Cefazolin sodium pentahydrate compound of new route
A technology of cefazolin sodium and compound, applied in the field of pharmaceutical synthesis, can solve the problems of low product purity, low yield and high cost, and achieve the effects of simplifying operation steps, reducing cost, and improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
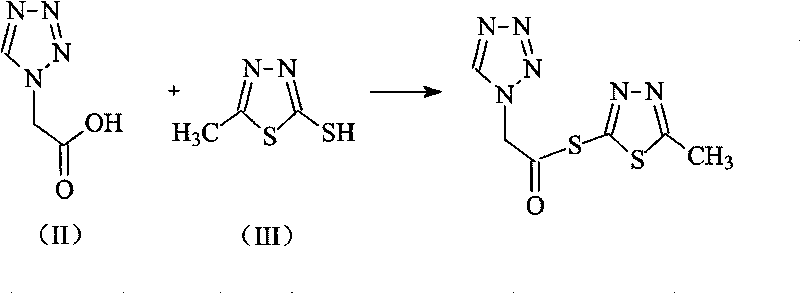
[0034] The synthesis of embodiment 1 tetrazoleacetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole
[0035] Add 275ml of isopropyl chloroformate to 1 liter of tetrahydrofuran solution containing 256 grams (2mol) of tetrazoleacetic acid, cool to 10°C, add 243ml of N-methylpiperidine, keep the reaction temperature not exceeding 15°C, Stir, add 264 grams of 2-mercapto-1,3,4-thiadiazole in 800ml of tetrahydrofuran solution, react at 15°C for 2 hours, then concentrate the reaction solution to a viscous state, add 2 liters of petroleum ether Vigorously stirred for 2 hours, a solid was formed. After continuing to stir for 2 hours, it was filtered to obtain 464 g of a yellow solid product, with a yield of 96%.
Embodiment 2 5
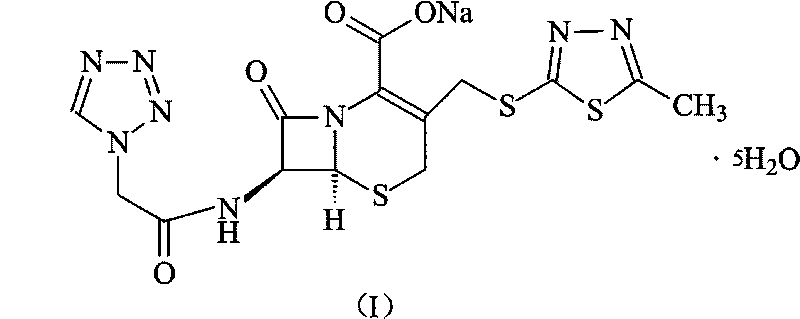
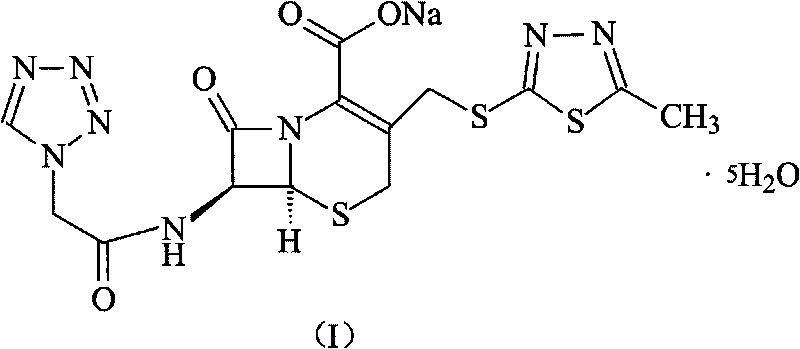
[0036] The synthesis of embodiment 2 cefazolin sodium pentahydrate
[0037] Under the protection of nitrogen, 272 grams of 7-ACA and sodium bicarbonate are added to water to form a clear solution, then add 242 grams of the product obtained in Example 1 (tetrazolium acetic acid 2-mercapto-5-methyl-1 , 3,4-thiadiazole) and 2 liters of acetone, then the mixed solution was reacted at 60°C for 4 hours, and the pH was adjusted to 7.5 with 3% aqueous sodium bicarbonate solution. After the reaction, 4L of ethanol was added to control the reaction The temperature was 15° C., stirred for 2 hours, crystals were precipitated, filtered, the filter cake was washed with ethanol, and dried at 40° C. for 3 hours to obtain 515 grams of cefazolin sodium pentahydrate, with a yield of 91%.
[0038] Elemental Analysis C 14 h 13 N 8 NaO 4 S 3 ·5H 2 o
[0039] Theoretical value C: 29.67%, H: 4.09%, N: 19.77%, O: 25.41%, S: 16.97%,
[0040] Experimental values C: 29.72%, H: 4.05%, N: 19.80%, O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com