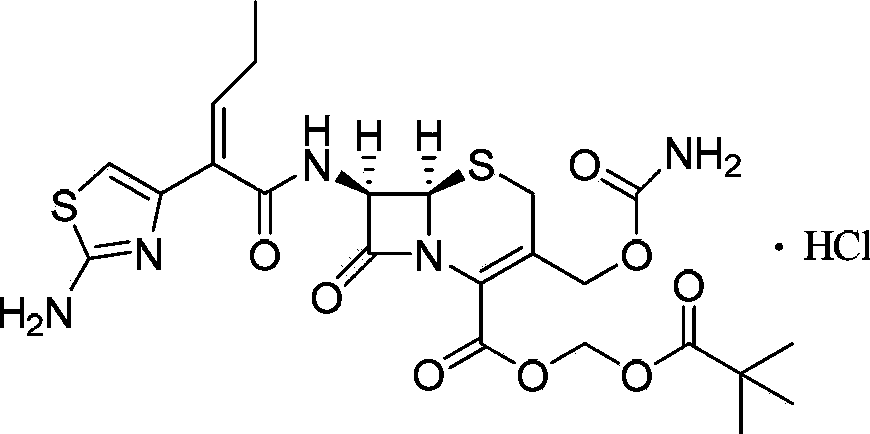
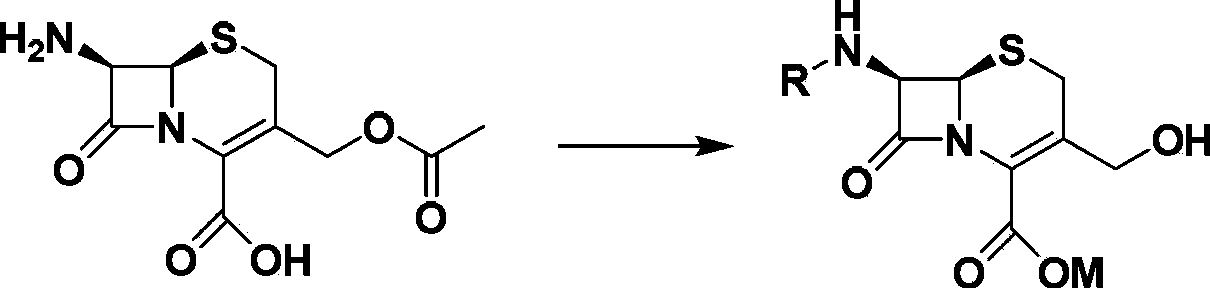
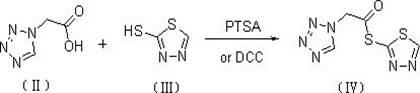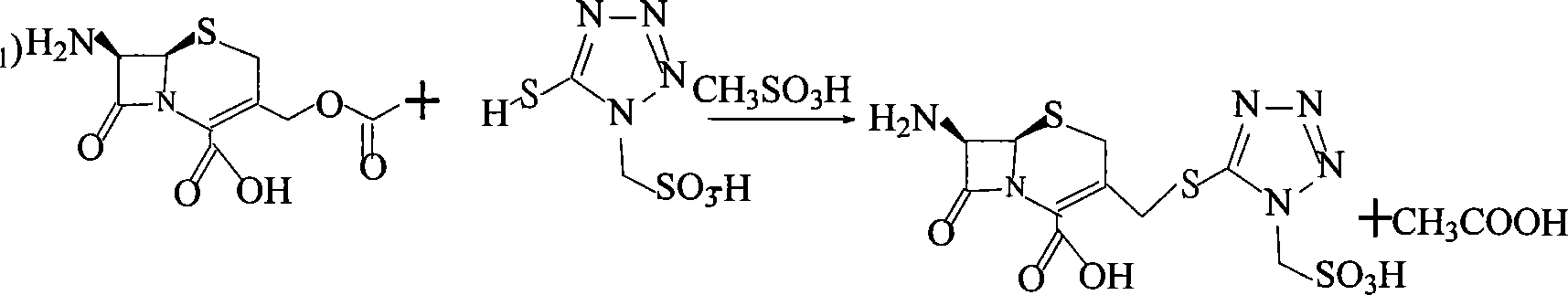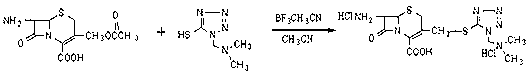Patents
Literature
150 results about "7-aminocephalosporanic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
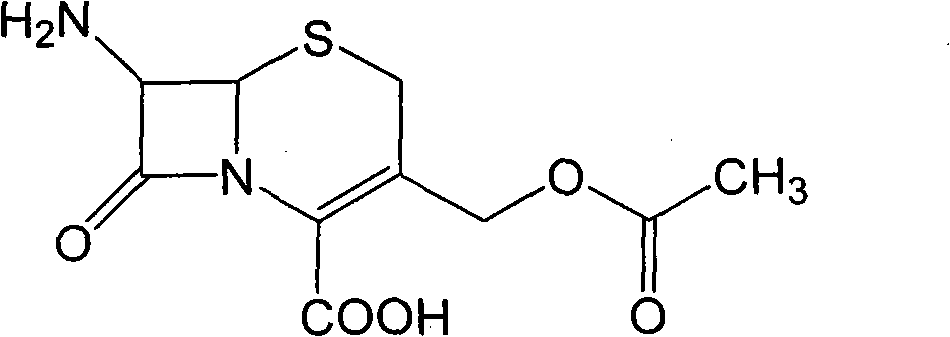
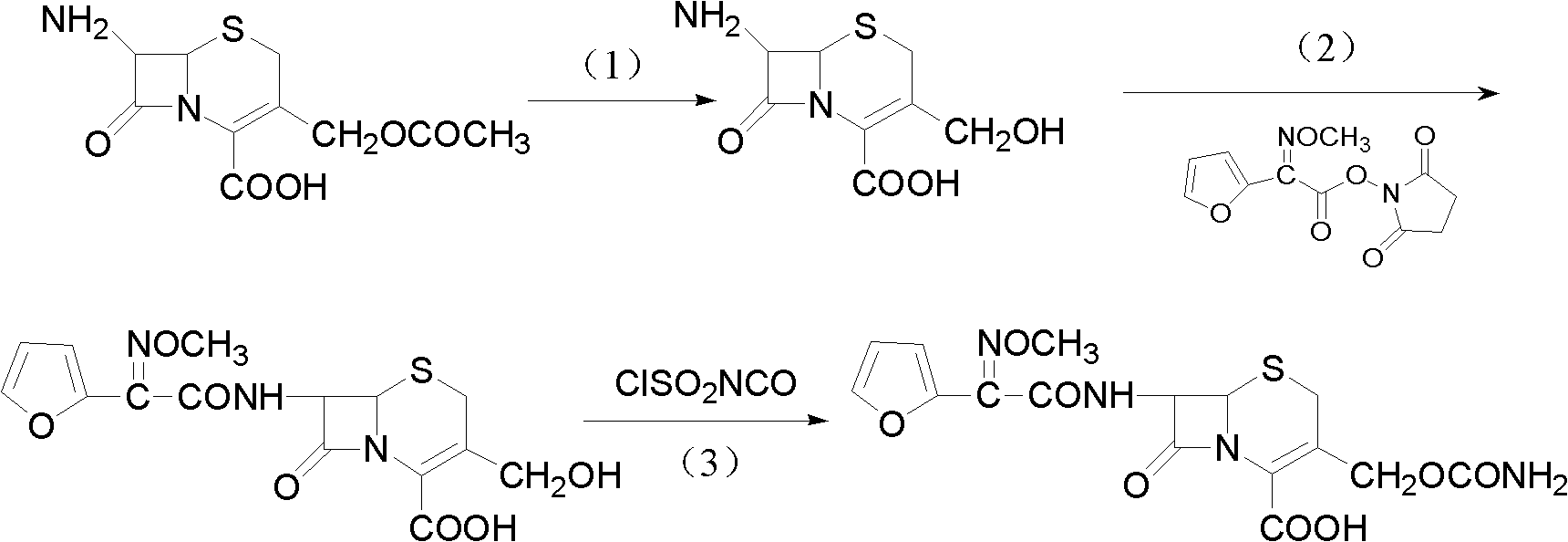
Preparation method of high-purity cefuroxime acid
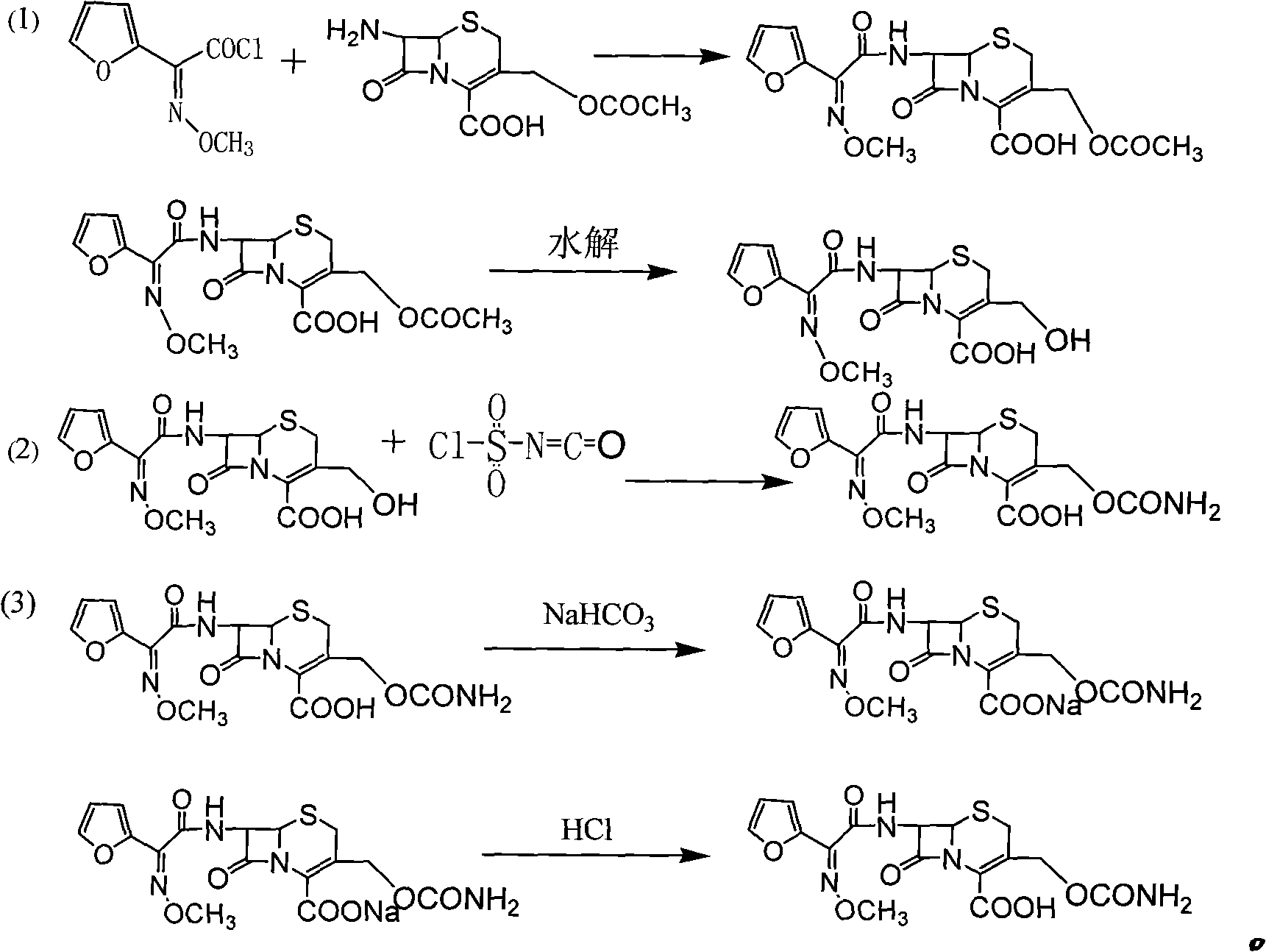
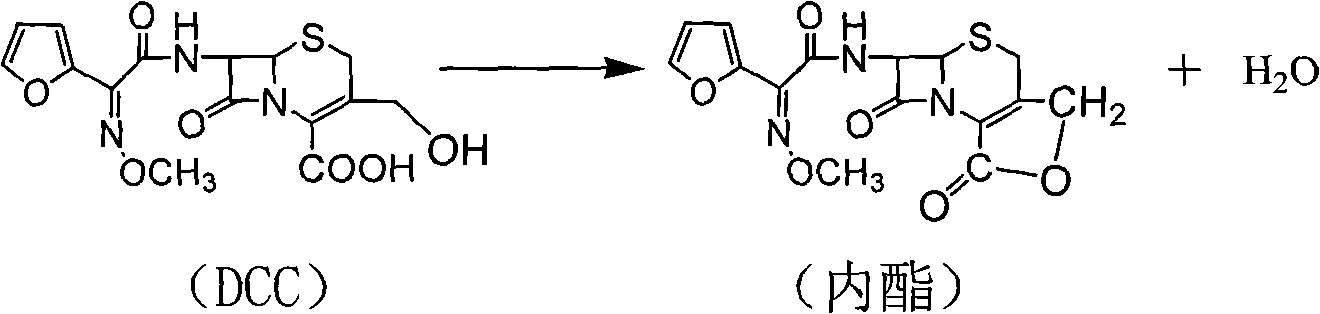
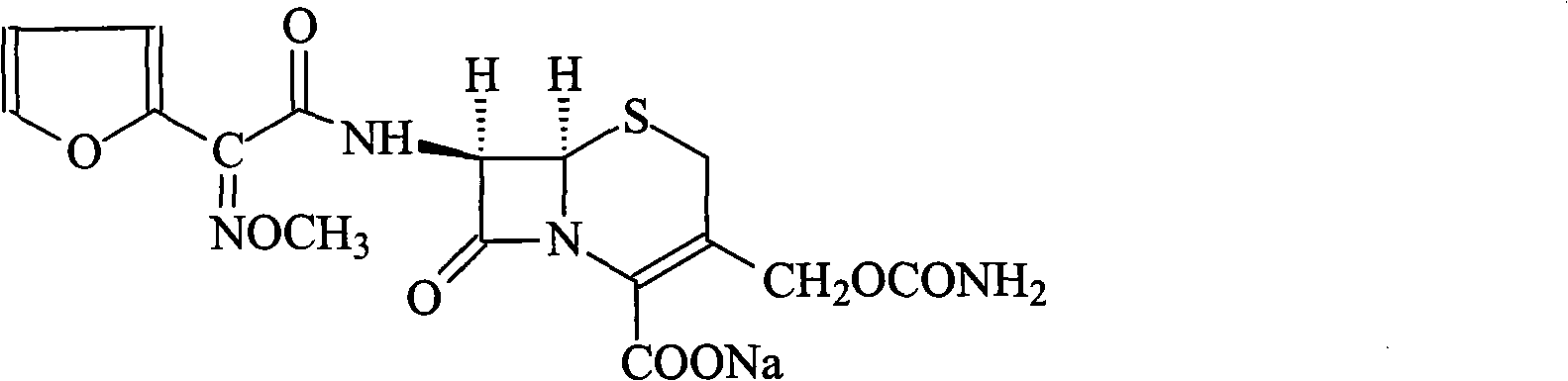
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
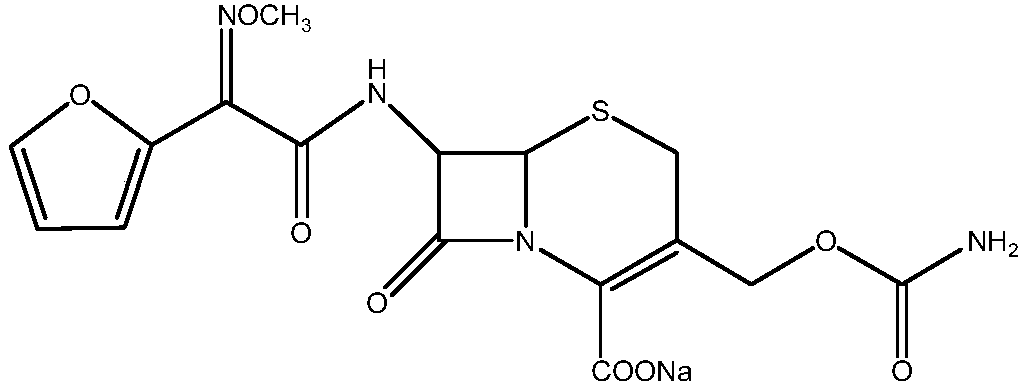
Method for synthesizing cefuroxime sodium
The invention relates to a method for synthesizing cefuroxime sodium, which comprises the following steps: 1, performing the N- acylation reaction of 3-deacetyl-7aminocephalosporanic acid and methoxyaminofuranyl ammonium salt which serve as raw materials and adjusting the pH value with hydrochloric acid to less than 7 in a mixed solvent phase to precipitate crystals to obtain 3-deoxyformyl cefuroxime acid; 2, performing the addition reaction of the 3-deoxyformyl cefuroxime acid and chlorosulfonyl isocyanate serving as a strong ammonia formylating agent in an organic solvent to obtain chlorosulfonyl cefuroxime acid, dehydrating the chlorosulfonyl cefuroxime acid to obtain the cefuroxime acid, decarburizing and concentrating the cefuroxime acid, crystallizing the cefuroxime acid in a solvent phase, and drying the crystals under vacuum to obtain a solid product of cefuroxime acid; and 3, dissolving the cefuroxime acid in alkaline solution, decarburizing the resulting product, crystallizing the resulting product in a mixed solvent phase, filtering crystals, and drying the crystals under vacuum to obtain the cefuroxime sodium.
Owner:哈药集团股份有限公司 +1
Cefuroxime sodium and preparation method thereof
The invention provides cefuroxime sodium and a preparation method thereof. The preparation method comprises the following steps that: (1) 7-aminocephalosporanic acid reacts with 2-(furan-2-base)-2-(methoxyimino) acetyl chloride to produce 3-deacety-7-aminocephalosporanic acid; (2) crystallization is conducted after the 3-deacety-7-aminocephalosporanic acid reacts with chlorosulfonyl isocyanate to produce cefuroxime acid; and (3) the cefuroxime acid is salified to obtain the cefuroxime sodium, wherein solvent for crystallization is selected from one or more of petroleum ether, normal hexane, cyclohexane, solvent oil and tetrahydrofuran. Since the preparation method adopts solvents such as petroleum ether for crystallization in the process of the preparation of the cefuroxime acid, the invention has the advantages that the yield of the product is effectively improved, the product purity is further improved, the impurity content is reduced, the product quality is compliant with Chinese Pharmacopoeia of version 2005, the operation of the method is simple, the raw materials can be easily obtained, the cost is relatively low and the industrial production can be realized easily.
