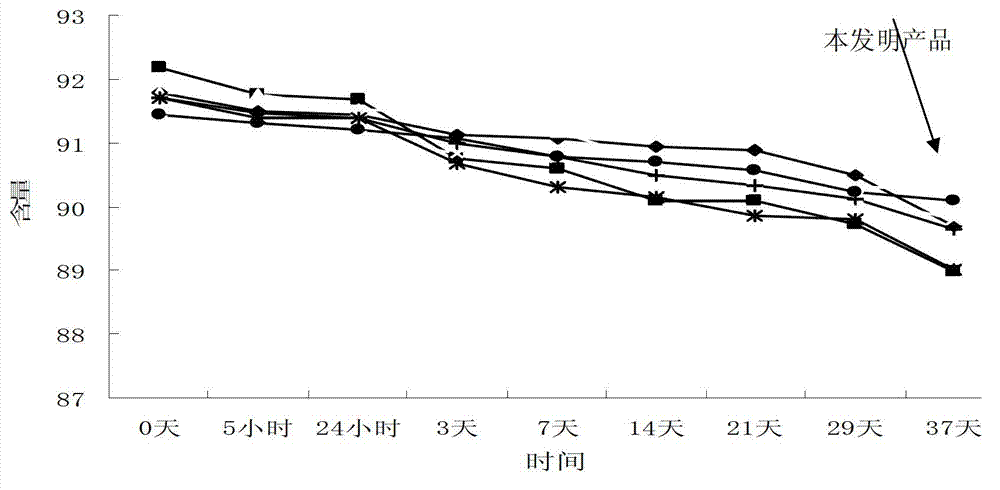
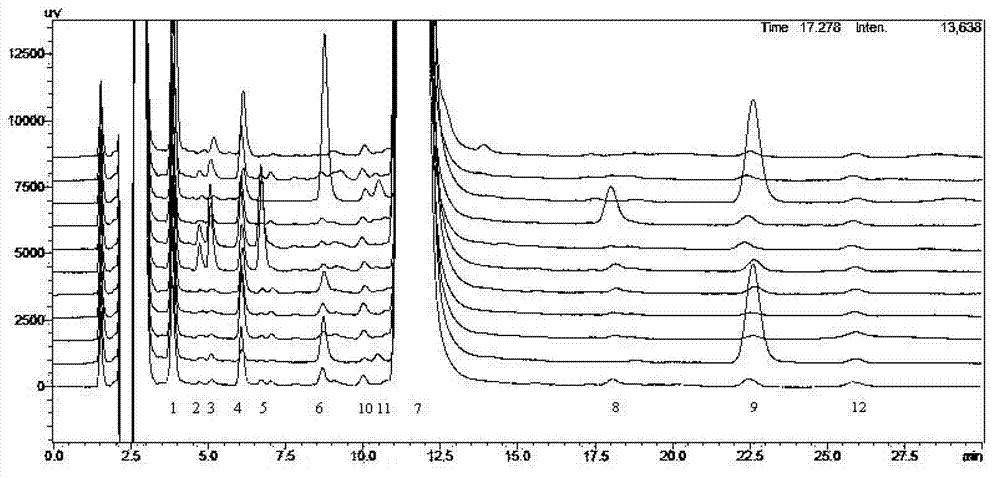
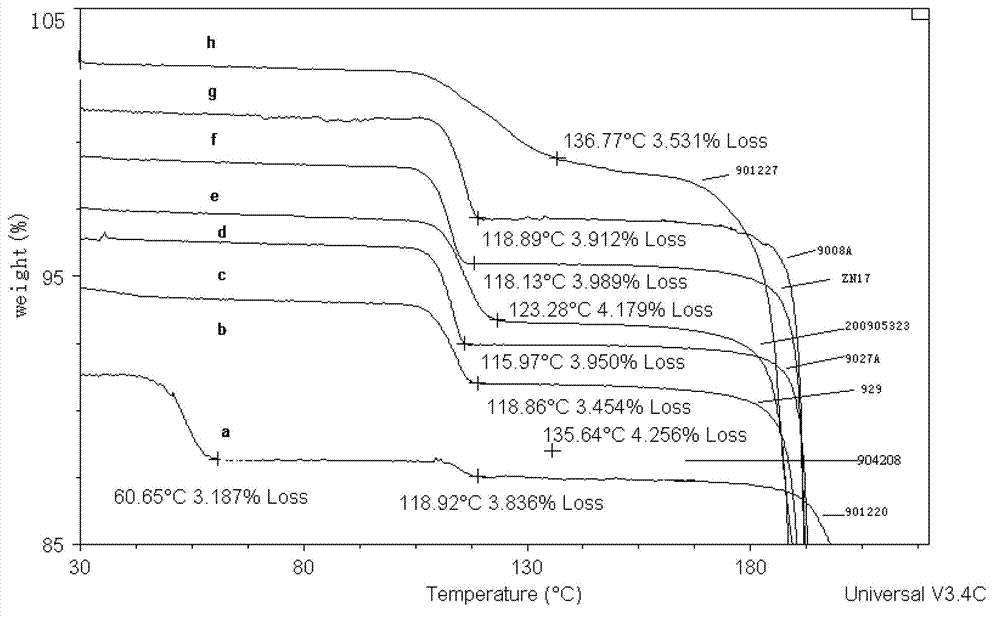
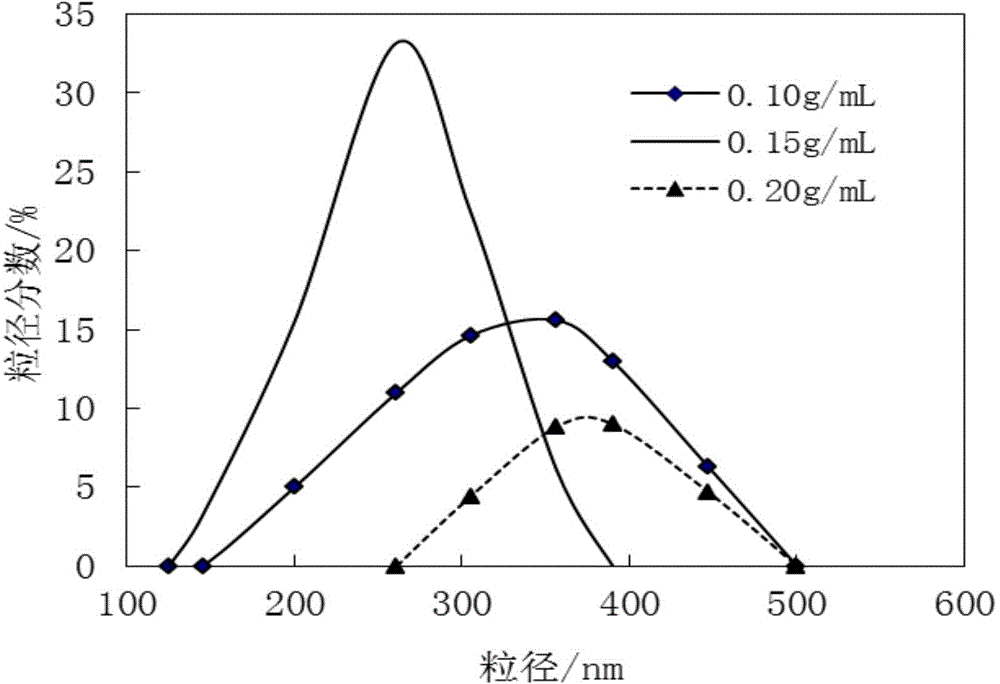
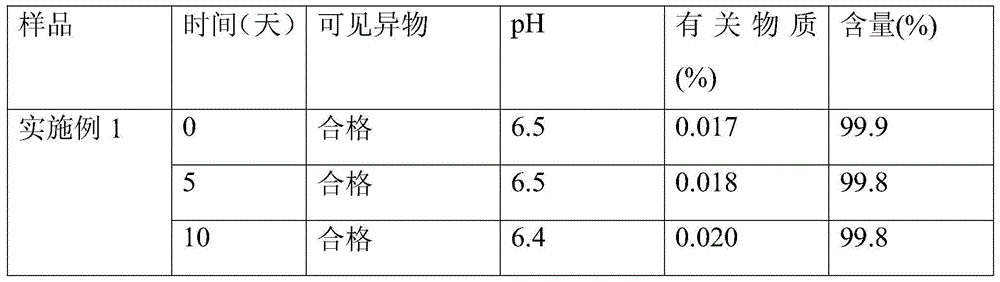
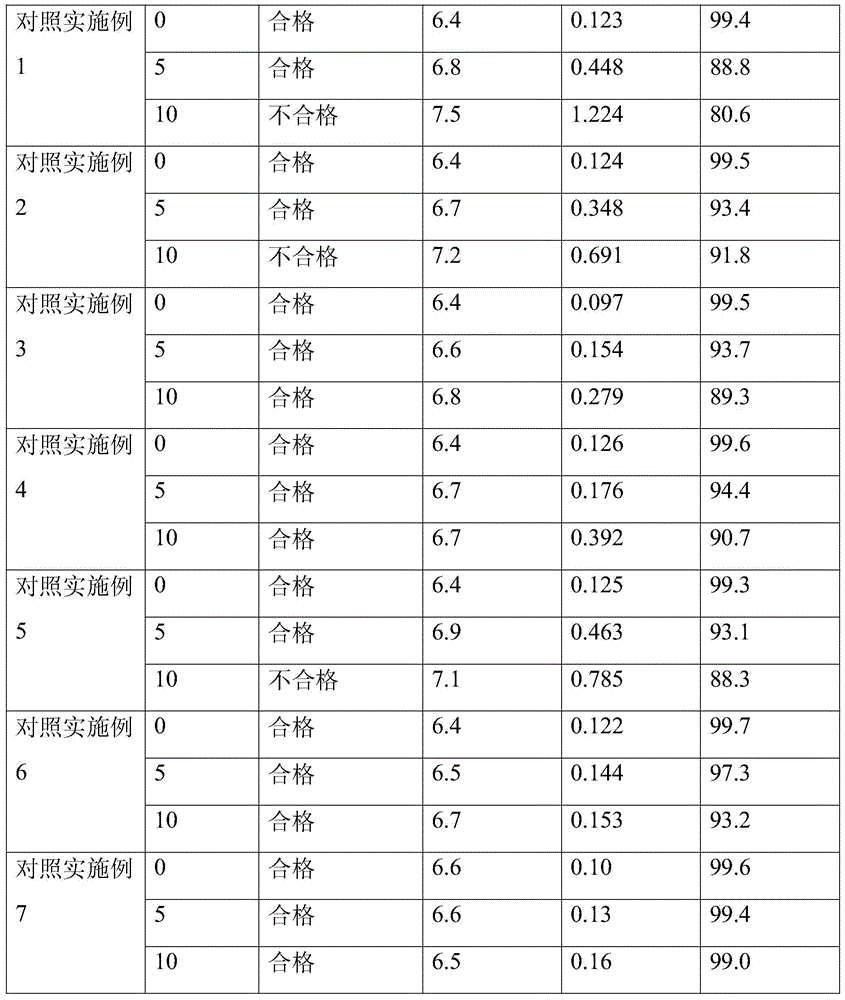
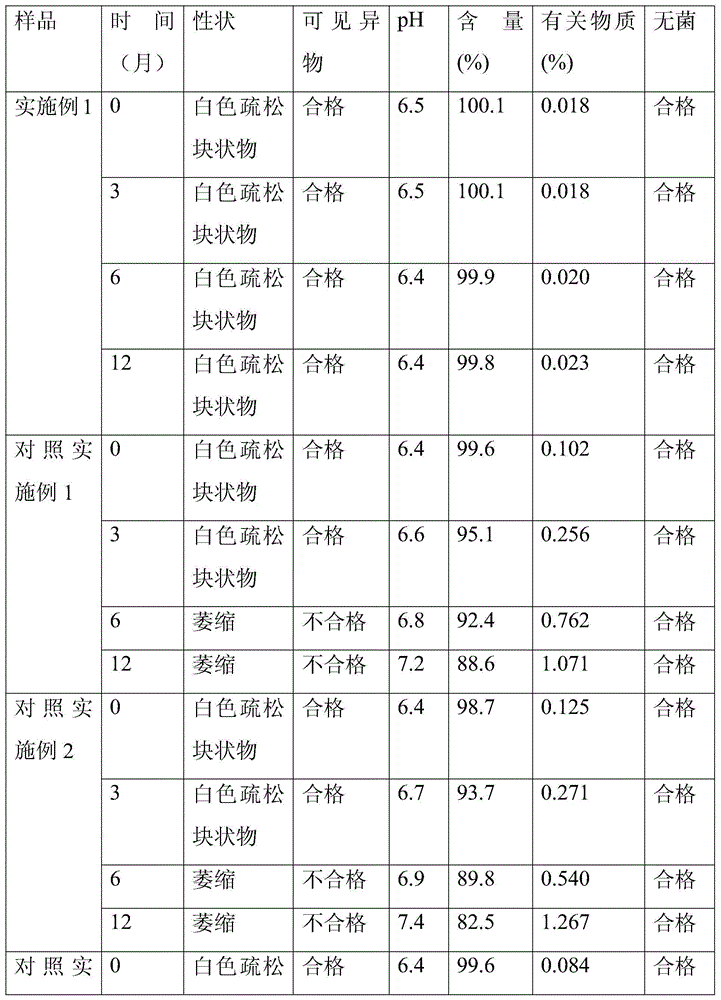
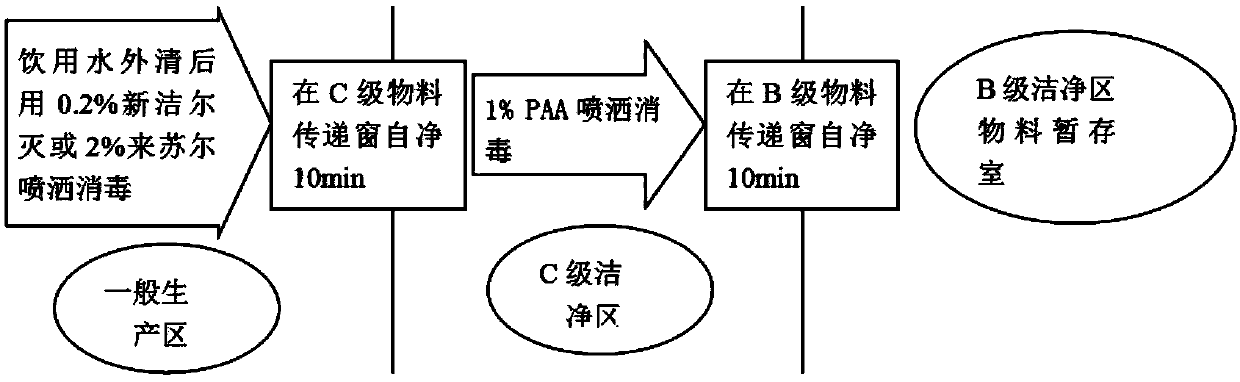
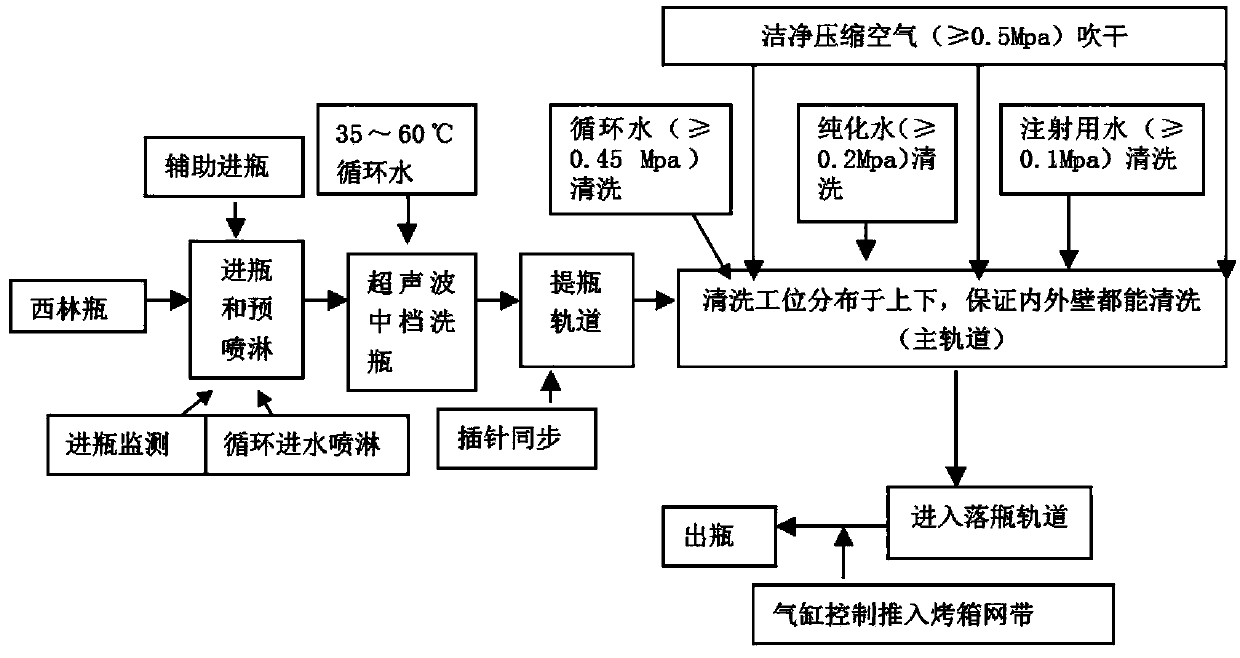
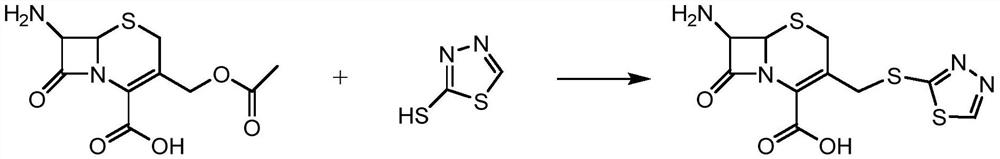
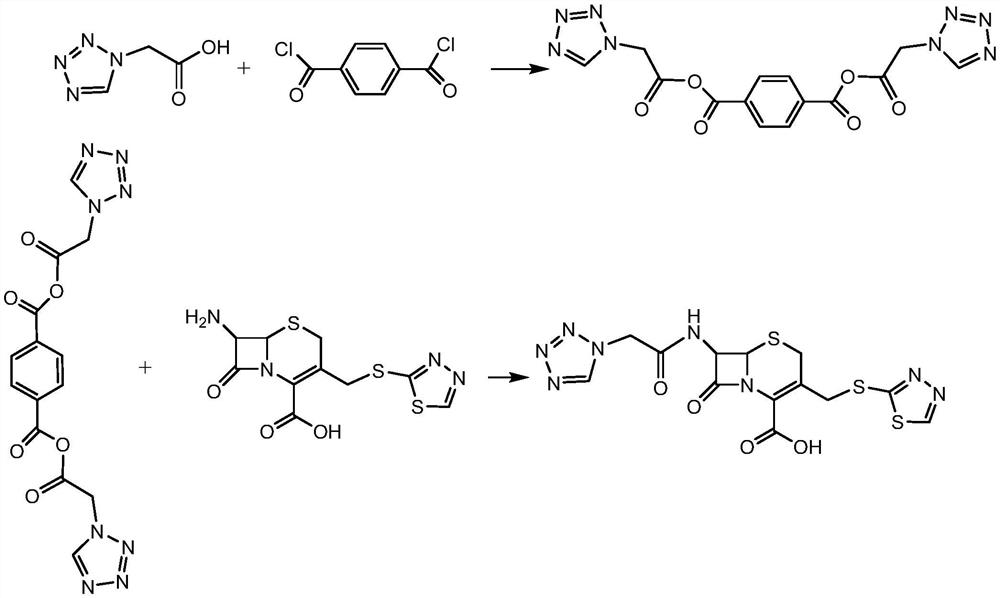
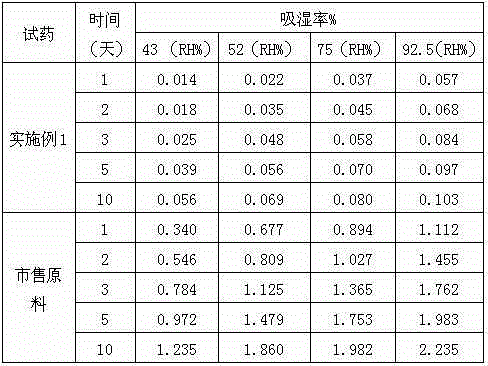
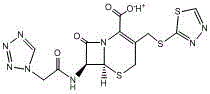
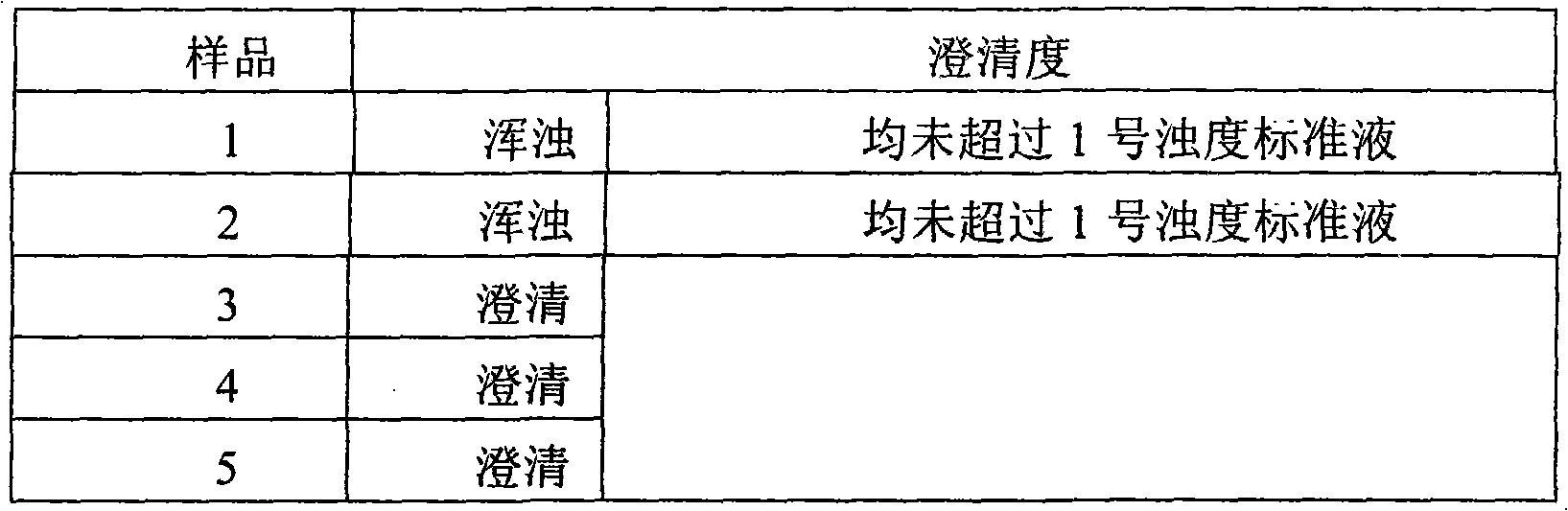
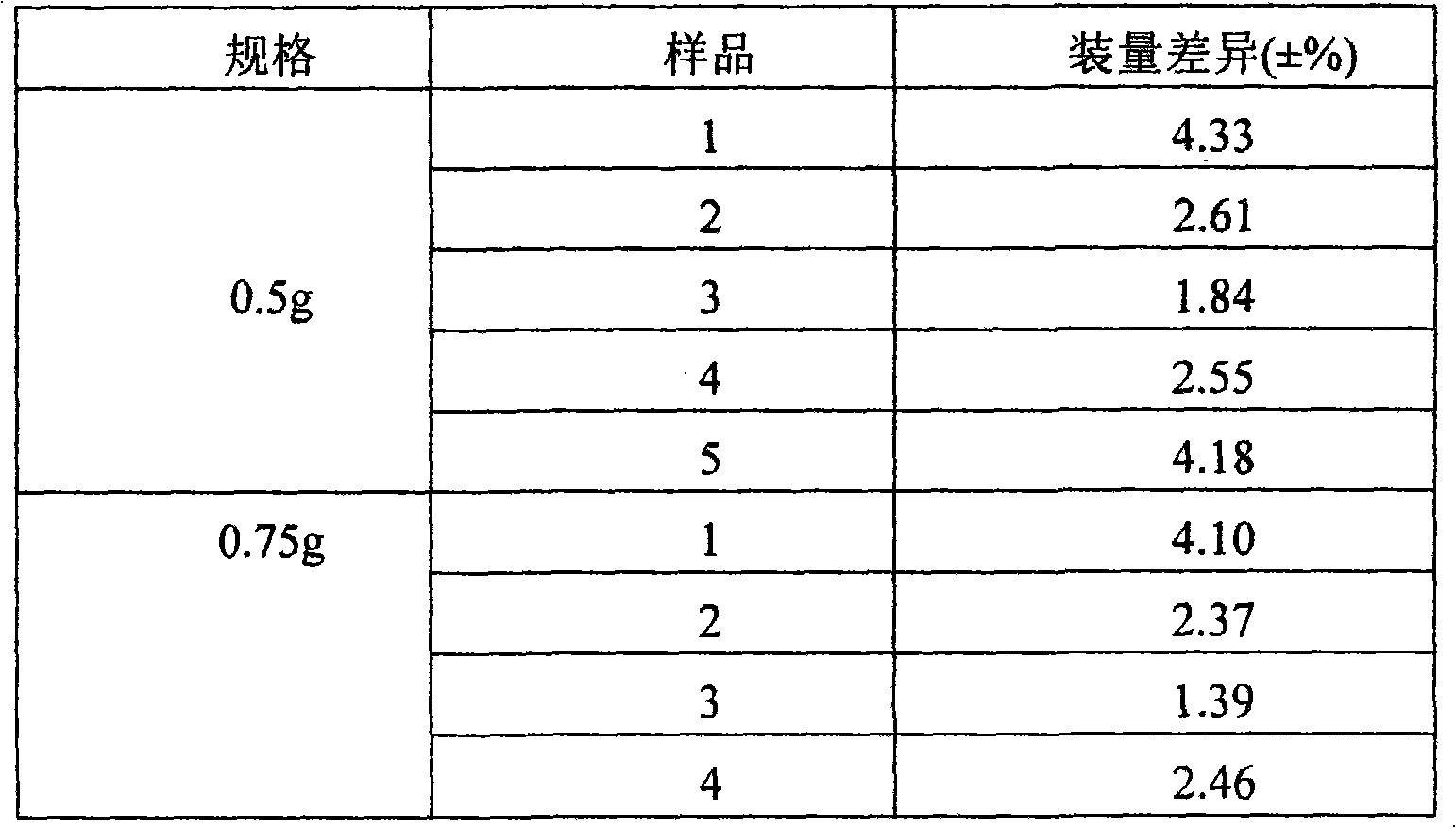
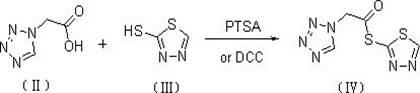Patents
Literature
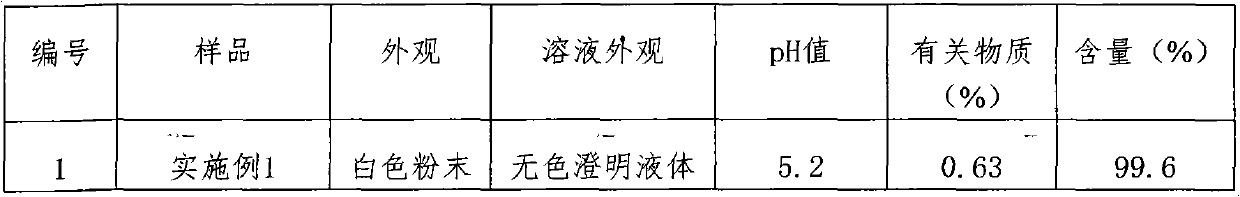
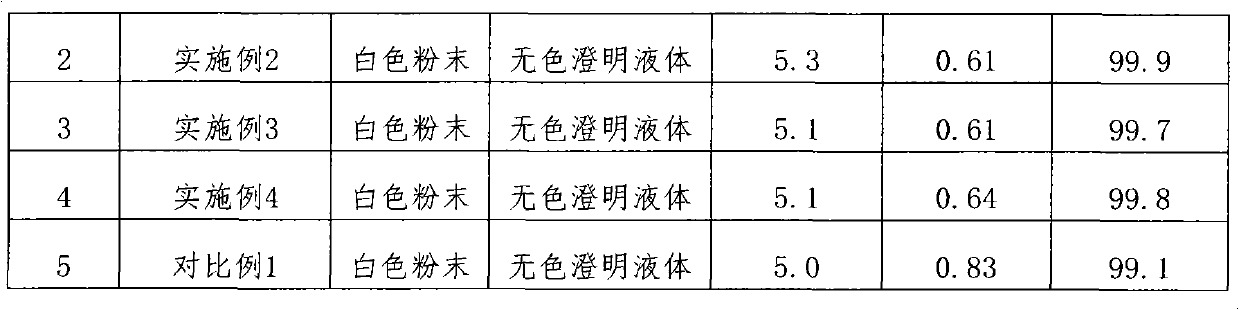
45 results about "Ceftezole Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
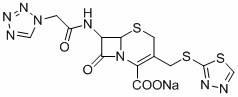
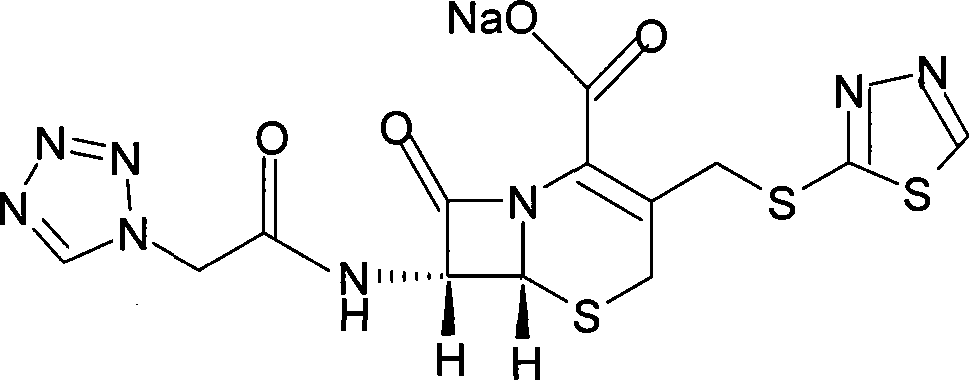
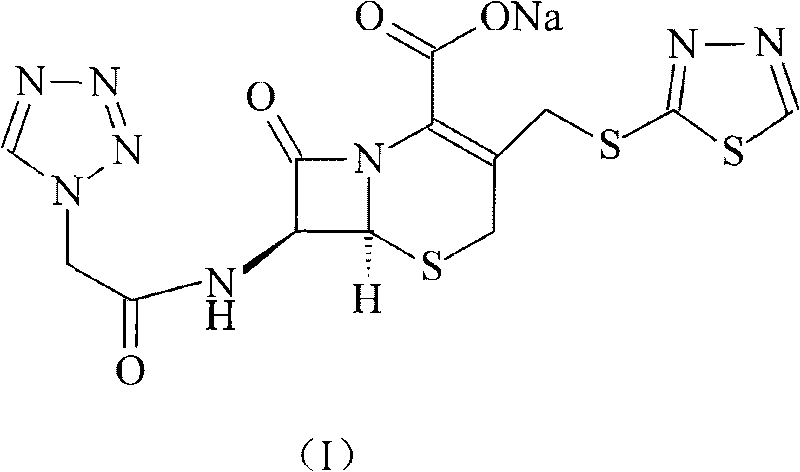
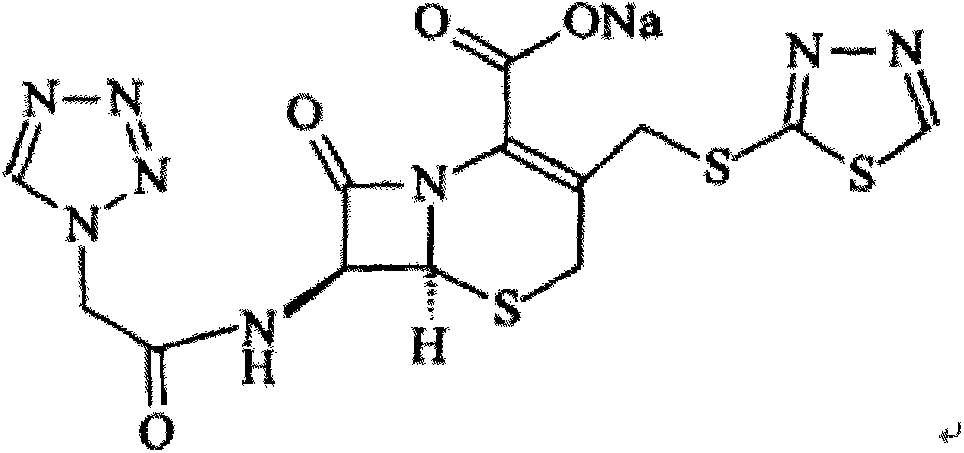
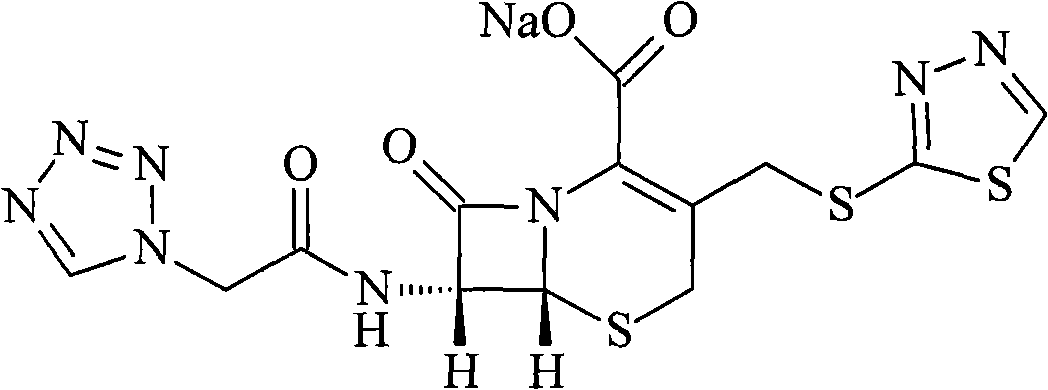
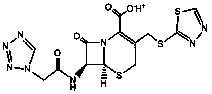
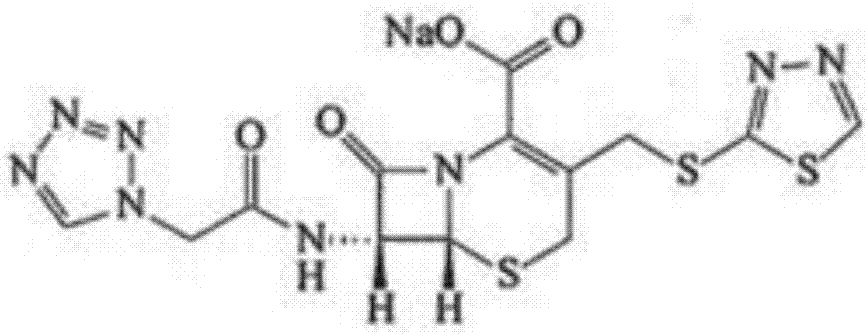
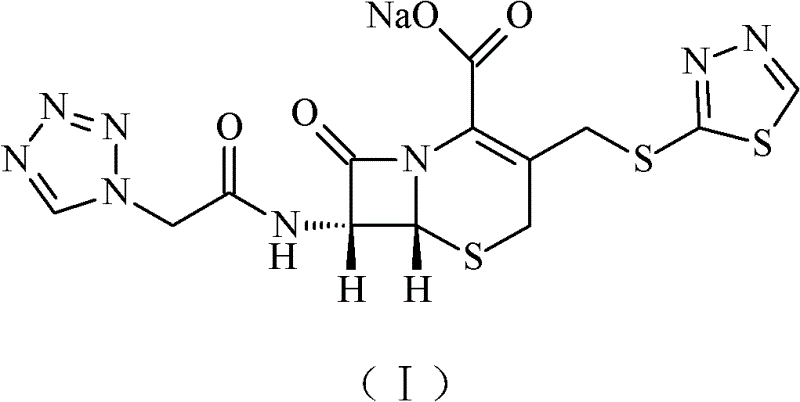
The sodium salt form of ceftezole, a semi-synthetic first-generation cephalosporin with antibacterial activity.
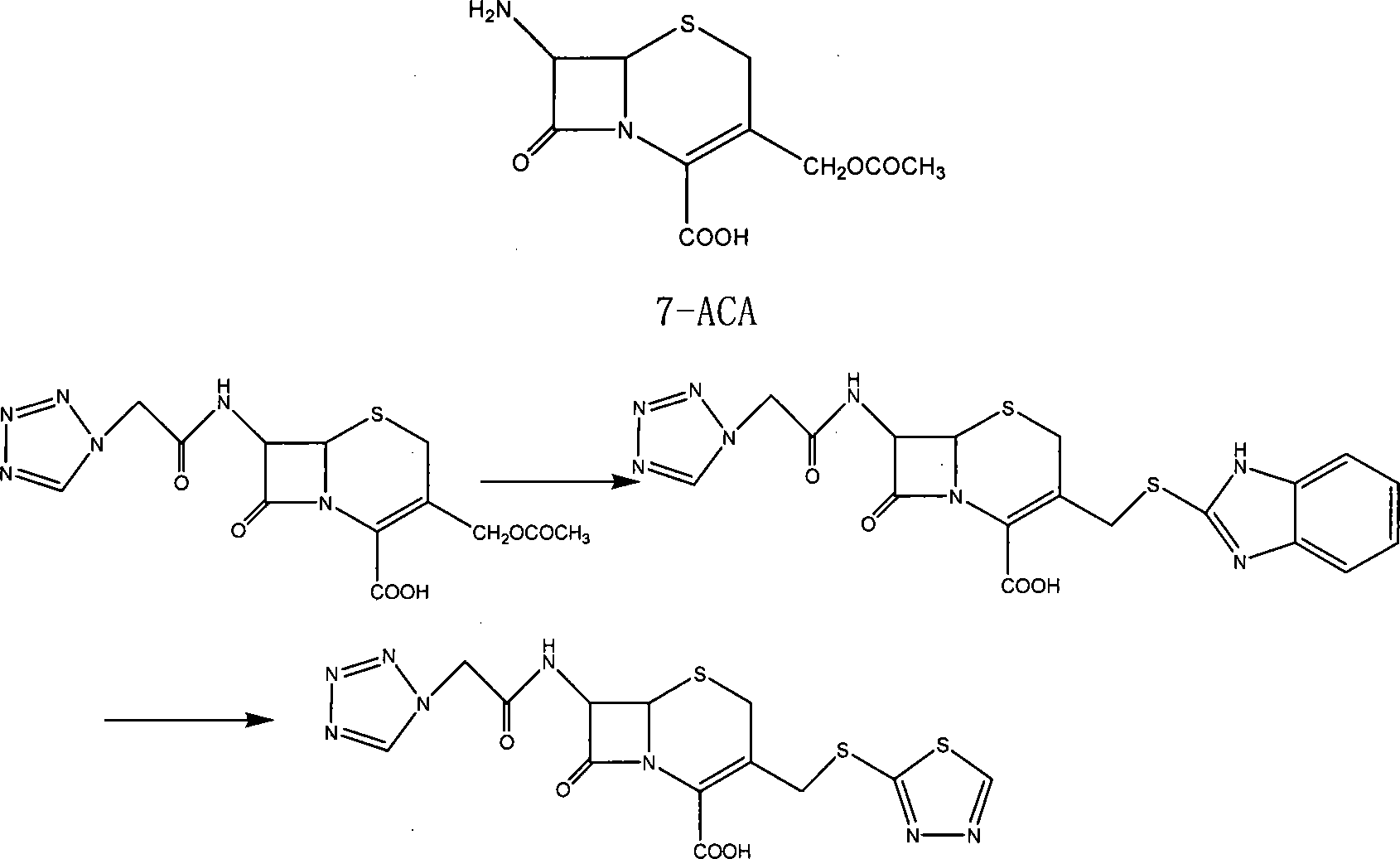
