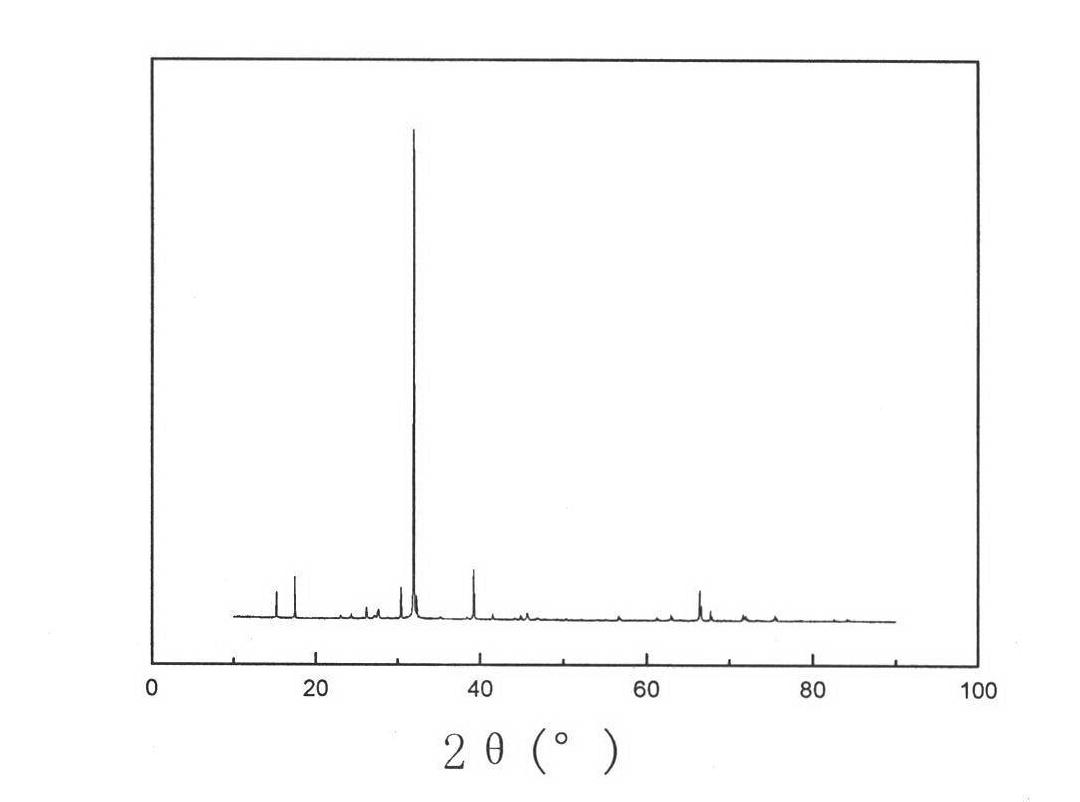
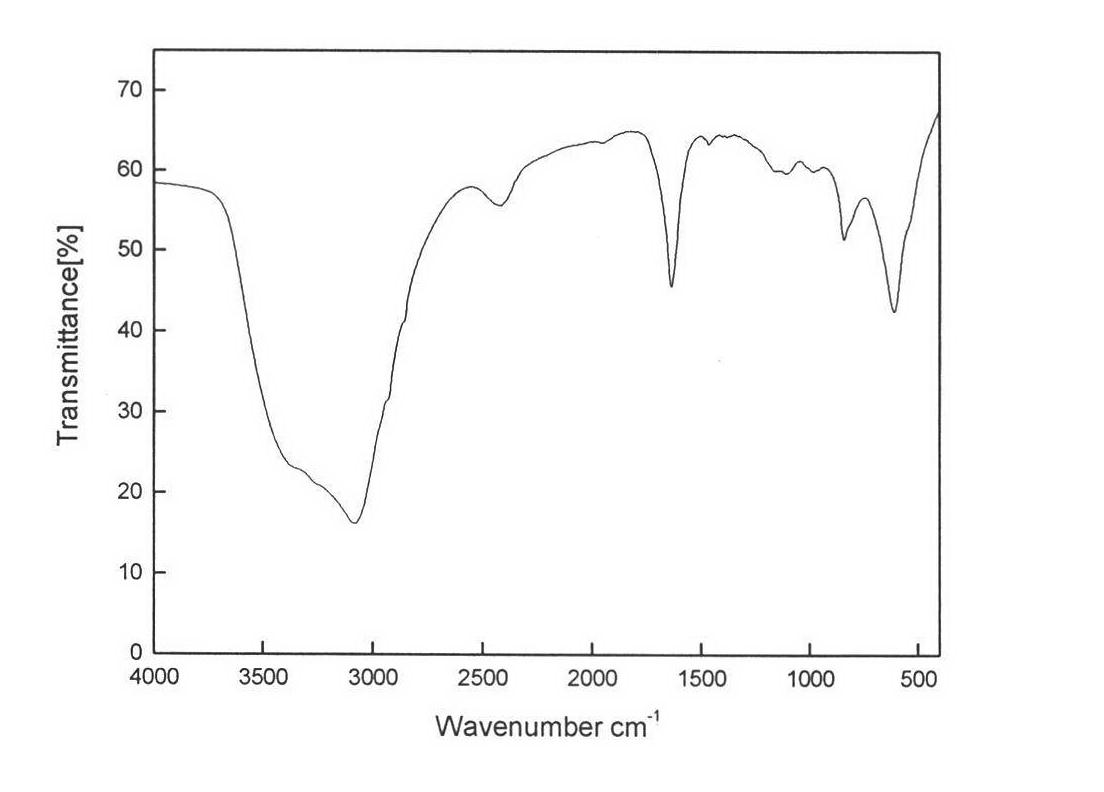
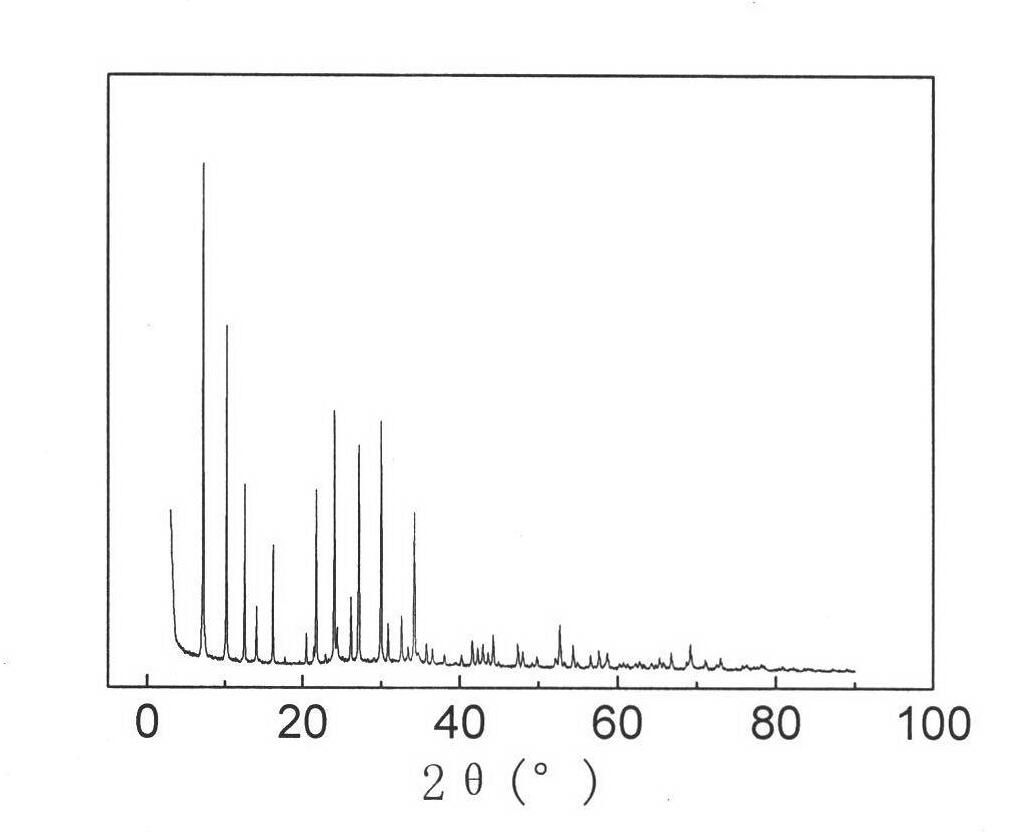
Comprehensive utilization method of hydrargillite-produced 4A zeolite waste residue
A technology of gibbsite and zeolite, which is applied in the direction of crystalline aluminosilicate zeolite, A-type crystalline aluminosilicate zeolite, chemical instruments and methods, etc., can solve the problems of comprehensive utilization that have not been seen, and achieve low equipment requirements and high reaction efficiency. The effect of mild conditions and sufficient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) acid leaching reaction
[0033] In the there-necked flask, add the gibbsite waste slag that is ground to 80 objects (the mass percentage of its main chemical composition is: Fe 2 o 3 14.30%, SiO 2 24.94%; Al 2 o 330 g of 38.25%) and 90 mL of 36% hydrochloric acid at a temperature of 90° C., fully stirred and reacted for 4 h, filtered to obtain 89.9 ml of filtrate, and then washed the filter residue with 70.1 ml of deionized water to obtain 70.1 ml of washing liquid. The wet filter residue was dried to remove water to obtain 7.40 g on a dry basis.