Owner:LIVZON PHARM GRP INC
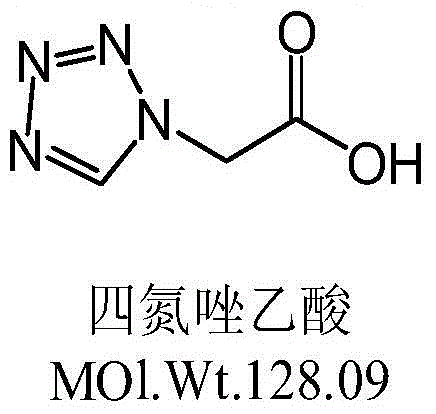
The preparation method of ceftezole sodium
InactiveCN102286001AReduce generationReduce pollutionOrganic chemistryCeftezole SodiumP-Toluenesulfonic acid
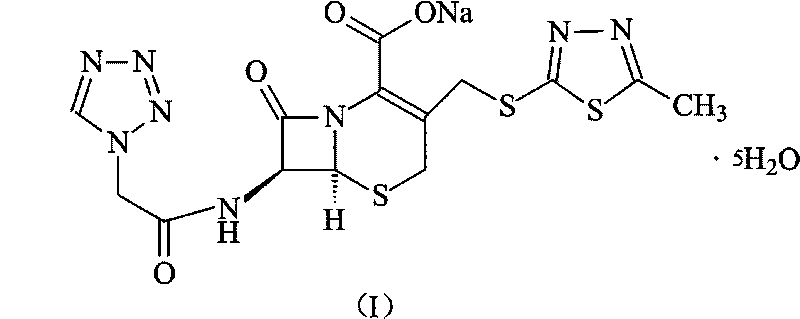
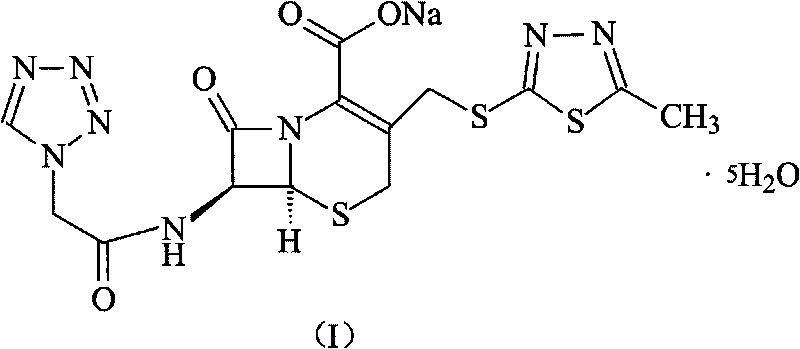
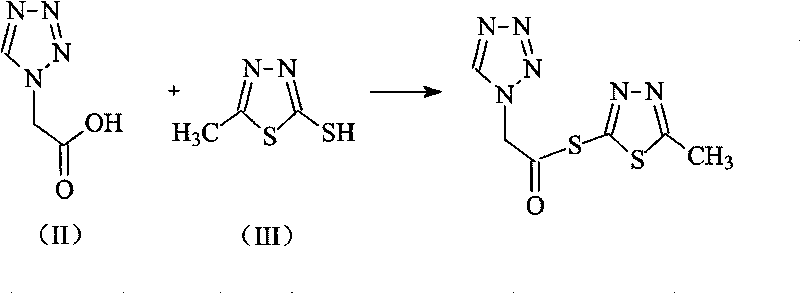
The invention discloses a preparation method of ceftizole sodium, which belongs to the field of medicinal chemistry. The method uses 1H-tetrazolium acetic acid and 2-mercapto-1,3,4-thiadiazole as raw materials, and generates 1H-tetrazolium acetic acid-1 under the catalysis of p-toluenesulfonic acid or dicyclohexylcarbodiimide ,3,4-thiadiazole-2-thioester (active ester), and then the active ester and 7-aminocephalosporanic acid are "one-pot" synthesis of ceftizole acid under the action of quaternary ammonium salt phase transfer catalyst, and then Sodium salt is generated, and further recrystallization and purification can obtain high-purity ceftiazole sodium. The preparation process is simple and feasible, the atom utilization rate is high, the product quality is good, and the industrial production requirements are met.
Owner:ZHENGZHOU UNIV
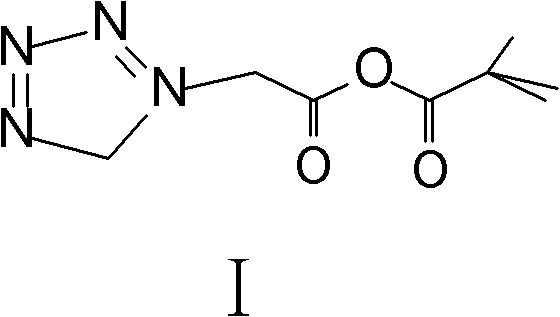
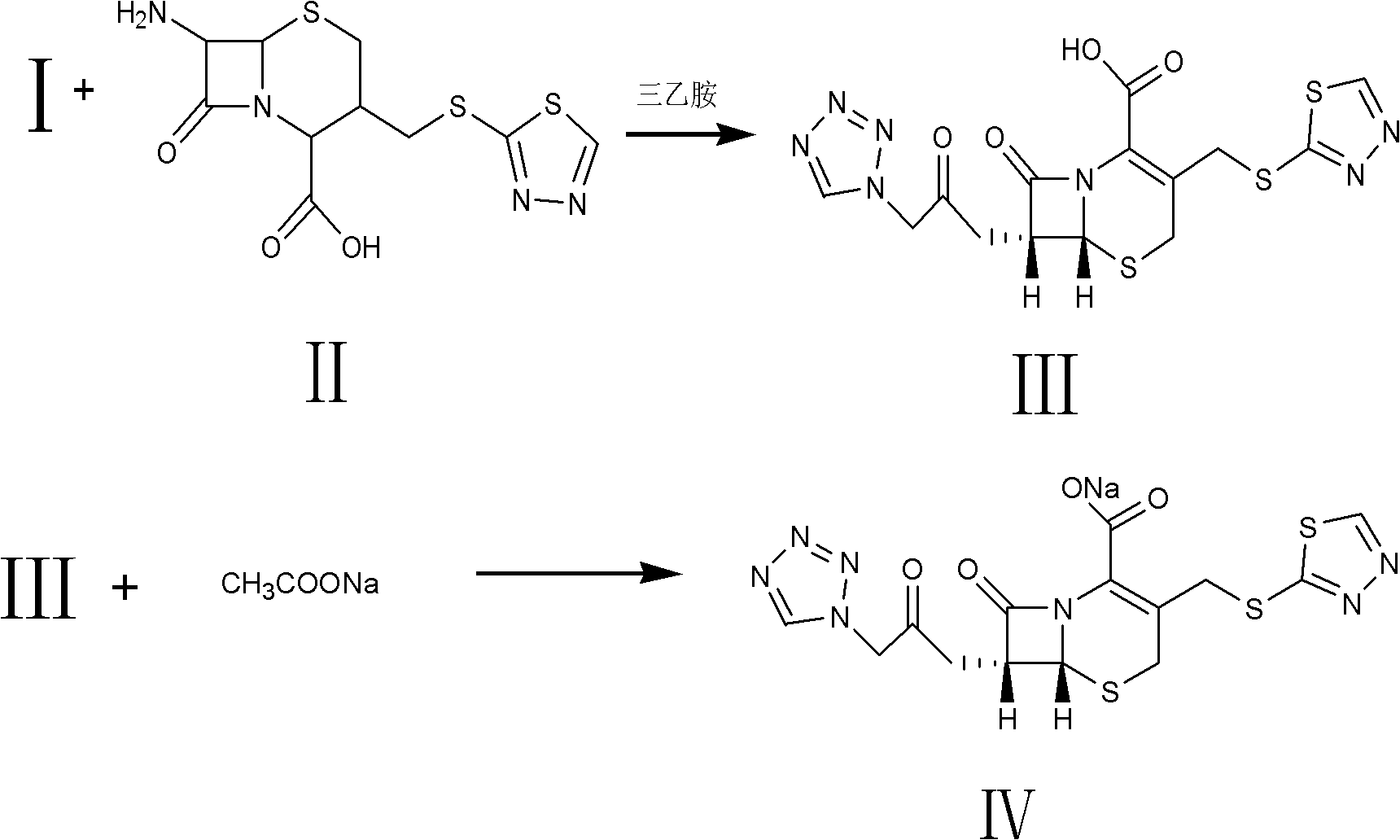
Method for preparing ceftezole sodium compound
ActiveCN102617606AReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumMethyl carbonate
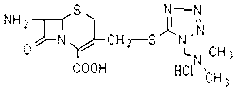
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
Method for preparing 7-aminocephalosporanic acid via enzymic method
ActiveCN103014114ALow impurity contentHigh purityOrganic chemistryFermentationFiltration membraneSorbent
The invention discloses a high-purity crystallizing method for preparing 7-ACA (aminocephalosporanic acid) via an enzymic method. The method comprises the following preparation processes: oxidizing cephalosporin C solution via oxidase and acylating cephalosporin C solution via acylase to obtain a 7-ACA crystallization solution; absorbing and separating the obtained 7-ACA crystallization solution via an adsorbent, carrying out membrane separation via an ultra-filtration membrane or a micro-filtration membrane, extracting and separating via an extracting agent in turn to obtain a purified 7-ACA crystallization solution; adding a crystallization accessory ingredient into the purified crystallization solution, adjusting PH via hydrochloric acid and crystallizing, then filtering, washing and drying to obtain high-purity 7-ACA crystallization particles. The invention can effectively reduce the impurity content in the 7-ACA crystal; the content of the impurity protein in the obtained 7-ACA crystal is less than 0.2% and the minimum content reaches 0.02%; and the endotoxin content is less than or equal to 0.5EU / mg and the minimum content reaches 0.01EU / mg.