The preparation method of ceftezole sodium
InactiveCN102286001AReduce generationReduce pollutionOrganic chemistryCeftezole SodiumP-Toluenesulfonic acid
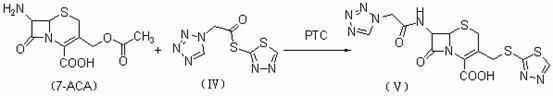
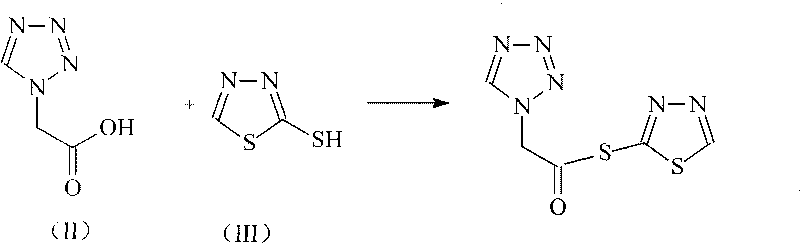
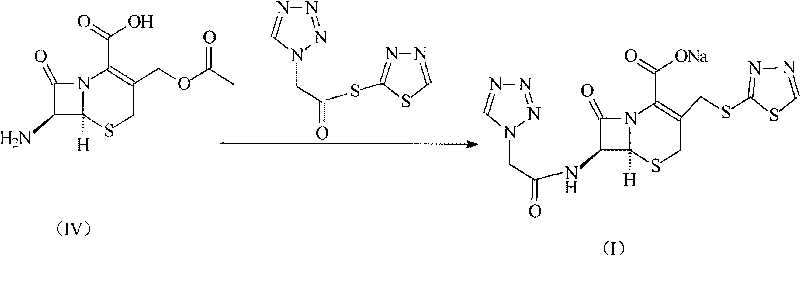
The invention discloses a preparation method of ceftizole sodium, which belongs to the field of medicinal chemistry. The method uses 1H-tetrazolium acetic acid and 2-mercapto-1,3,4-thiadiazole as raw materials, and generates 1H-tetrazolium acetic acid-1 under the catalysis of p-toluenesulfonic acid or dicyclohexylcarbodiimide ,3,4-thiadiazole-2-thioester (active ester), and then the active ester and 7-aminocephalosporanic acid are "one-pot" synthesis of ceftizole acid under the action of quaternary ammonium salt phase transfer catalyst, and then Sodium salt is generated, and further recrystallization and purification can obtain high-purity ceftiazole sodium. The preparation process is simple and feasible, the atom utilization rate is high, the product quality is good, and the industrial production requirements are met.
Owner:ZHENGZHOU UNIV
Ceftezole sodium powder injection and synthesizing method thereof
ActiveCN101229129AHigh reaction yieldImprove responseAntibacterial agentsPowder deliverySodium bicarbonateCeftezole Sodium
