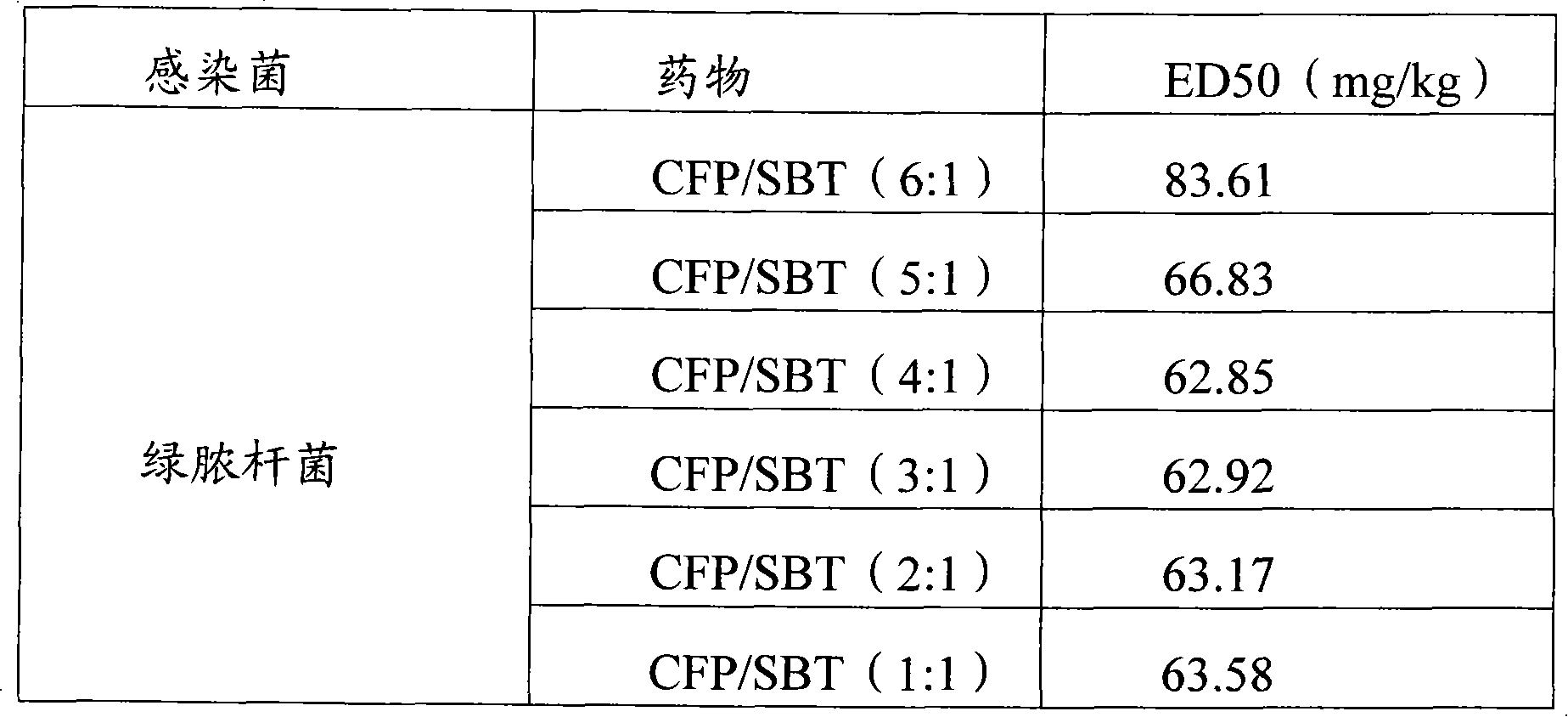
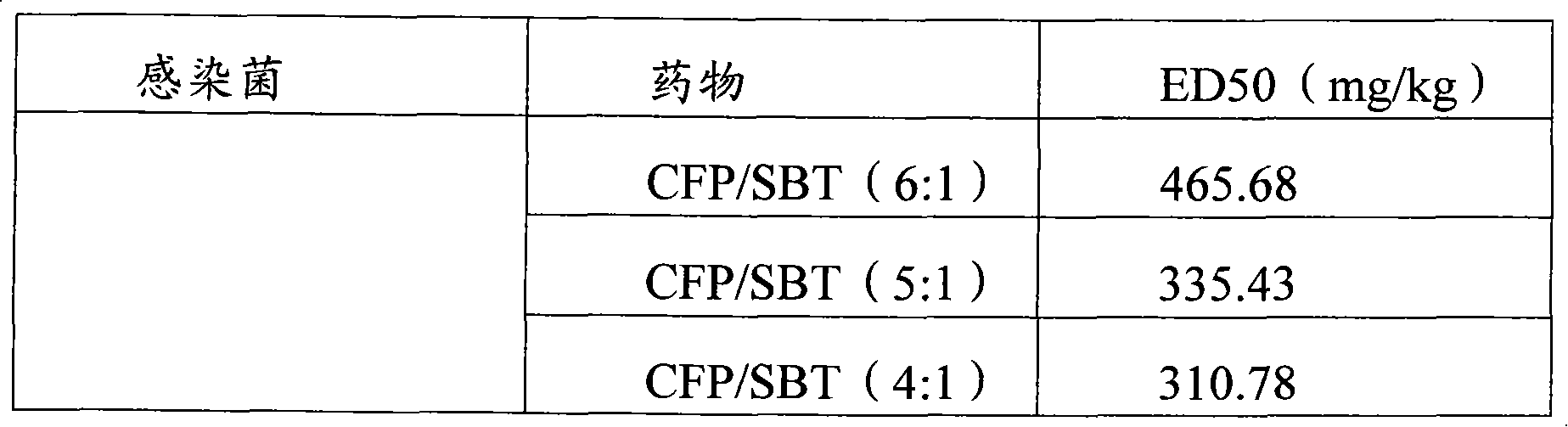
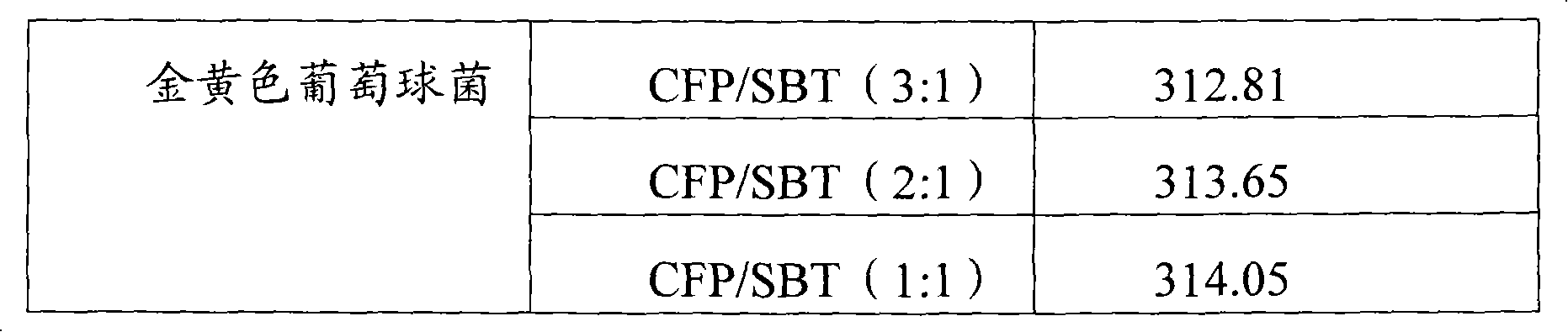
Infection contamination resistance pharmaceutical combination containing optimum proportioning cefoperazone natrium and sulbactam sodium and method of preparing the same
A technology of cefoperazone sodium and sulbactam sodium, which is applied in the direction of anti-infective drugs, drug combinations, active ingredients of heterocyclic compounds, etc., can solve the problems of increased cost of anti-infection treatment, aggravated kidney damage, and high price of sulbactam sodium. Achieve the effect of eliminating the poor control of drug dose and the chance of bacterial infection, which is conducive to flexible selection and improvement of therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Mix the sterile powder of 0.5g cefoperazone sodium and the sterile powder of 0.25g sulbactam sodium in a mixer under aseptic conditions, dissolve each sample with 5ml water for injection, measure the pH value, and control the pH value at 4.5, make 1 bottle of powder injection of cefoperazone sodium and sulbactam sodium composition according to the standard powder injection process.
Embodiment 2
[0049] Mix 0.75g cefoperazone sodium sterile powder and 0.25g sulbactam sodium sterile powder in a mixer evenly under aseptic conditions, dissolve each sample with 5ml water for injection, measure the pH value, control the pH value at 5.5, press A standard powder injection or a powder injection of a combination of cefoperazone sodium and sulbactam sodium made into one bottle.
Embodiment 3
[0051] Mix 0.8g cefoperazone sodium sterile powder and 0.20g sulbactam sodium sterile powder in mixer under aseptic condition, dissolve with 5ml water for injection after each sampling, measure pH value, pH value is controlled at 6.5, The powder injection of the cefoperazone sodium and sulbactam sodium composition of 1 bottle is made by standard powder injection process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com