Owner:华北制药河北莱欣药业有限公司
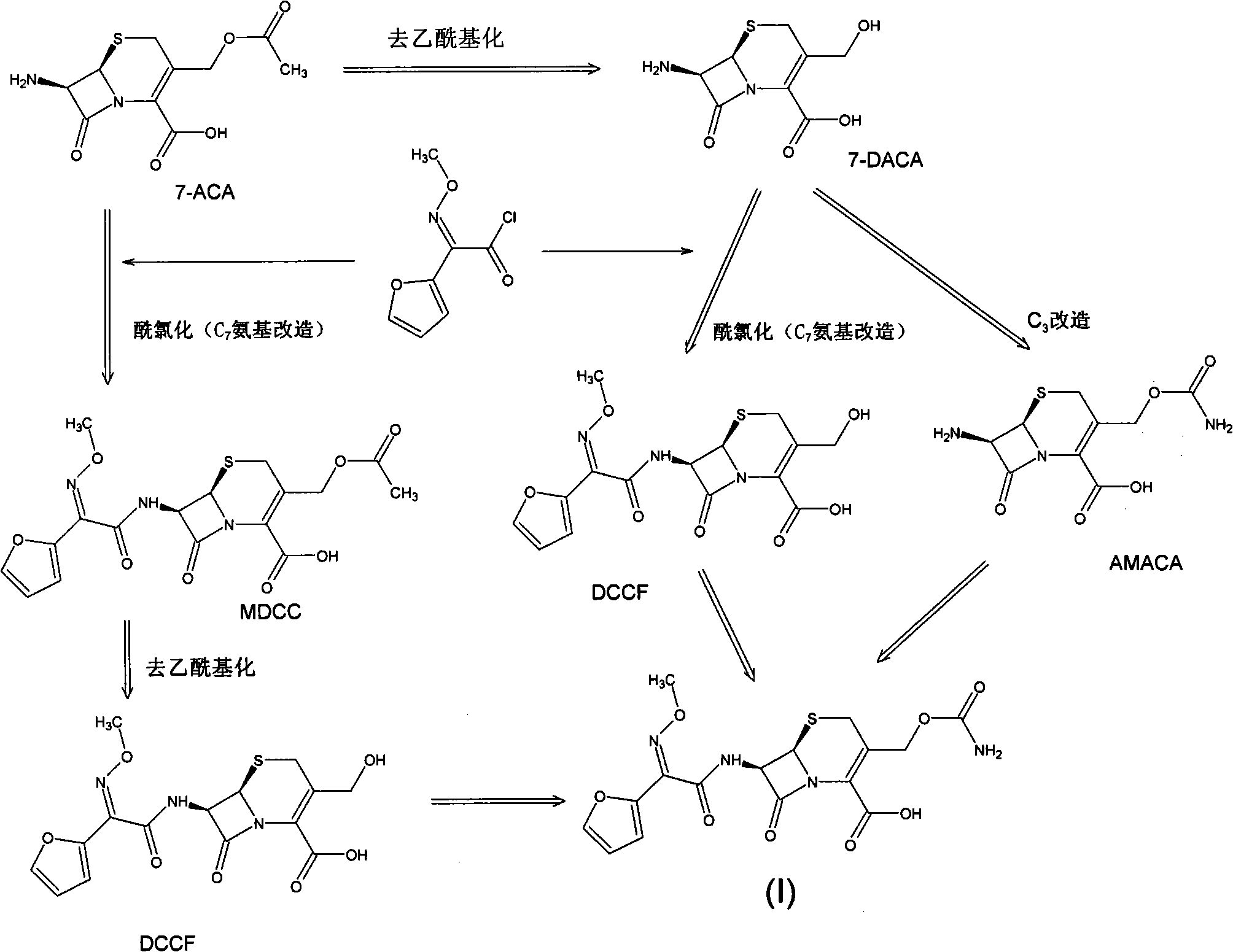
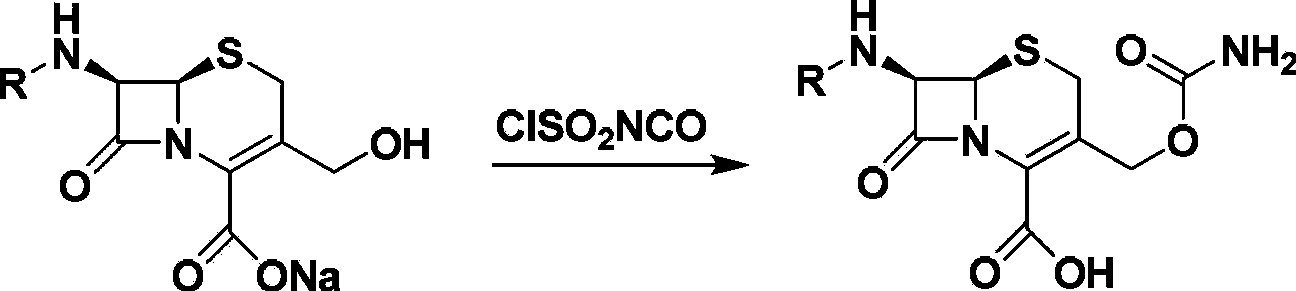
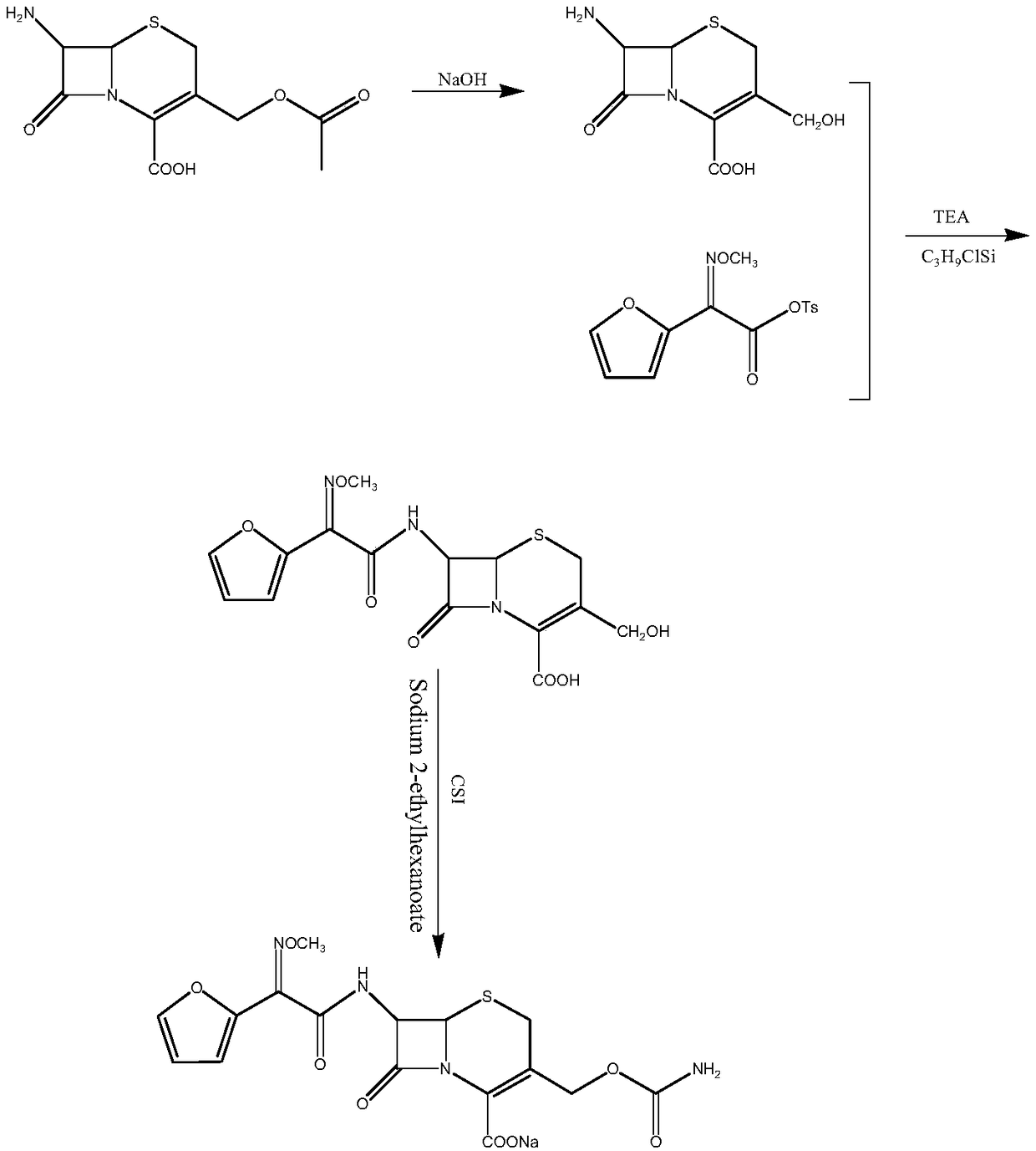
Method for preparing cefuroxime acid
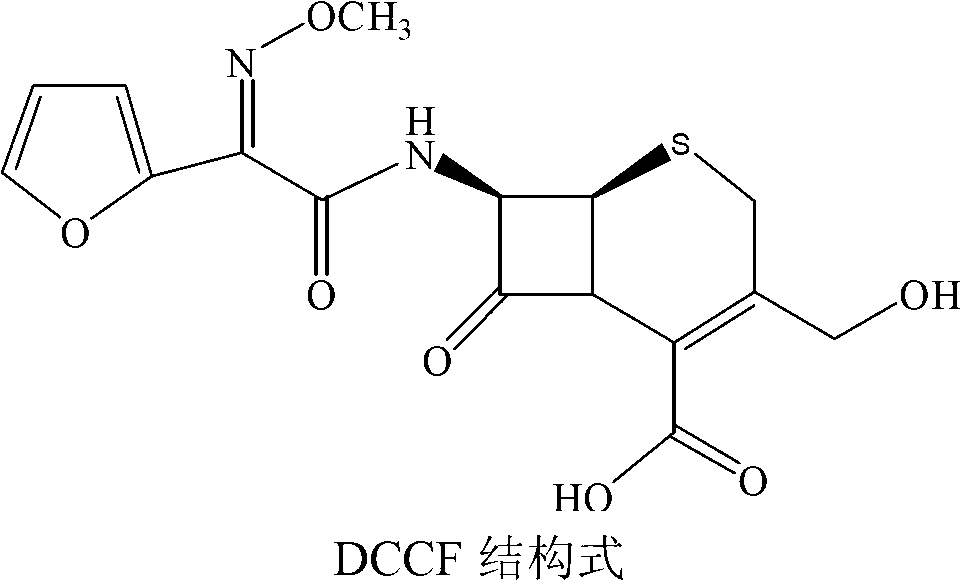
The invention discloses a method for preparing cefuroxime acid. The method comprises the following steps of: performing a chloride acylation reaction on 7-aminocephalosporanic acid (7-ACA) and methoxyiminofuran acetate serving as raw materials; performing deacetylation to synthesize DCCF; performing nucleophilic addition on the DCCF and chlorosulfonyl isocyanate (CSI) serving as a strong carbamoyl reagent to obtain chlorosulfonyl cefuroxime; and hydrolyzing the chlorosulfonyl cefuroxime to obtain cefuroxime acid. In the preparation method, the preparation process is simple, and the cefuroximeacid is crystallized by adopting aqueous solution, so that the loss of organic solvents is reduced; simultaneously, aids are added selectively in the reaction process to improve the quality of products, so that finished products with high purity and yield and good colors are obtained. The purity of the cefuroxime acid prepared by the method is more than 98.5 percent, and the weight yield is approximately 100 percent.
