Lyophilized viper antivenin and preparation method thereof
An anti-venom and viper venom technology, applied in the field of anti-venom and preparation technology, can solve the problems of strong toxicity, large difference in antigen specificity, large amount of detoxification, etc., achieve high titer, reduce the incidence of adverse reactions, guarantee therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
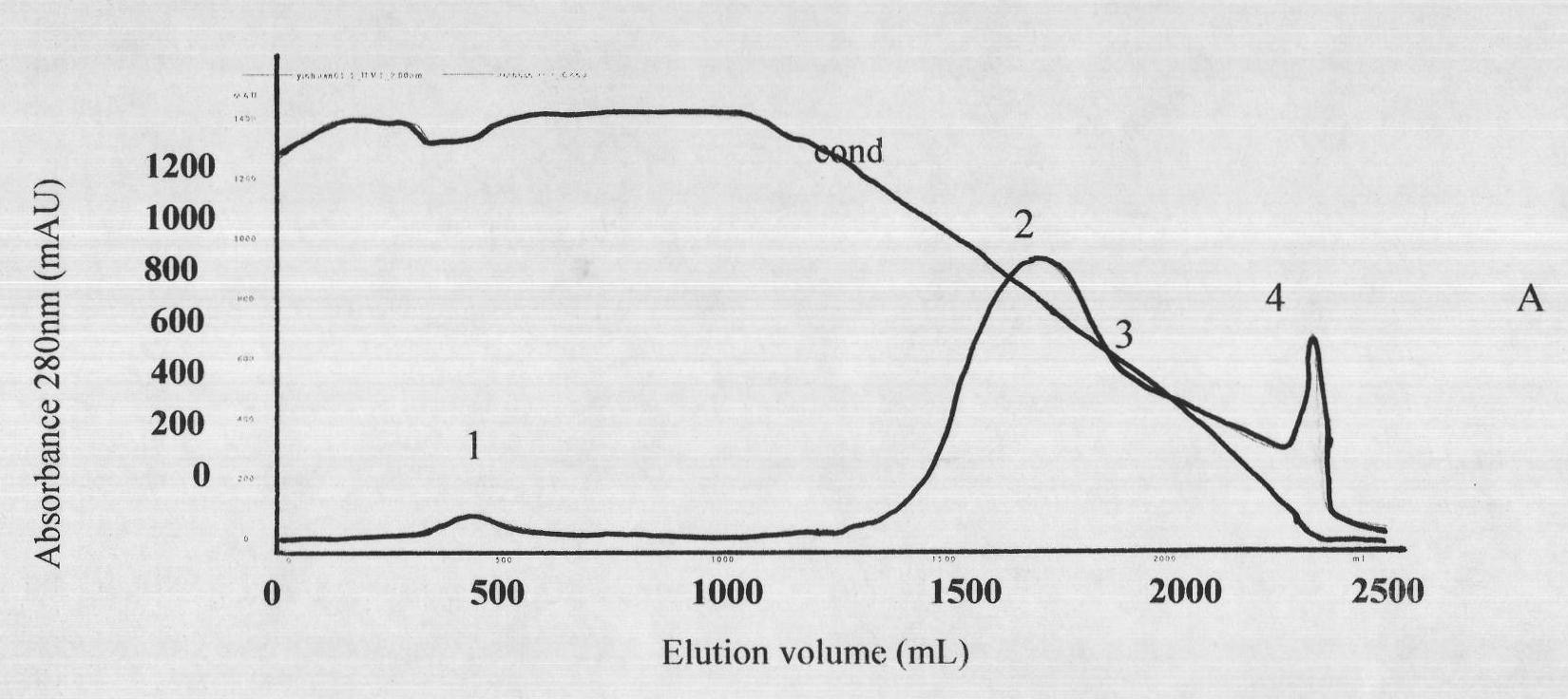
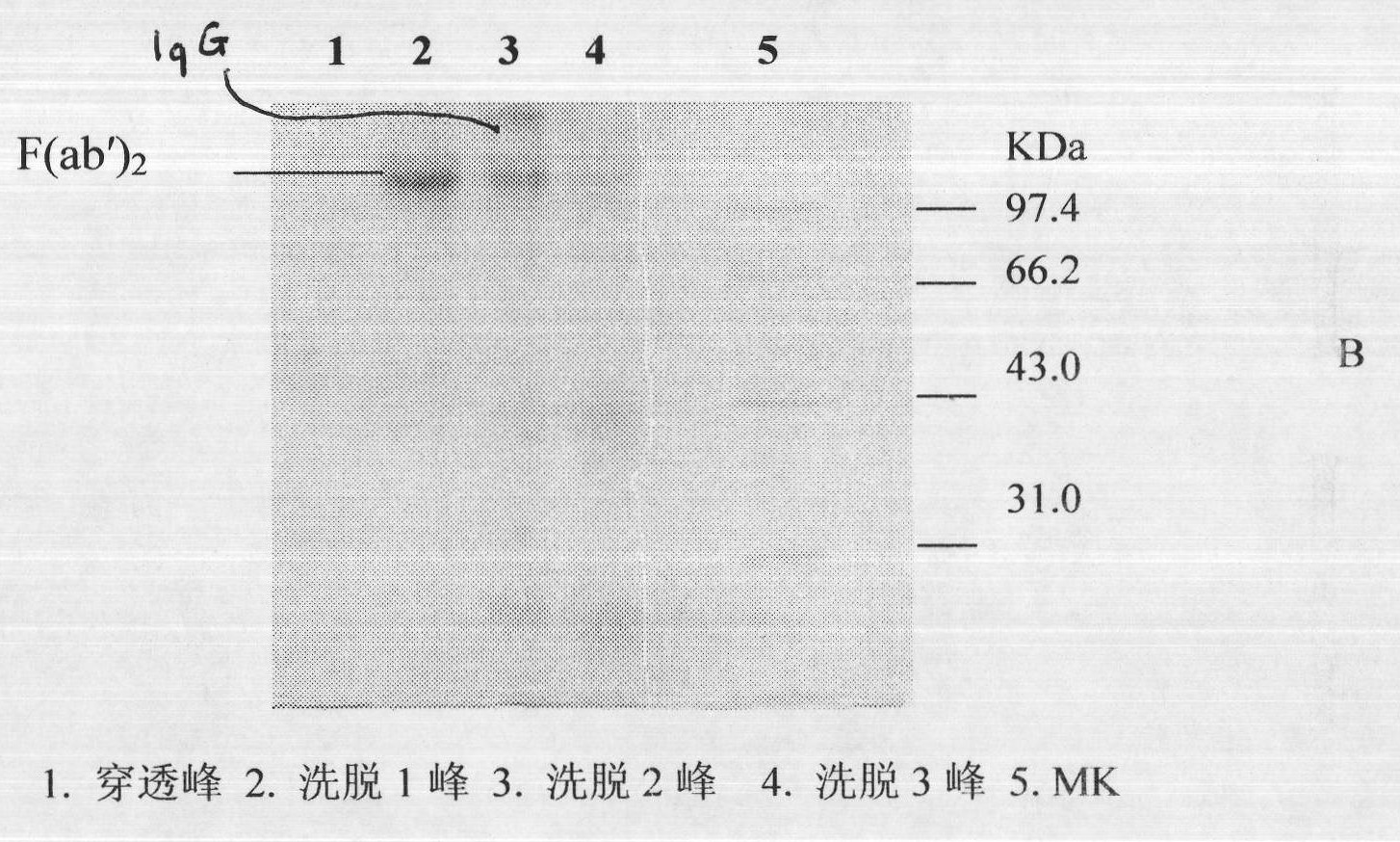
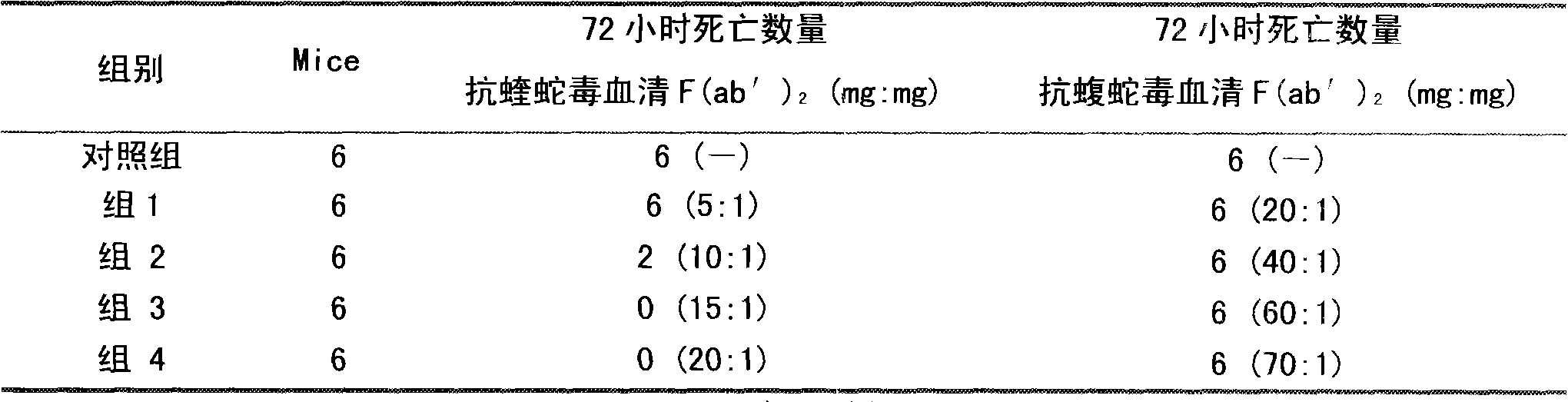
[0023] Two to three lyophilized products of viper subspecies were purchased from different places in southern China.
[0024] Mix the lyophilized poisonous products of different viper subspecies from the above origins, and detect LD 50 As an immunogen; the horse used for immunization is a Kunming pony that has passed the quarantine inspection according to the relevant provisions of the three relevant provisions of the "Chinese Pharmacopoeia" in 2010; the mouse used in the experiment is a Kunming white mouse, SPF grade, weighing 18-20g; pepsin has been tested for class A substances Qualified, specific activity 1:3000.
[0025] (1) Immunization of horses and collection of immune plasma
[0026] Before immunization, the horses were immunized twice with concentrated tetanus toxoid, and after the viper venom was gradually detoxified with formaldehyde, an adjuvant was added (the concentration of the venom was 0.2 mg / ml). Immunization is divided into basic immunity and hyperimmunit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com