[0034] (2) Synthesis of polyaluminum ferric chloride from the filtrate
[0035] Put 160 mL of the mixed solution of acid leaching filtrate and lotion obtained in step (1) into a three-necked flask, stir and raise the temperature to 65°C, add 48 mL of 4 mol / L sodium hydroxide solution, adjust the pH to 2.0, and react for 4 hours; After filtering, the filtrate was evaporated to dryness to obtain a solid product, polya...
Embodiment 2
[0049] Embodiment 2: (recycling of embodiment 1 synthesis 4A mother liquor)
[0050] (1) acid leaching reaction
[0051] Same as Example 1(1)
[0052] (2) Polymerization reaction to produce polyaluminum ferric chloride
[0053] Same as Example 1(2)
[0054] (3) Wet slag plus alkali roasting activates iron removal
[0055] Same as Example 1(3)
[0056] (4) Hydrothermal synthesis of 4A zeolite
[0057] The mother liquor after synthesizing 4A zeolite decomplexation is 194mL altogether, wherein alkali content is 6.745g by NaOH, Si is by SiO 2 Calculated as 1.025g (equivalent to 2.084g of sodium silicate), Al is expressed as Al 2 o 3 Calculated as 0.5126g (equivalent to 0.8242g of sodium aluminate). This mother liquor needs to add 6mL deionized water, 14.42g alkali fusion roasting activator that crosses 80 mesh sieves, 1.255g sodium hydroxide and 9.176g sodium aluminate, can make reaction solution synthesize 4A with (4) in embodiment 1 The system is the same. First add 6m...
Embodiment 3
[0064] (1) acid leaching reaction
[0065] Same as Example 1(1)
[0066] (2) Polymerization reaction to produce polyaluminum ferric chloride
[0067] Same as Example 1(2)
[0068] (3) Wet slag plus alkali roasting activates iron removal
[0069] Take 7.40 g of the wet slag obtained in step (1) and dry it to remove water, pass through 80 meshes, add 14.38 g of sodium carbonate and 0.22 g of sodium chloride, mix well, and burn at 800 ° C for 2 hours to obtain alkali fusion 17.5g of the activated substance was calcined.
[0070] (4) Hydrothermal synthesis of 4A zeolite
[0071] First, dissolve 10g of sodium aluminate and 7g of sodium hydroxide in 100mL of deionized water, mix the two solutions after they are completely dissolved, then take the mixed solution and place it in a three-necked flask with a constant temperature water bath at 40°C, and quickly add 17.5 g passed through an 80-mesh sieve and 7.8 mL of triethanolamine and alkali fusion roasting activated product, stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com