Owner:国药集团致君(苏州)制药有限公司
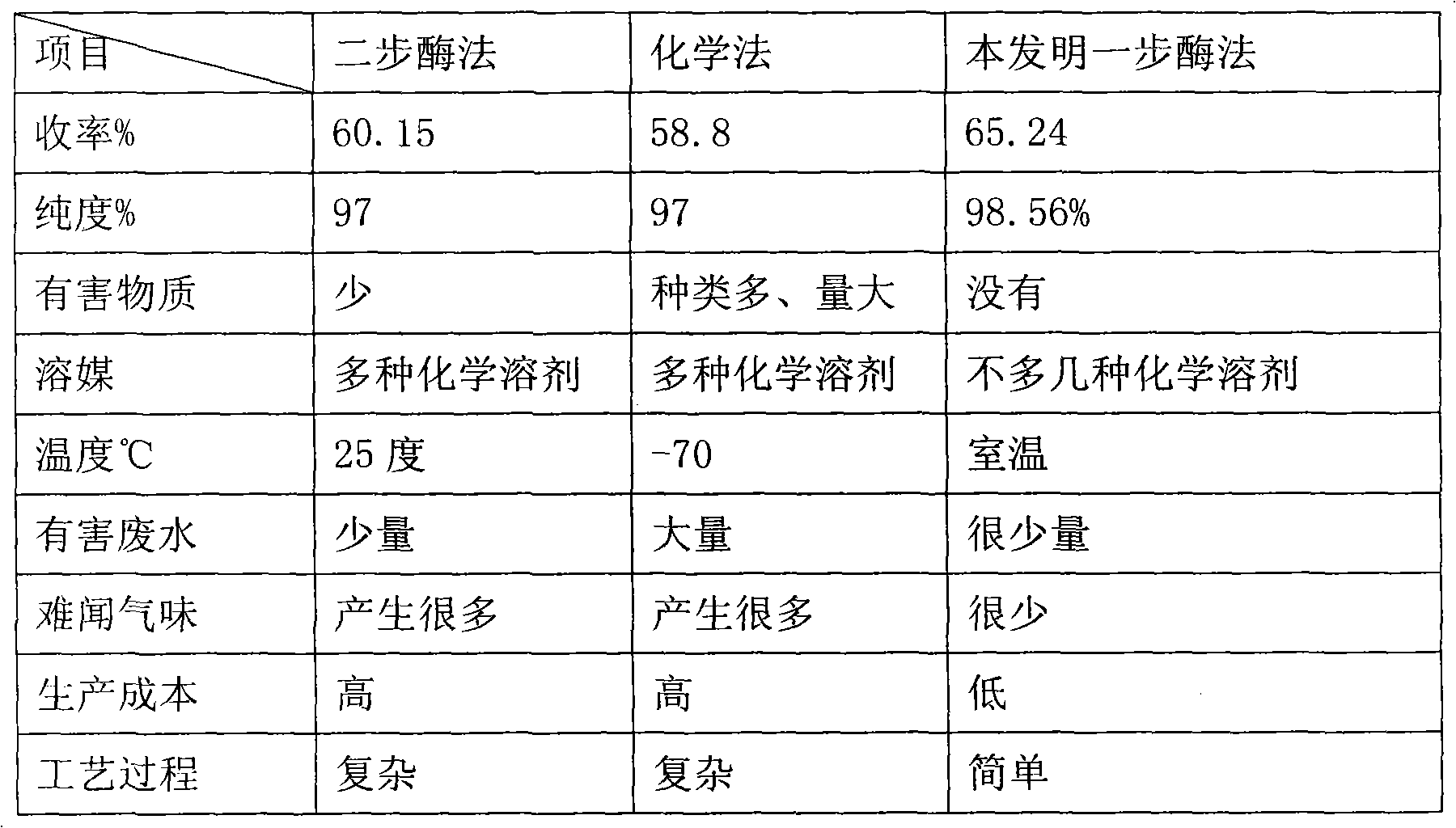
One-step enzymatic method for preparing 7-aminocephalosporanic acid
The invention relates to a one-step enzymatic method for preparing 7-aminocephalosporanic acid. The method for preparing the 7-aminocephalosporanic acid comprises four steps of: 1, treating fermentation liquid of cephalosporin C; 2, performing enzymolysis of the cephalosporin C under the action of acyltransferase of the cephalosporin C to obtain the 7-aminocephalosporanic acid; 3, treating the lysate of 7-aminocephalosporanic acid obtained through the enzymolysis; and 4, crystallizing and drying the 7-aminocephalosporanic acid.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Method for preparing cefonicid or its medicinal salt and intermediate
ActiveCN101085781AProduced noHigh post-processing costOrganic chemistryDecreased energyEconomic benefits
The invention provides a method for preparing cephalosporin nixi or its salt and its intermediate. The invention employs alkyl sulfonic acid to replace BF3 used in curretn technique as catalyst for reaction of 1- methanesulfonic acid- 5- mercapto- 1, 2, 3, 4- tetrazole acid or its salt and 7-aminocephalosporanic acid or its salt. It is characterized by reduced cost, decreased energy consumption, less toxic waste gas discharge and apparent economic benefit.
Owner:SHANDONG SALUBRIS PHARMA +1
Method for preparing 3-descarbamoyl-cefuroxime acid
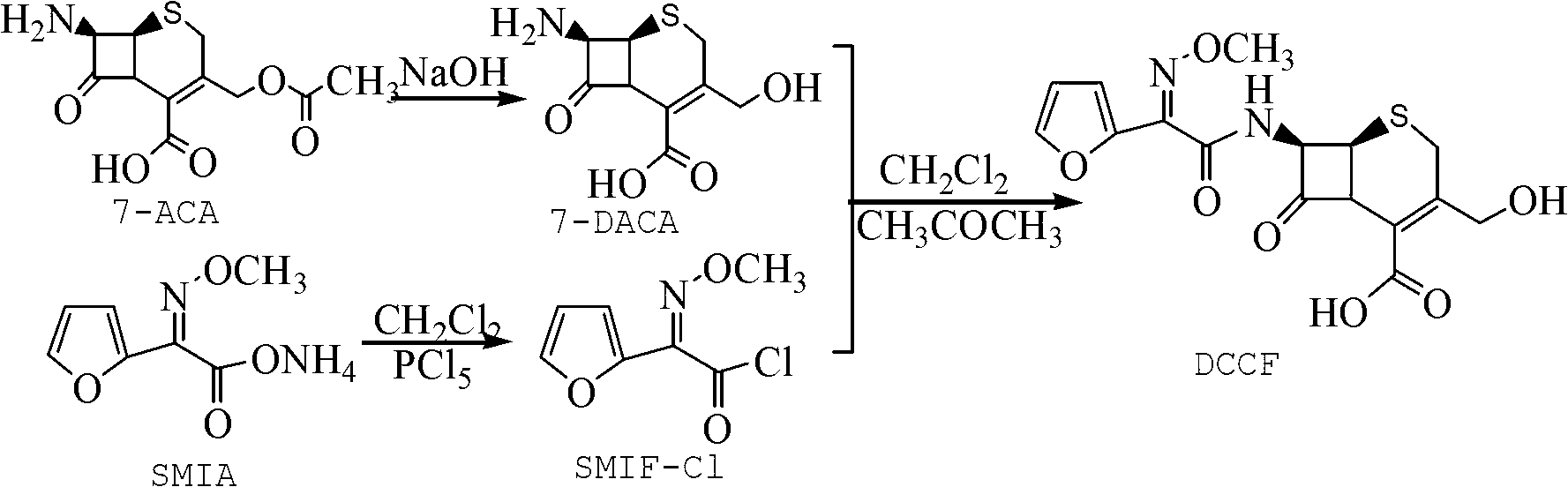
The invention relates to a method for preparing 3-descarbamoyl-cefuroxime acid. The method comprises the following steps of: (1) dissolving 7-aminocephalosporanic acid (7-ACA) in water or a methanol solution to prepare a 7-ACA solution, and performing hydrolysis reaction to obtain a 3-deacetyl-7-aminocephalosporanic acid (7-DACA) solution; (2) dissolving an acylchlorination reagent in a solvent, adding a cosolvent, adding (Z)-2-methoxyimino-2-(furyl-2-yl)acetic acid ammonium salt (SIMA), reacting, filtering, adding water or performing rotary evaporation to remove the excessive acylchlorination reagent, performing vacuum rotary evaporation, adding a homogenization reagent, and dissolving to obtain a (Z)-2-(furyl-2-yl)-2-(methoxyimino)-acetyl chloride (SMIF-Cl) solution; (3) adding the homogenization reagent into the 7-DACA solution, dripping the SMIF-Cl solution, regulating the pH value, preserving heat and reacting to obtain a reaction solution; and (4) decolorizing the reaction solution, regulating the pH value, adding purified water, growing a crystal, filtering, and performing vacuum drying. Reactants form a homogeneous system by adding the homogenization reagent, so that the contact area of the reactants is expanded, the reaction rate is increased, and reaction time is shortened.
Owner:SHANDONG UNIV
Process for preparing 3-deacetylate-7-aminocephalosporanic acid
ActiveCN102321721AReduce pollutionShort process routeFermentationCracking reactionCephalosporin Acylase
The invention discloses a process for preparing 3-deacetylate-7-aminocephalosporanic acid, belonging to the technical field of medicines. The method disclosed by the invention comprises the following steps of: directly catalyzing 3-deacetylate cephalosporin C extract with cephalosporin acylase, then acidifying and crystallizing, washing and drying to obtain solid 3-deacetylate-7-aminocephalosporanic acid. The method only requires one-step normal temperature cracking reaction and one crystallizing process, process rout is short, pollution is less, mole yield of product is more than 86%, and the method disclosed by the invention is applicable to industrialization production.
Owner:石药集团内蒙古中诺药业有限公司
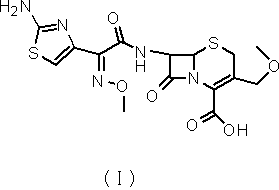
Green synthetic method for cefotaxime acid
InactiveCN101550149AReduce usageRealize multiple recycling and applicationAntibacterial agentsOrganic chemistryCefotaximeOrganic base
The invention discloses a green synthetic method for cefotaxime acid showed in formula (I), which comprises the following steps: adding 2-methyl tetrahydrofuran, water, 7-Aminocephalosporanic acid showed in formula (II), AE-active ester showed in formula (III) and organic base into a reaction vessel; reacting at the temperature of 0-40 degrees for 1-2 hours; adjusting system pH value to 1-3 by diluted hydrochloric acid after the reaction; precipitating solid, filtering to obtain filter cake and filtrate; and drying the filter cake to obtain cefotaxime acid. the organic base is organic amine or pyridine compound; the ratio of the 7-Aminocephalosporanic acid, the AE-active ester and the organic base is 1.0:1.0-1.8:0.01-1.5. The invention prepares cefotaxime acid in 2-methyl tetrahydrofuran which is a green solvent, avoids the using of poisonous harmful solvent such as dichloromethane; has the advantages of mild condition, good product quality, high yield and low production cost; achieves higher industrial value and potential social economic benefit.
Owner:ZHEJIANG UNIV OF TECH +1
Mother liquid recovery process for 7-aminocephalosporanic acid production
The invention provides a mother liquid recovery process for 7-aminocephalosporanic acid production, which comprises the following steps of: a. after being collected, adjusting pH of centrifugal mother liquid to be 7.0 to 8.0 with alkali, reducing the temperature to be 0 to 8 DEG C; b. concentrating the pH-adjusted centrifugal mother liquid with the concentration time of 10 to 15 times; c. treating concentrated liquid with depigmentation; d. placing the depigmented into an acylation tank, using acylase to have cracking reaction under the condition of pH equal to 7.5 to 8.5 and the temperature of 19 to 28 DEG C, and re-cracking non-fully reacted glutaryl 7-aminocephalosporanic acid in the liquid into 7-aminocephalosporanic acid; e. filtering acylase from the cracking reaction liquid, reducing the temperature to be 0 to 8 DEG C, and slowly adding acid for crystallization; f. carrying out solid-liquid separation for crystal with a centrifugal machine, washing the obtained crystal with water or organic solvent, and drying under the temperature of 40 to 50 DEG C.