The invention which provides a ceftezole sodium powder injection and a synthesis method for the powder injection consists of more stable ceftezole sodium crystals; in the method, tetrazoleacetic acid, N, N'- dicyclohexylcarbodiimide and 7-amino cefgadoleic acid with the proportion of 1.4 to 1.7:1 to 1.3:1 make reactions in dimethyl sulfoxide; ceftezole is prepared by causing a react intermediate to react with thiadiazole thiol of 1.1 to 1.3 times; the ceftezole sodium is prepared in sodium bicarbonate water solution after acetone is re-crystallized. The method is easy in operation and has the total yield of 55.5 percent.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for preparing ceftezole sodium compound
ActiveCN102617606AReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumMethyl carbonate
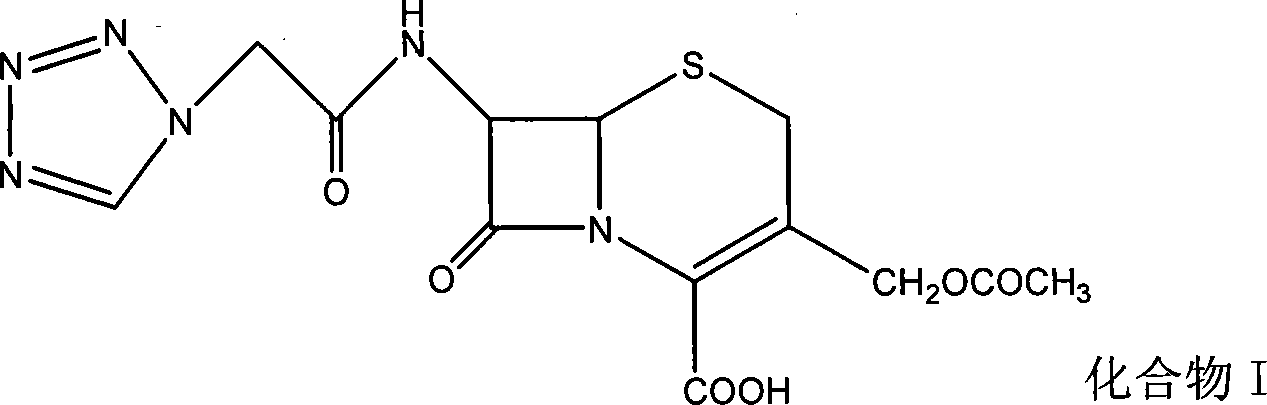
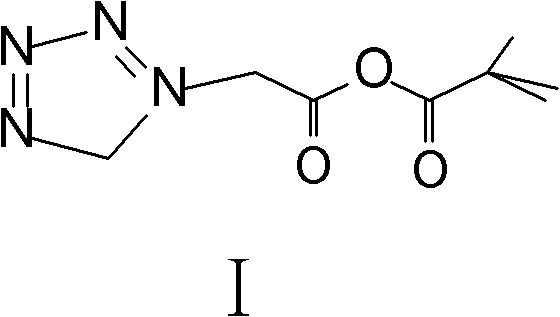
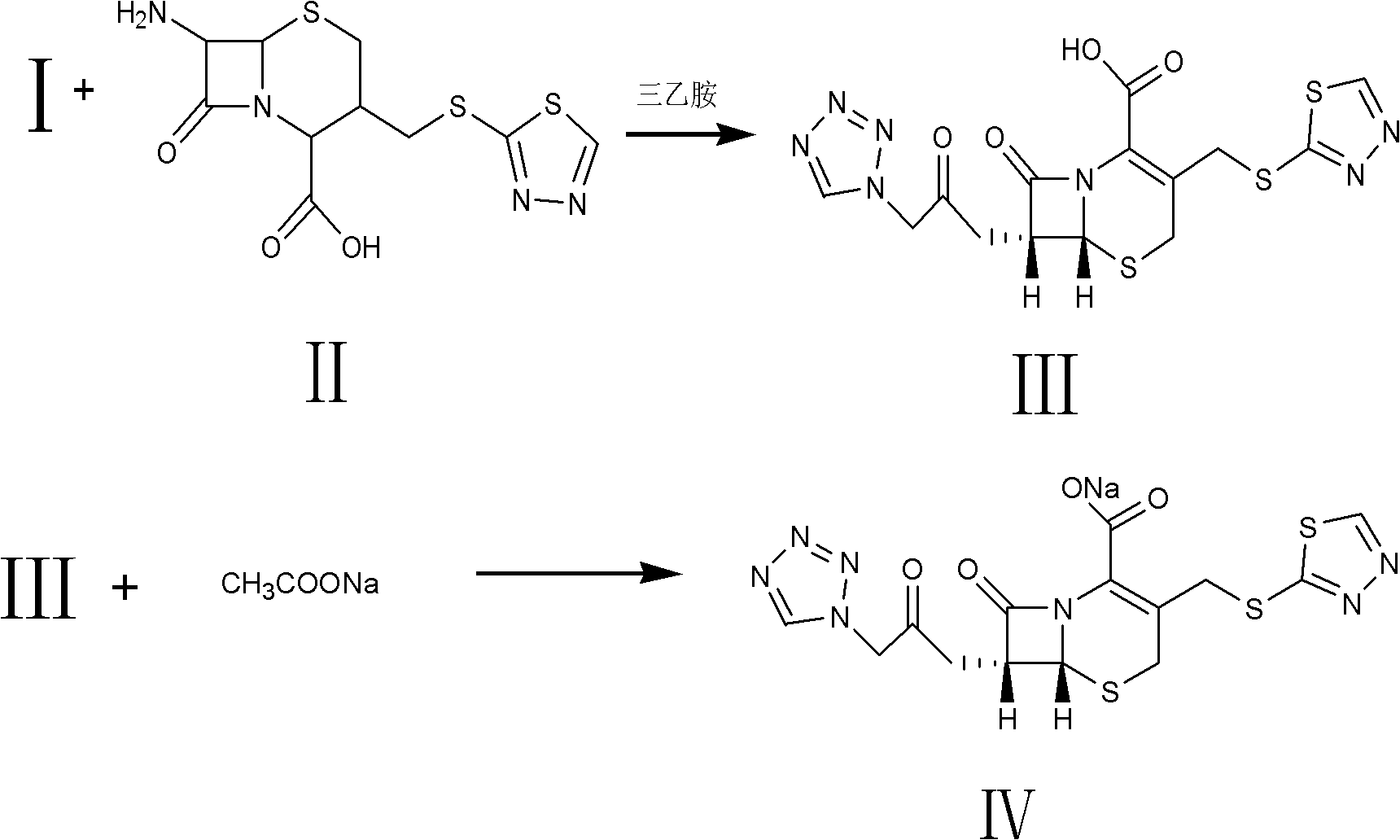
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
Crystallization method of ceftezole sodium
InactiveCN102775426AReduce contentIncrease humidityOrganic chemistryActivated carbonCeftezole Sodium
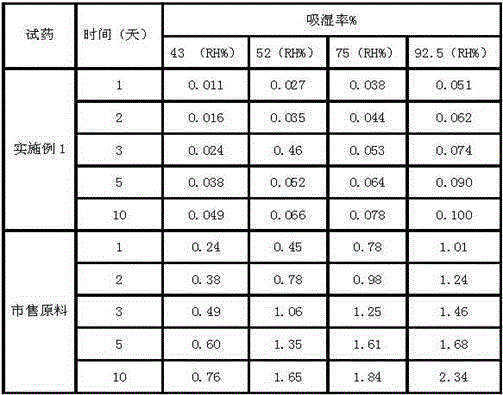
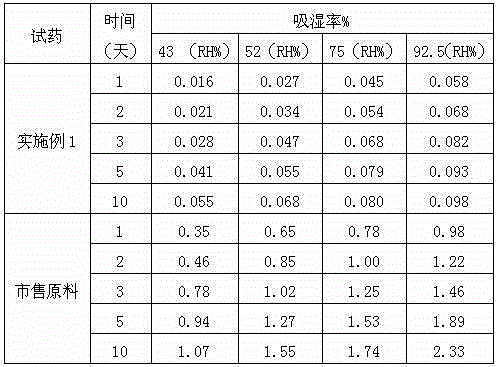
