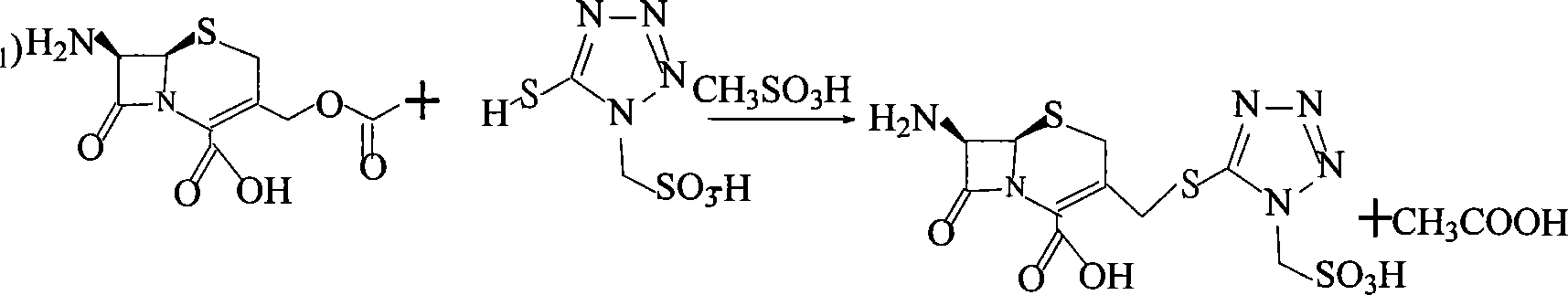Method for preparing cefonicid or its medicinal salt and intermediate
A technology of cefanixime and medicinal salts, which is applied in the field of compound preparation, can solve the problems of corroding equipment, consuming manpower, energy, and increasing the burden of environmental protection, and achieves high processing costs, shortening the reaction cycle, and saving operation time and manpower cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 BP1 acid
[0034] Add 300mL of methanesulfonic acid and 115ml of dichloromethane into the dry reaction kettle, add 52g of TSA acid under stirring, and stir for 30 minutes until completely dissolved. Cool down to 20°C, add 76.5g of 7-ACA, and react at 25°C for 60 minutes. After the reaction was completed, 382.5 ml of water and 612 ml of methanol were added at room temperature. Adjust the pH to 2.5-3.0 with sodium bicarbonate, and stir at room temperature for 30 minutes. After filtering, the filter cake was washed twice with a mixed solution of methanol and water. The material was filtered and dried in vacuum at 35° C. to constant weight to obtain about 108 g of product BP1 acid. (yield 96%, HPLC purity 99%)
Embodiment 2
[0035] The preparation of embodiment 2 cefnixin sodium
[0036] 1) Acetylation: Add BP1 acid (100 g) to a mixture of pure water (800 ml) and tetrahydrofuran (600 ml), stir well, and cool down to 2°C. Then add 35ml of dilute ammonia (1:1) to dissolve it completely. Control the temperature at 0°C, add D-(-)-formylmandelic acid chloride solution (53g of D-(-)-formylmandelic acid chloride solution is dissolved in 200ml of tetrahydrofuran), and add dilute ammonia water (1:1) dropwise during the process Maintain the pH between 6.5 and 7.0. After the addition, the temperature was controlled at 0°C for 30 minutes. Then the reaction solution was washed twice with dichloromethane, adding 150 ml of dichloromethane each time.
[0037] 2) Deformylation: Add HCl (35-40ml) dropwise to the above aqueous solution, adjust the pH to 1.0, keep warm at 30°C for 20 hours, then cool down to 20°C, and decolorize with 10g of activated carbon for 30 minutes.
[0038] 3) Extraction: filter, and wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com