Owner:焦作健康元生物制品有限公司
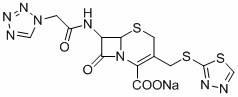
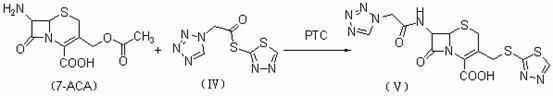
Synthetic method of cefazolin acid
The invention relates to a synthetic method of cefazolin acid. According to the synthetic method, 7-aminocephalosporanic acid is taken as a raw material, and is reacted with 1H-tetrazole-1-acetic acid in a solvent so as to obtain an intermediate; the intermediate is reacted with 5-mercapto-1-methyltetrazole under base catalysis at solution states without crystallization, washing, and drying so as to obtain cefazolin acid. One-pot method is adopted; the synthetic method is simple; solvent application amount is low; environmental pollution is low; product yield is high; cost is low; and the synthetic method is suitable for industrialized production.
Owner:QILU ANTIBIOTICS PHARMA
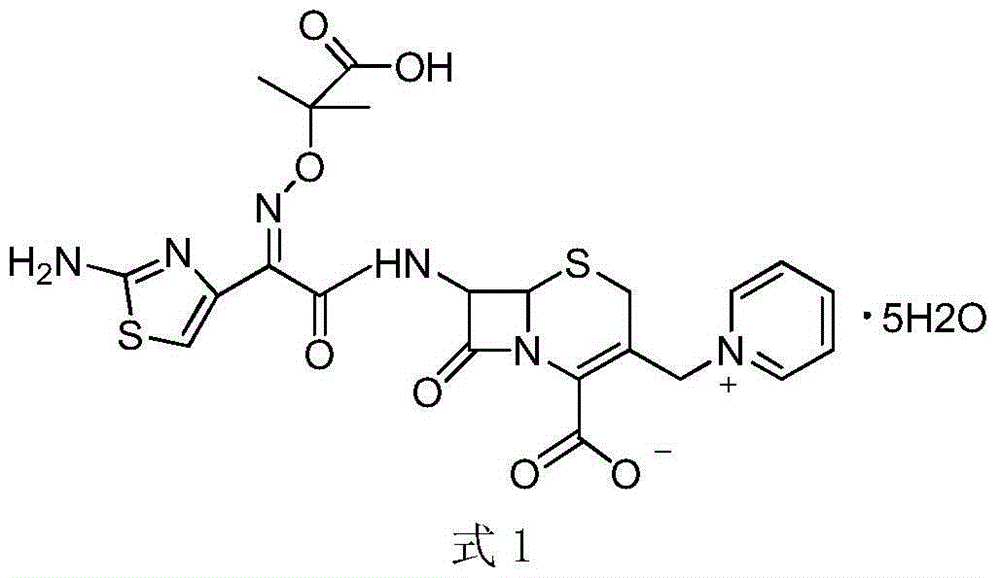
Method for preparing ceftazidime by one-pot process
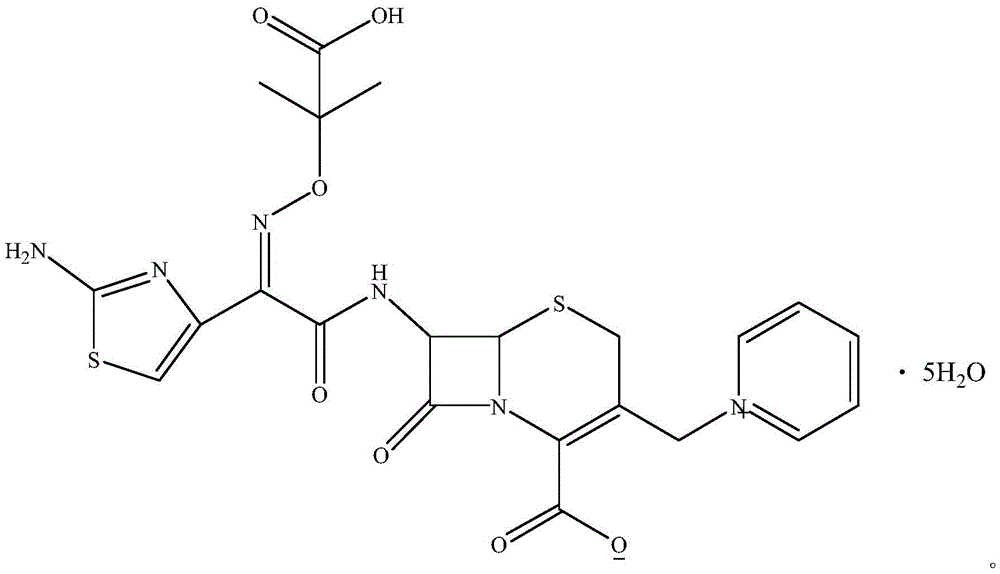
The invention relates to a method for preparing ceftazidime by a one-pot process. The method comprises the following steps: by using 7-aminocephalosporanic acid as the raw material, carrying out silanization reaction and iodination reaction, reacting with pyridine, directly adding the liquid into ceftazidime side chain acyl chloride hydrochloride to perform acylation reaction without separation to obtain ceftazidime iodate, adding the liquid into a concentrated hydrochloric acid-water mixed solution to perform deprotection, extracting to stratify, and regulating the pH value of the water phase with an alkaline solution to obtain ceftazidime (6R,7R)-7-[[(2-amino-4-thiazolyl)-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4,2,0]octyl-2-ene-3-methylpyridine pentahydrate. The method has the advantages of high yield, low cost, mild technological conditions, controllable technical process, high safety and low energy consumption, and is simple to operate.
Owner:QILU ANTIBIOTICS PHARMA
Cefazolin sodium pentahydrate compound of new route
The invention relates to a cefazolin sodium pentahydrate compound of a new route, and particularly provides a method for synthesizing the cefazolin sodium pentahydrate compound. The method comprises the following steps: synthesizing tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester by using tetrazolyl acetic acid and 2-mercapto-5-methyl-1,3,4-thiadiazole as raw materials; and generating cefazolin sodium through the reaction between the tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester and 7-aminocephalosporanic acid. Compared with that the method for synthesizing tetrazole acetic acid 2-mercapto-1,3,4-thiadiazole ester in the prior art uses expensive reagents such as trifluoroacetic anhydride, aluminium trimethide or DCC and the like as a condensation agent, the method improves the synthesis method, not only simplifies the operation steps, but also, surprisingly, greatly improves the product yield and the purity, reduces the cost, and lays a foundation for industrialization.
Owner:HAINAN MEIDA PHARMA
One-pot method for preparing ceftazidime
The invention discloses a one-pot method for preparing ceftazidime. The one-pot method includes the steps that 7-aminocephalosporanic acid, ceftazidime side chain acyl chloride hydrochloride and pyridine are weighed, placed in an ultrasonic wave reactor and ultrasonically oscillated for 2-3 h at the temperature of minus 18 DEG C-minus 15 DEG C and the power of 750-1000 W, the pH value is adjusted to 4.1-4.2, acetone is added into a reaction solution, and a white solid is separated, washed with cold water at the temperature of 0 DEG C-5 DEG C and dried to obtain ceftazidime. In the method, pyridine serves as an acid-binding agent and a catalyst for an acyl chloride and amidogen reaction, a reactant and reaction solvent, other reagents are not added any more, and the workload of impurity research in the later period of new drug research is greatly reduced. By means of environment-friendly ultrasonic oscillation, after the reaction is completed, the processing process is simple, the long crystal growing process is avoided, and meanwhile the possible influence on product quality by acetone residual amount due to acetone washing after cold water washing is avoided. The yield and the purity are high, and the method is suitable for industrial production.
Owner:上海博速医药科技有限公司
Method for recovering 7-aminocephalosporanic acid (7-ACA) from 7-ACA mother liquor
The invention discloses a method for recovering 7-aminocephalosporanic acid (7-ACA) from 7-ACA mother liquor. The method comprises the following steps of a, adjusting a pH value of a 7-ACA mother liquor to 7 by alkali and carrying out condensation treatment at concentration multiple of 4-5, b, controlling a temperature in a range of 5-10 DEG C, adsorbing the concentrated 7-ACA mother liquor by LXT-032 resin, and carrying out resolving by a resolving agent, c, adding acid into the material liquid subjected to resolving and carrying out crystallization, and d, filtering the crystals, and carrying out washing and drying to obtain a 7-ACA finished product. The method for recovering 7-ACA has the advantages of simple processes, low cost, energy saving, environmental protection, high 7-ACA product yield and high purity.
Owner:华北制药河北莱欣药业有限公司
Process for the preparation of furaca
Owner:AUROBINDO PHARMA LTD
Synthetic method of cefcapene pivoxil hydrochloride
The invention discloses a synthetic method of cefcapene pivoxil hydrochloride. The synthetic method comprises following steps: 7-aminocephalosporanic acid (7-ACA) is taken as a raw material, and is subjected to 'one-pot reaction' so as to realize 3-esterolysis, and 7-amino protection is carried out; an obtained product is subjected to 'one-pot reaction' so as to realize 3-amidation, and 7-deprotection is carried so as to obtain an important intermediate of cefcapene pivoxil; the intermediate and a derivative of cefcapene pivoxil with an acid side chain are subjected to condensation, and an obtained cefcapene pivoxil precursor with a 4-acid group is subjected to esterification and acidification so as to obtain cefcapene pivoxil hydrochloride finished products. According to the synthetic method, 'atom economy' principle is fully used so as to shorten reaction route, and increase reaction total yield; and it is beneficial for synthetic technology improvement and optimization of cefcapene pivoxil hydrochloride.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing cefquinome sulfate
InactiveCN103275103AReduce manufacturing costMild reaction conditionsOrganic chemistryCefotaximePhosphoric acid
The invention discloses a method for preparing cefquinome sulfate. The method comprises the steps as follows: 7-aminocephalosporanic acid is taken as a raw material; a C-3 bite ester group is hydrolyzed under the action of alkali and reacts with 2-(2-Amino-4-thiazolyl)-(z)-methoxyiminoacetic, thiobenzothiazole ester, so that an intermediate A is obtained; an iodo substance 3-iodine methyl cefotaxime is obtained through the intermediate and potassium iodide under the action of phosphoric acid; the 3-iodine methyl cefotaxime reacts with 5, 6, 7, 8-tetrahydroquinoline, so that an intermediate B of cefquinome hydriodate is obtained; and finally, the intermediate B of cefquinome hydriodate reacts with sulfuric acid under the action of an alkaline anion exchange resin, so that the cefquinome sulfate is obtained. According to the method for preparing the cefquinome sulfate, the 7-aminocephalosporanic acid which is cheap and easy to obtain is taken as the raw material, so that the use of iodotrimethylsilane which is expensive and prone to decompose when contacted with light and water is avoided, and the production cost is reduced; and the reaction condition is mild, the operation is simple, the yield is high, and the cefquinome sulfate is suitable for industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for synthesizing cefotiam
InactiveCN102898441AHigh yieldSuitable for industrial production applicationsOrganic chemistryCefotiamWhite powder
The invention discloses a method for synthesizing cefotiam. The method comprises the following steps of: reacting acetonitrile, 7-aminocephalosporanic acid and 1- (2-bimethylaminoethyl)-1H-5-mercapto-tetrazole raw materials to obtain a 7-amino-3-[1-(2-bimethylaminoethyl)-1H-tetrazol-5-yl)thiomethyl]cefozopran dihydrochloride; reacting the product with pure water and isopropanol, decoloring the product of the reaction to obtain 7-amino-3-[1-(2-bimethylaminoethyl)-1H-tetrazole-5-yl)thiomethyl]cefozopran hydrochloride; reacting the compound and processing the product of the reaction to obtain formylamino cefotiam; reacting formylamino cefotiam with isopropanol, pure water and hydrochloric acid to obtain coarse cefotiam; and refining twice to obtain finished cefotiam. The method has the advantages that the prepared cefotiam is white powder and the cefotiam yield is as high as 33%; and the method is suitable for industrial production application.
Owner:南通康鑫药业有限公司
Extraction method of cephalosporin C
ActiveCN104278071AHigh light transmittanceReduce lossesOrganic chemistryFermentationUltrafiltrationTransmittance
An extraction method of cephalosporin C mainly comprises the steps of primary ultrafiltration, secondary ultrafiltration, macroporous resin adsorption, weakly alkaline resin adsorption and nanofiltration concentration. An ultrafiltration combination technology is adopted to directly filter a fermentation liquid, and the pH value of the obtained clarified filtrate is adjusted by sulfuric acid. The technology effectively reduces the loss of cephalosporin C in the acid adjustment process. The filtrate obtained through the ultrafiltration combination technology has the advantages of good light transmittance, and thorough removal of proteins and other impurities, and the method has the advantages of product quality improvement, increase of the extraction yield of a resin workshop section, and prolongation of the recovery cycle and the service life of resin, and obtained 7-ACA (7-aminocephalosporanic acid) has good quality and good stability.