The invention relates to a crystallization method of ceftezole sodium. The crystallization method comprises the steps of: dissolving ceftezole acid in a solvent, carrying out nitrogen washing protection, adding a salifying agent to adjust pH until the solid is completely dissolved, adding activated carbon, carrying out titanium rod filtering, carrying out ultrafiltration, adding the solvent in filter liquor under the ultrasonic wave condition, carrying out gradient cooling to separate out crystals, filtering, washing, carrying out suction filtration, and carrying out vacuum drying to obtain the ceftezole sodium. The crystallization method adopts an ultrasound crystallographic orientation method to be combined with a gradient cooling solvent crystallization method to obtain the ceftezole sodium which obviously improves hygroscopicity, is high in purity, even in particles and good in batch repeatability and is a stable type I crystal form, and thus stability and safety of products are obviously improved.
Owner:天津新丰制药有限公司
Synthesis process of ceftezole sodium
InactiveCN102010430ASimple stepsHigh yieldOrganic chemistryCeftezole SodiumHigh volume manufacturing
The invention relates to a synthesis process of ceftezole sodium, comprising the following process steps of: firstly, synthetizing ceftezole; then, synthetizing the crude product of ceftezole sodium; and finally, refining the crude product of the ceftezole sodium. The synthesis process of the ceftezole sodium has the advantages of simple steps, higher yield and lower processing cost and is suitable for mass production.
Owner:JIANGSU HI STONE PHARMA
Ceftezole sodium compound with novel route
InactiveCN101735250AAvoid reactionAvoid processing powerOrganic chemistryAcetic acidCeftezole Sodium
The invention relates to a cephalo class compound, in particular to a Ceftezole sodium compound which belongs to the technical field of medicine. Tetrazolyl acetic acid is adopted to condense with 2-mercapto-1,3,4-thiadiazole to generate tetrazolyl acetic acid 2-mercapto-1,3,4-thiadiazole ester, and the tetrazolyl acetic acid 2-mercapto-1,3,4-thiadiazole ester and 7-aminoce-phalosporanic acid (7-ACA) react to directly obtain a target compound of Ceftezole sodium. The multi-step reaction and the complicated postprocessing of the prior art are avoided, the problems existing in the prior art are solved, the steps are simplified, the cost is lowered, and the yield is enhanced greatly.
Owner:HAINAN LINGKANG PHARMA CO LTD
Ceftezole sodium agent and preparation method thereof
InactiveCN103271878AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryCeftezole SodiumArginine
