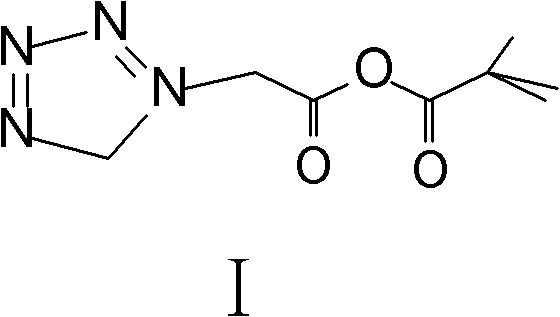
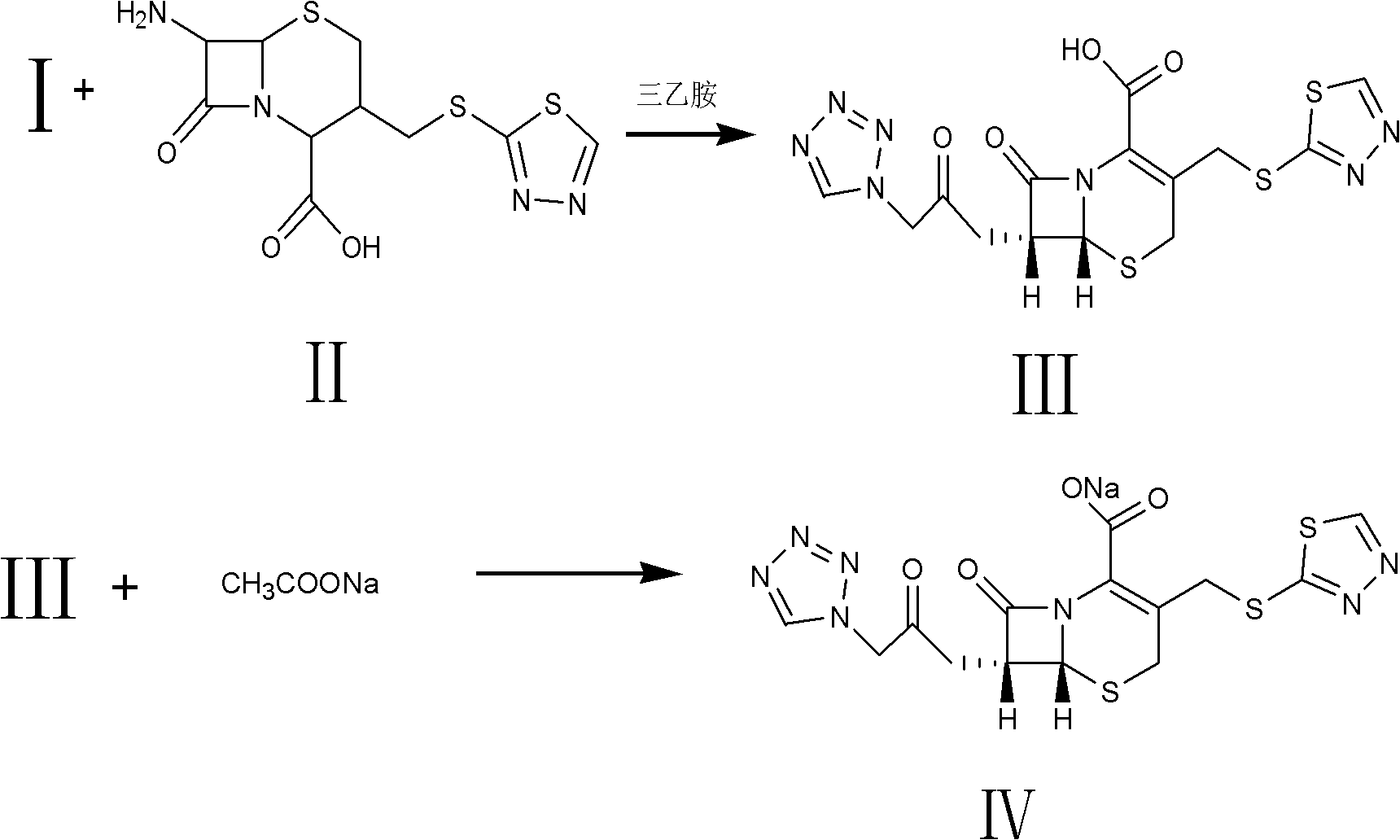
Method for preparing ceftezole sodium compound
A technology of ceftezole sodium and ceftezole, which is applied in the field of preparation of antibacterial compounds, can solve problems such as environmental pollution and processing difficulties, and achieve the effects of reducing costs, reducing pollution, and controlling product turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1. Ceftezole three-position intermediate TZT (II) is synthesized
[0034] Add 480g of dimethyl carbonate and 500g of boron trifluoride dimethyl carbonate complex to the reaction kettle, add 80g of demethiadiazole and 170g of 7-ACA under stirring conditions, and react at 40°C±5°C for 60 minutes, measure 7-ACA residual was less than 1.0%. Add 1000ml of water into the condensate, stir for 60 minutes, add Na 2 CO 3 The solution was filtered to pH = 3.5, filtered, washed and dried to obtain II with a purity greater than 98.9% and a yield of 106%.
[0035] Step 2. acid anhydride preparation (I)
[0036] Add 500ml of dichloromethane to the reaction kettle, add 78.5g of tetrazoleacetic acid, cool to -10°C with liquid nitrogen, add 120ml of triethylamine dropwise within 10 minutes under stirring, and stir until the tetrazoleacetic acid is completely dissolved. Cool down to -35±5°C, add 5.4ml of pyridine and 71.5ml of pivaloyl chloride, react at -20±2°C for 50 minutes, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com