Owner:SHANGHAI KAIXIN ISOLATION TECH CO LTD
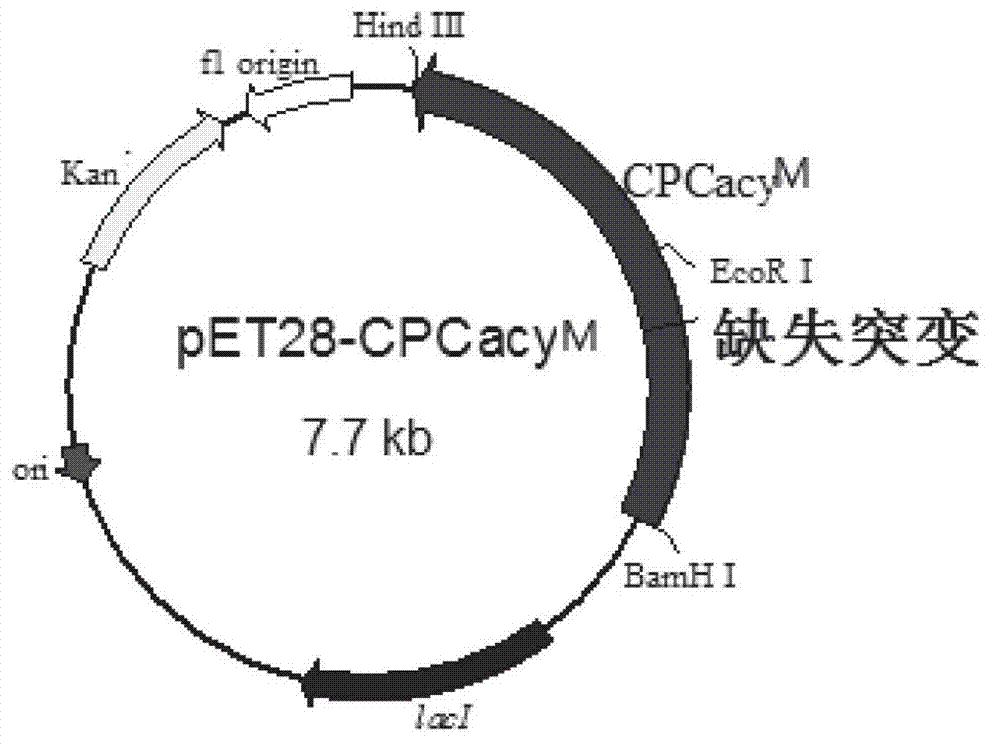
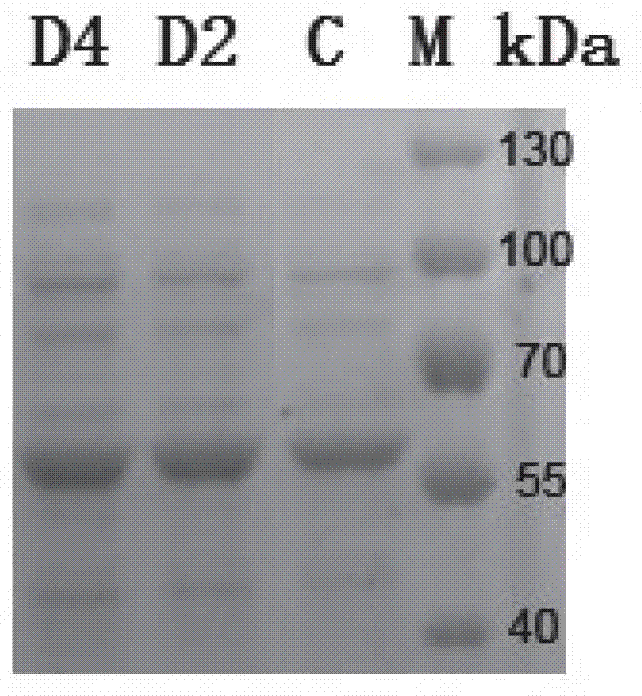
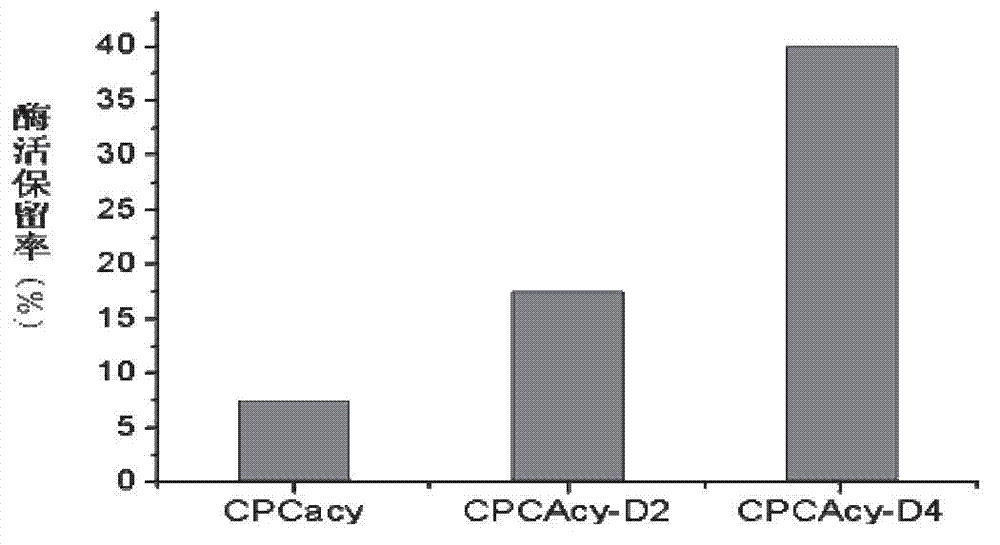
Mutated cephalosporin C acylase
The invention discloses a product-resistant inhibitory mutated cephalosporin C acylase, a gene carrier and a transformant of the enzyme, and application of the enzyme in one-step enzymatic production of 7-aminocephalosporanic acid, belonging to the technical field of enzyme engineering and biotechnology industry. The amino acid sequence of the enzyme is obtained by conducting deletion mutation on the amino acid sequence of cephalosporin C acylase coded by the gene sequence SEQ ID NO:1 to obtain enzyme CPCAcy-D2 and CPCAcy-D4. The amino acid sequences are shown as SEQ ID NO:2 and SEQ ID NO:3, respectively. The invention also discloses the gene carrier, the transformant, and the application of the enzyme. The enzyme has high expressing activity, and also the tolerance of the product is improved significantly, so that CPC is catalyzed efficiently to produce 7-ACA.
Owner:TSINGHUA UNIV
Synthesis method of cefpodoxime proxetil intermediate
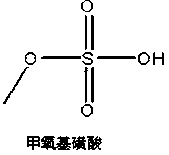
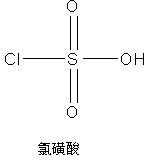
InactiveCN105669701AFew stepsLow costOrganic chemistryBulk chemical productionChlorosulfuric acidDesorption
The invention discloses a synthesis method of a cefpodoxime proxetil intermediate, namely (6R,7R)-7-[2-(2-amino-4-thiazolyl)-(Z)-2-(methoxyimino)acetamido]-3-methoxymethyl-8-oxo-5-thio-1-azabicyclo[4.2.0]oct-2-ene-2-methanoic acid. The synthesis method includes: enabling chlorosulfonic acid and methanol to react to prepare methoxy sulfonic acid; under the action of the methoxy sulfonic acid and dimethylformamide, etherifying 7-ACA (7-aminocephalosporanic acid) and trimethyl borate prior to aftertreatment, adding into a water and methanol solution reversely to guarantee that the obtained intermediate isn't sticky and is loose, drying and grafting with AE active ester so as to obtain a target product, namely the cefpodoxime proxetil intermediate. The synthesis method of the cefpodoxime proxetil intermediate has the advantages that synthesis steps of 3-position and 7-position protection and desorption of 7-ACA can be omitted, so that low step cost, high yield and high purity are achieved, all materials are cheap and available, and industrial production and little pollution are benefited.
Owner:陕西思尔生物科技有限公司
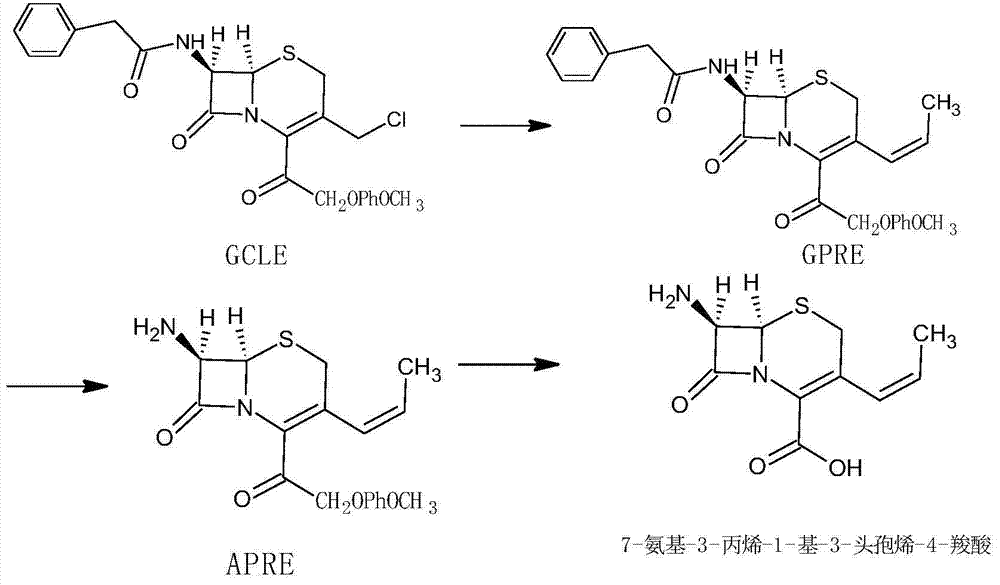
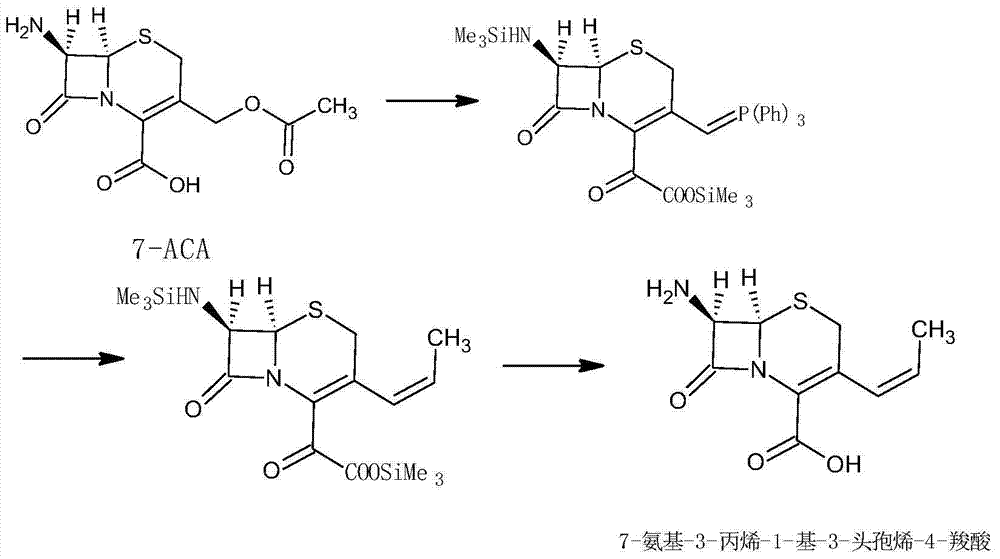
Synthesis method of high-purity 7-amino-3-propylene-1-yl-3-cephem-4-carboxylic acid
The invention relates to a synthesis method of high-purity 7-amino-3-propylene-1-yl-3-cephem-4-carboxylic acid. The method takes deacetylated-7-aminocephalosporanic acid (D-7-ACA) as a starting raw material, and the starting raw material is reacted with ethyltriphenyl phosphonium bromide through oxidization to generate the 7-amino-3-propylene-1-yl-3-cephem-4-carboxylic acid. The synthesis method has a simple process, low cost and high product yield; the purity is more than 99% and the synthesis method is suitable for industrial production.