The invention relates to a ceftezole sodium agent and preparation method thereof, especially relates to a ceftezole sodium injection for treating microbe infection, and preferably relates to a freeze-drying powder injection. The mezlocilin sodium for injection mainly comprises the following components: ceftezole, and accessory sorbitol and arginine. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is sorbitol, and sodium hydroxide or hydrochloric acid is used for adjusting pH valve.
Owner:张宏民
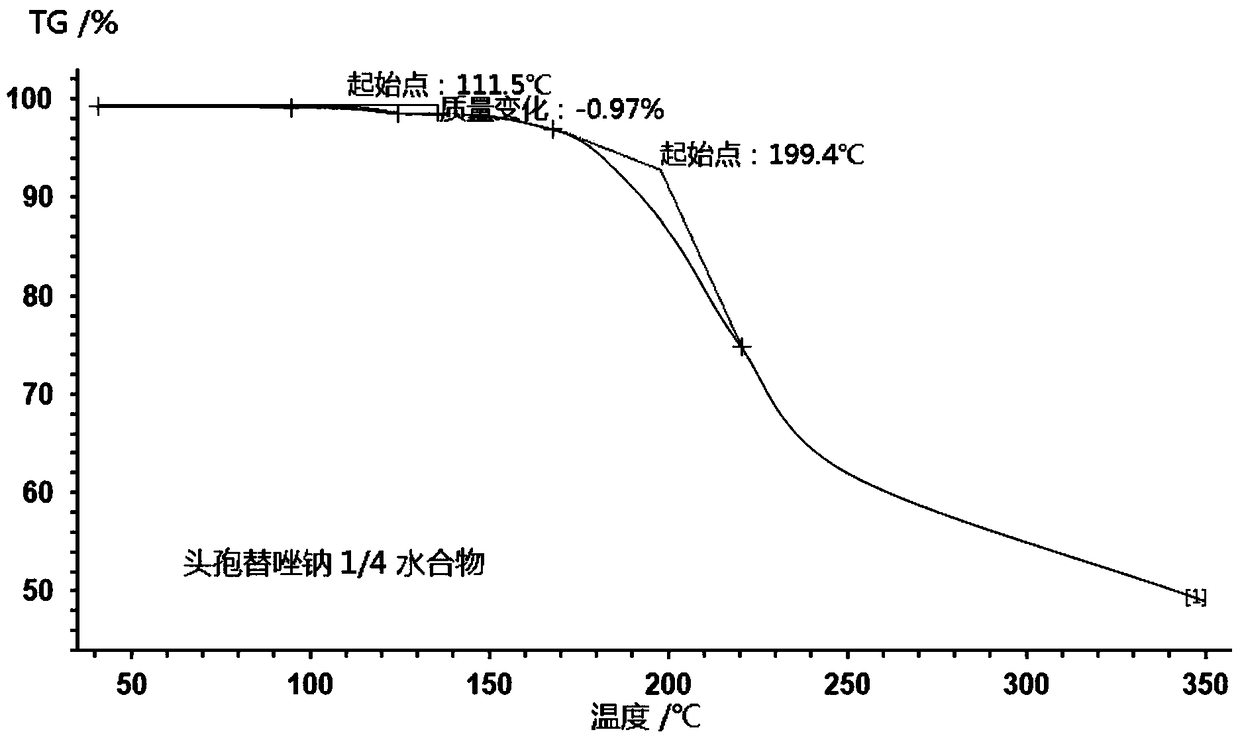
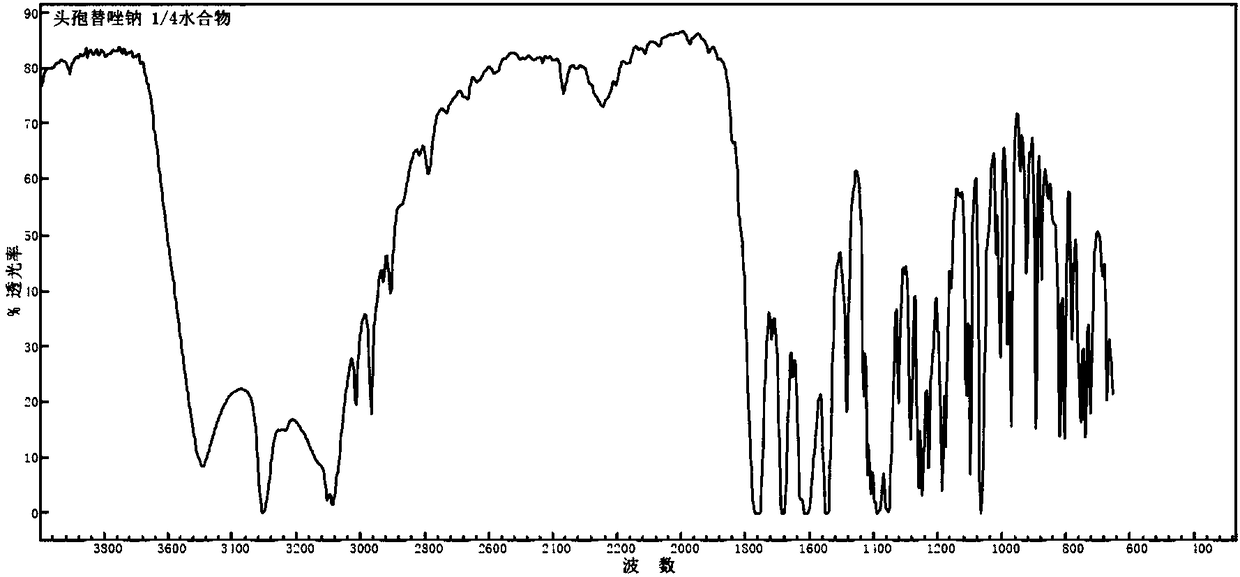
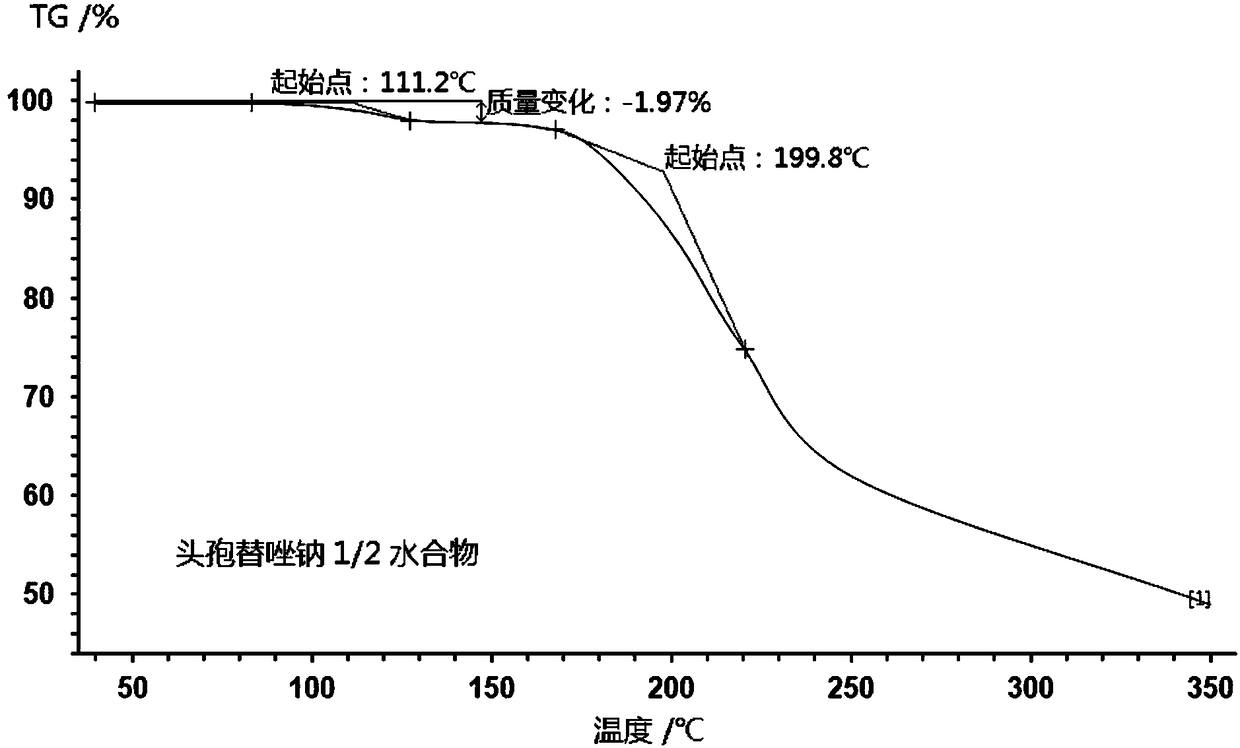
1/4 water ceftezole sodium compound
The invention discloses a 1 / 4 water ceftezole sodium compound and a preparation method of the 1 / 4 water ceftezole sodium compound, and each molar of ceftezole sodium contains 1 / 4 molar of water. The inventor dissolves ceftezole acid into a mixed solution of acetonitrile and water, the pH and the temperature of the solution are controlled, dichloromethane is added to crystalize, and the 1 / 4 water ceftezole sodium compound is prepared. The 1 / 4 water ceftezole sodium compound is good in stability, high in purity, low in hygroscopicity, and good in flowability.
Owner:刘兆娟
Ceftezole sodium liposome lyophilized preparation and preparation method thereof