Owner:QILU ANTIBIOTICS PHARMA
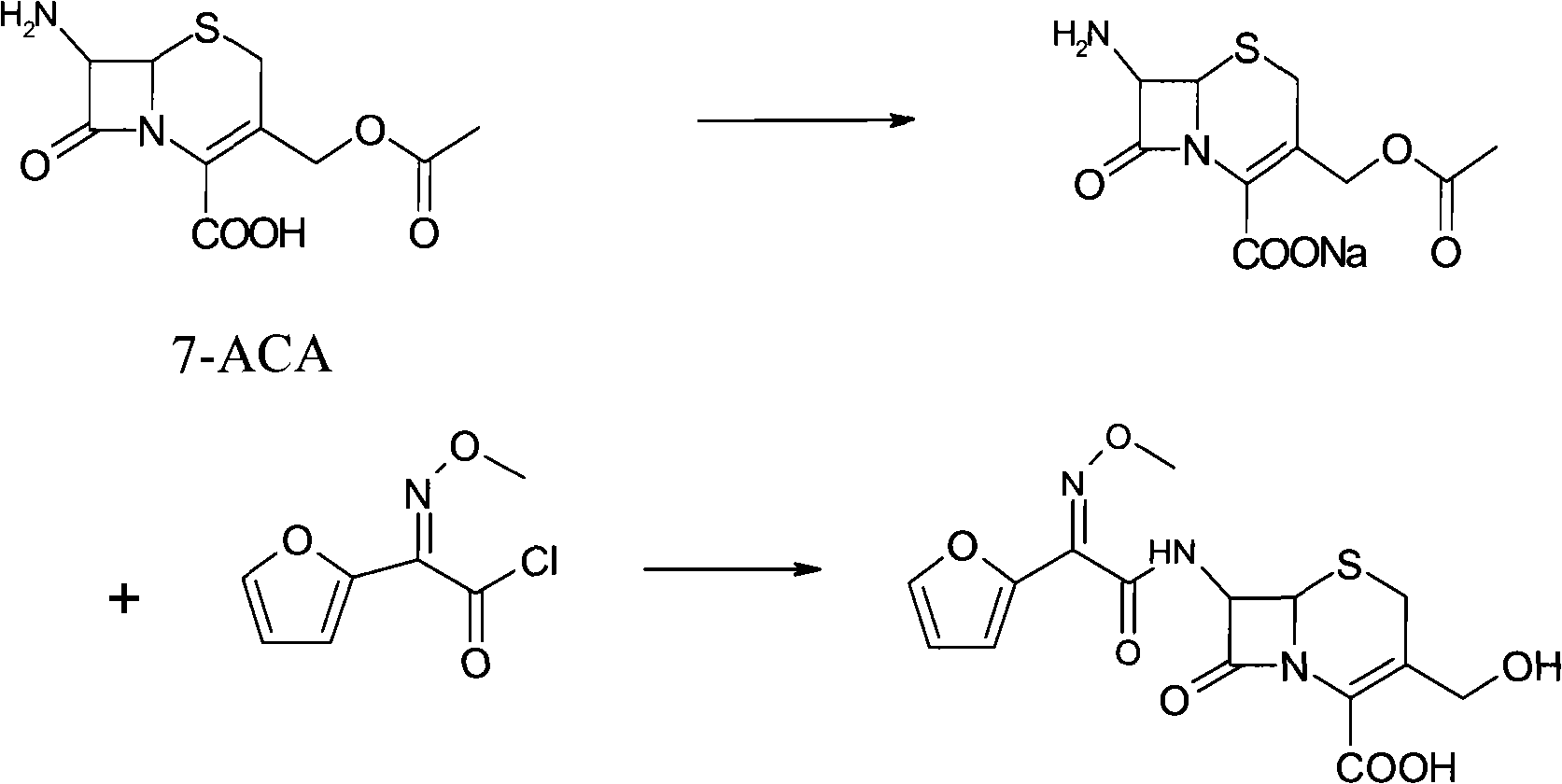
Cefuroxime sodium synthesizing method
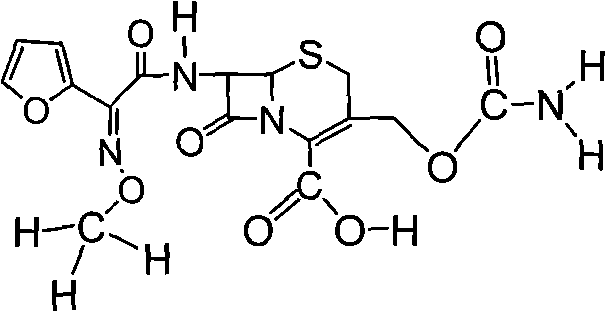
The invention relates to the technical field of pharmaceutical and chemical industries and particularly discloses a cefuroxime sodium synthesizing method. The synthesizing method comprises the steps:dropwise adding alkali solution into 7-aminocephalosporanic acid aqueous solution for hydrolysis reaction, and then subjecting the mixture and (Z)-2-furyl-2-methoxyimino acetic acid p-toluene sulfonicanhydride to amidation to obtain 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid; dissolving the 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid into organic solvents to sequentially perform nucleophilic addition and hydrolysis reaction with chlorosulfonyl isocyanate, then adding sodium iso-octoate solution, and performing devitrification, filtering, washing and drying to obtain cefuroxime sodium. According to the synthesizing method, raw materials are wide and easy to obtain, the cost is low, the steps are simple, operation is simple, and side reaction is less.
Owner:湖北凌晟药业股份有限公司 +1
Colibacillus expression carrier and its application
ActiveCN1912127AHigh yieldLow costFermentationVector-based foreign material introductionGlutaryl-7-aminocephalosporanic acidMutant
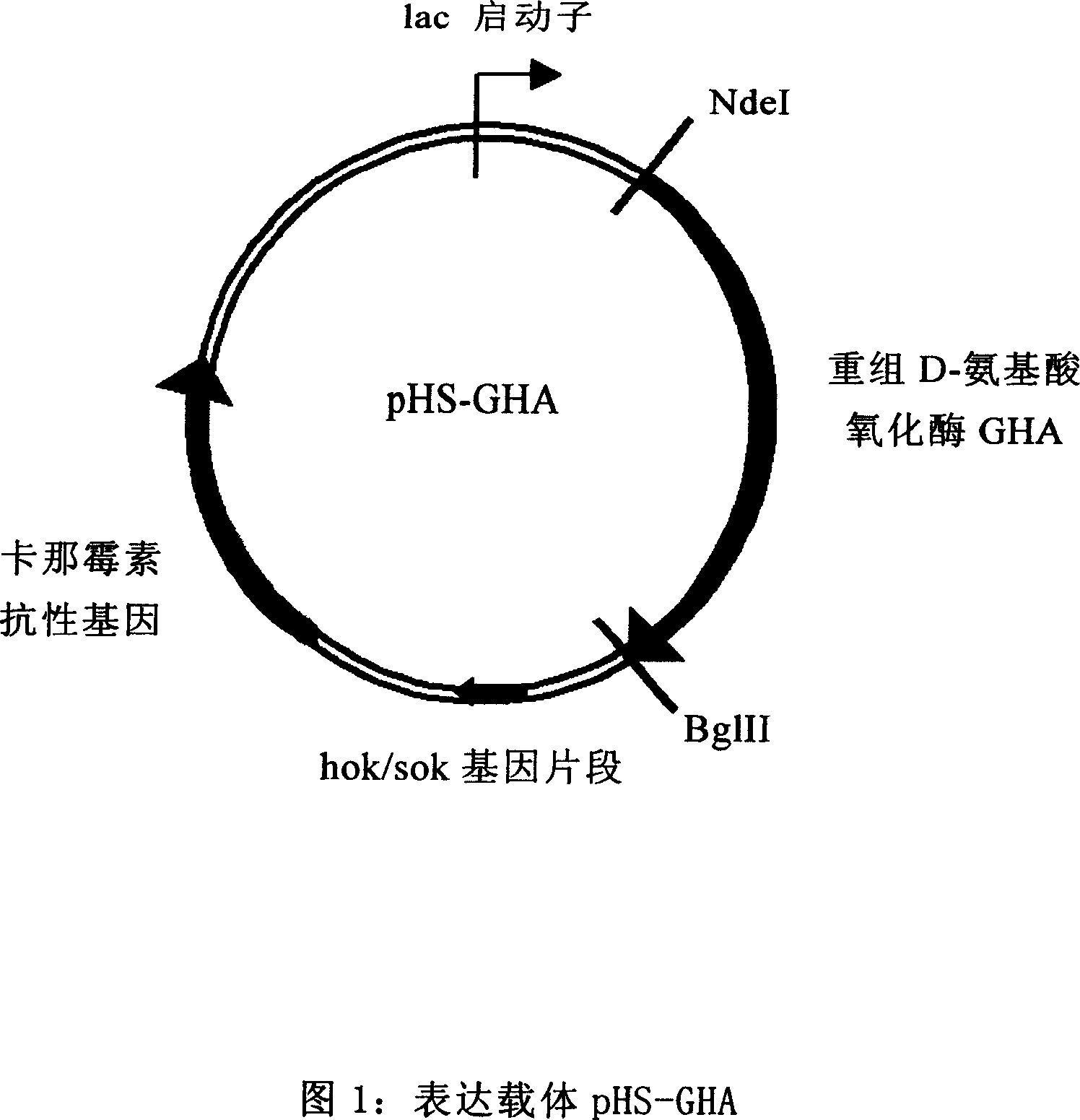
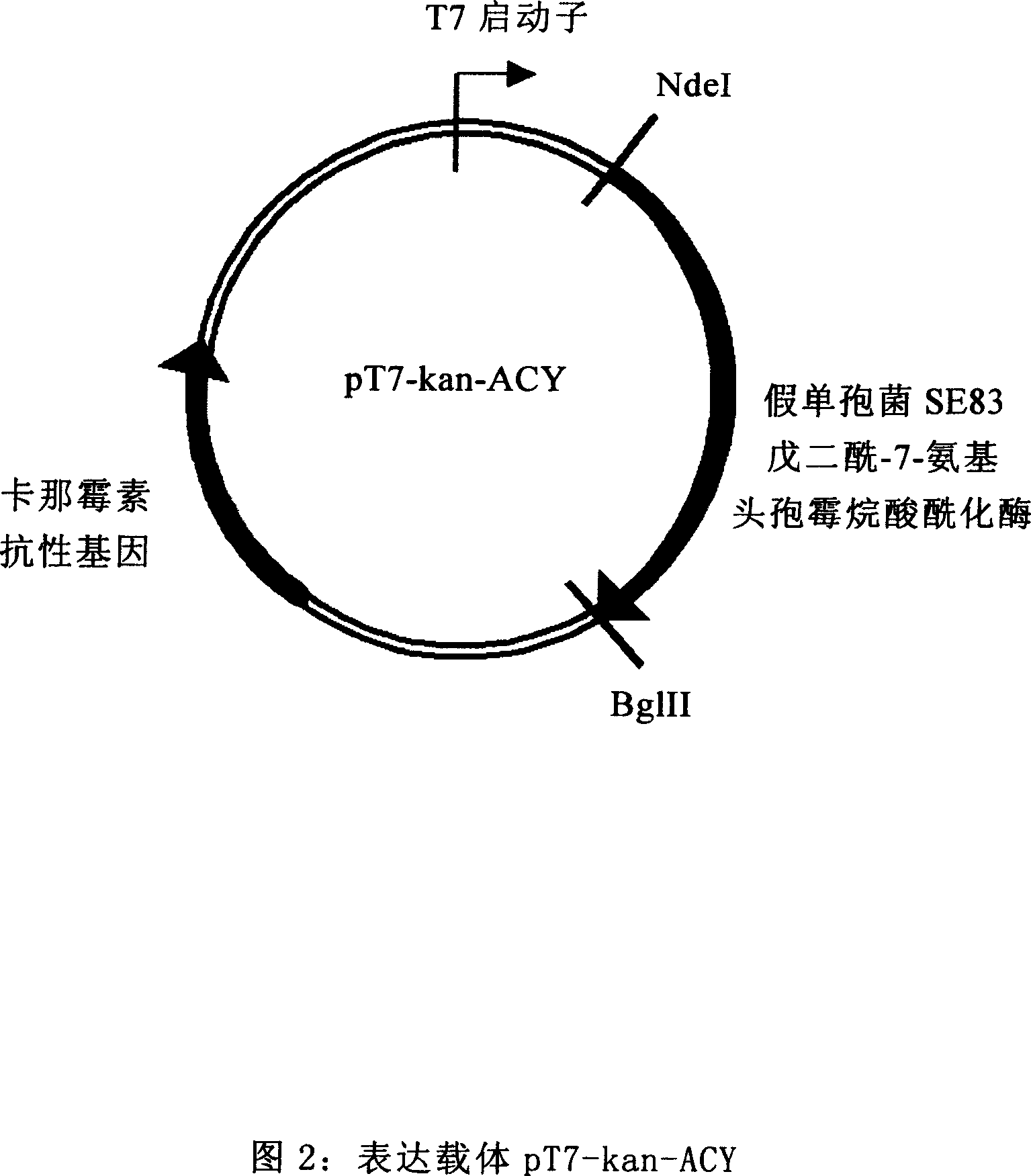
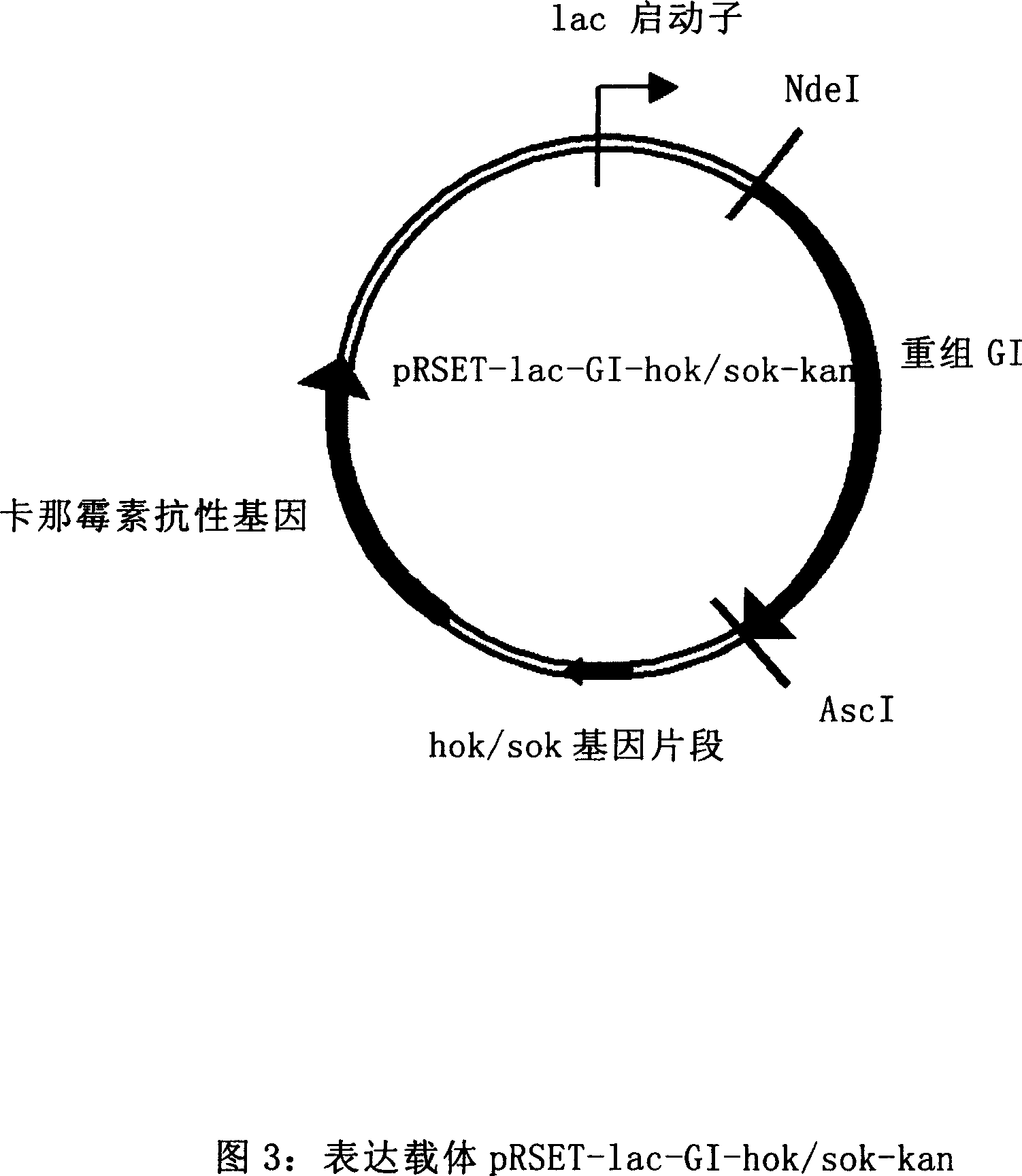
The invention provides Escherichia coli expression vector and its using method. The expression vector is formed by vector pRSET-A whose ampicillin resistant gene is replaced by kanamycin resistant gene. And it can be used to express the enzyme needed in making 7-aminocephalosporanic acid but no beta-lactamase reduced its yield. The invention also provides two concrete expression vectors that pHS-GHA and pT7-kan-ACY whose gene products are respectively D-amino acid oxidase mutant GHA and Pseudomonas sp.SE83 glutaryl-7-aminocephalosporanic acid acidylating enzyme.
Owner:常熟盈赛生物科技有限公司 +1
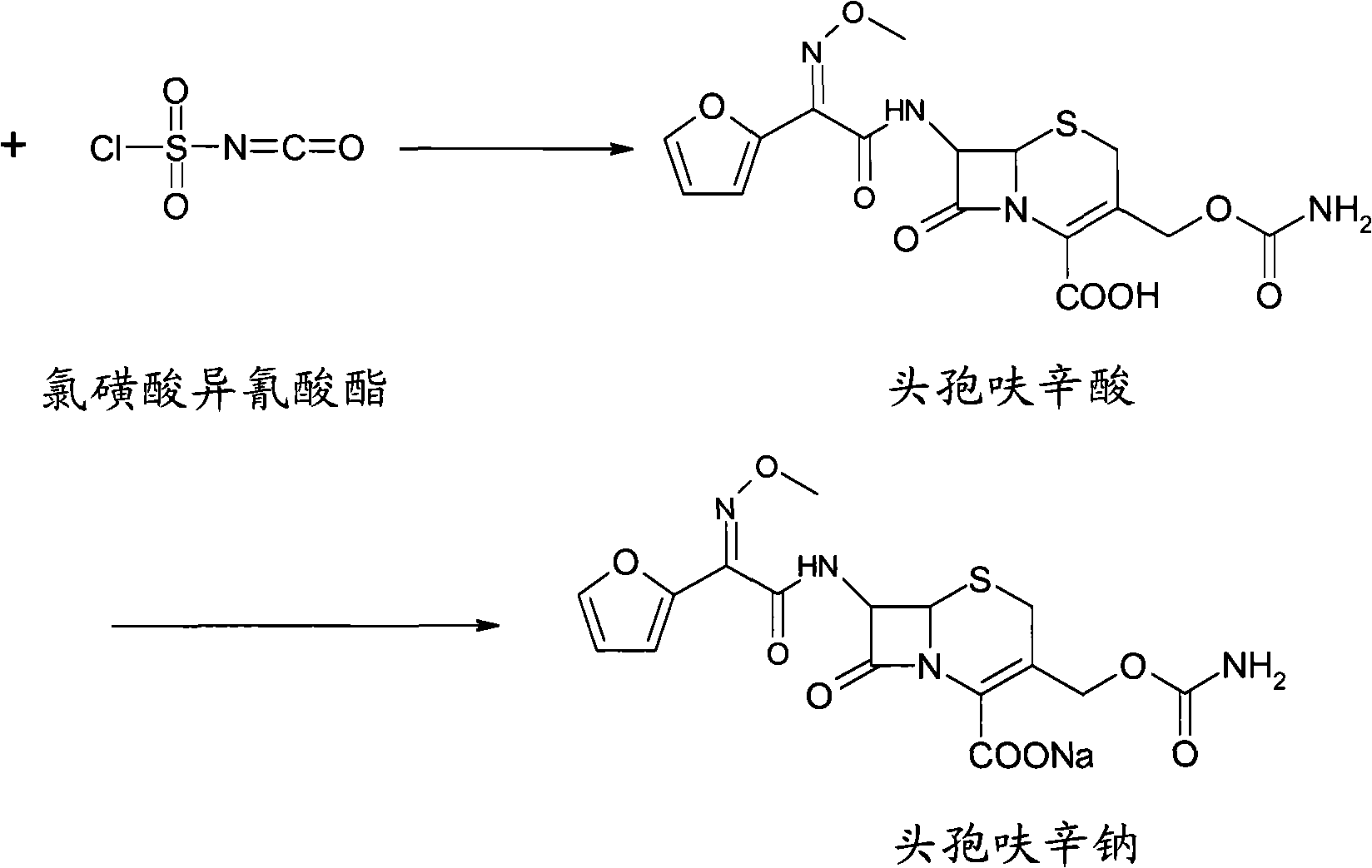
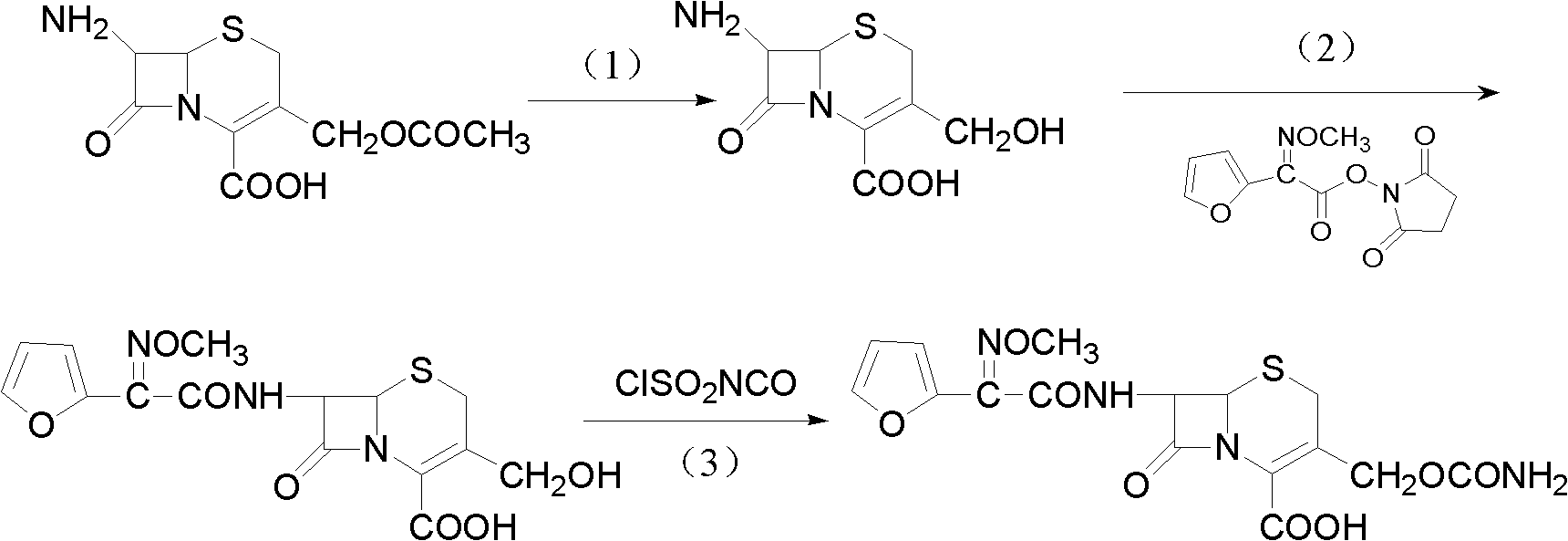
Method for preparing cefuroxime acid
ActiveCN102093390AEasy to operateReduce the discharge of three wastesOrganic chemistryAcetic acid7-ACA
The invention provides a method for preparing cefuroxime acid. The method comprises the following steps: (1) selectively hydrolyzing 7-aminocephalosporanic acid (7-ACA) with aqueous alkali so as to obtain 3-deacetylation-7-aminocephalosporanic acid (7-DACA); (2) condensing 2-(2-furyl)-2-(methoxyimino)acetic acid-(2,5-dioxo-pyrrolidyl)-1-ester and 7-DACA so as to obtain 3-decarbamyl-cefuroxime acid (DCCF); and (3) modifying 3-hydroxymethyl of DCCF with chlorosulfonyl isocyanate so as to obtain the cefuroxime acid. In the method, low-temperature selective hydrolysis is carried out by using inorganic base to remove the 3-ester group of 7-ACA so as to prepare 7-DACA; C7-amino modification is carried out by an active ester method so as to obtain DCCF; and the 3-hydroxymethyl of DCCF is modified into carbamoyl methoxyl so as to obtain the cefuroxime acid. The method has the advantages of less emission of three wastes and high yield, is simple and convenient to operate, and is suitable for industrial production.
Owner:蚌埠丰原涂山制药有限公司
Preparation method for 3-deacetyl-7-aminocephalosporanic acid
The invention discloses a preparation method for 3-deacetyl-7-aminocephalosporanic acid (ACA), and belongs to the technical field of medicines. The preparation method adopts a two-enzyme two-step method and comprises the following steps: concentrating cephalosporin C (CPC) sodium salt liquid for acid modulation, performing catalytic cracking through immobilized CPC acylase and immobilized deacetyl esterase in sequence, and finally performing acid modulation and crystallization to obtain D-7-ACA. According to the preparation method, the preparation process is simple to carry out, a D-7-ACA product is high in purity and high in reaction yield, the immobilized CPC acylase and the immobilized deacetyl esterase can be repeatedly utilized, the service life of the immobilized CPC acylase reaches up to 600-700 batches, the service life of the immobilized deacetyl esterase reaches up to 800-1,000 batches, and the production cost is greatly reduced; the method is of great significance on industrialized enlarged production.
Owner:华北制药河北莱欣药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com