InactiveCN102525924AImprove stabilityImprove securityAntibacterial agentsOrganic active ingredientsCeftezole SodiumCholesterol
The present invention provides a ceftezole sodium liposome lyophilized preparation and a preparation method thereof. According to the preparation method, ceftezole sodium, an excipient and a blank film material of a neutral lecithin and cholesterol are mixed, an ultrasonic treatment is performed, and treatments of volume metering, sterilization, subpackaging and freeze drying are performed to obtain the ceftezole sodium liposome lyophilized preparation. With the ceftezole sodium liposome lyophilized preparation prepared by the method of the present invention, disadvantages of the existing formations are overcome, the quality is stable, and the safety of the drug application in clinic is enhanced.
Owner:双鹤药业(海南)有限责任公司
Ceftezole sodium compound and novel method thereof
The invention relates to a ceftezole sodium compound and a novel method thereof. The method provided by the invention achieves the refining aim by acidification reaction, activated carbon adsorption, electroosmosis device processing and preparation of chromatographic columns, so as to finally obtain a high-purity ceftezole sodium compound, overcomes the defects of low purity of the existing raw materials, improves the product quality of the preparation, reduces the toxic and side effects, and ensures the clinical safety of the medicine at the same time.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method for type I ceftezole sodium crystal
The invention discloses a type I ceftezole sodium crystal and a preparation method thereof. The preparation method is characterized by comprising the following steps of mixing sodium salt solution with ceftezole acid to obtain an aqueous solution of ceftezole sodium, decolorizing with activated carbon and filtering; dropping the aqueous solution of ceftezole sodium in mixed solution of organic solvent I and water by stirring; when needle-like precipitates are found, reducing the stirring rotating speed and growing the crystal at room temperature; continuously dropping the organic solvent I and organic solvent II, growing the crystal at low temperature and raising the stirring rotating speed; filtering, flushing the crystal with the mixed solution of the organic solvent I and the water and performing vacuum drying to obtain the type I ceftezole sodium crystal. According to the preparation method for the type I ceftezole sodium crystal, provided by the invention, the single type I ceftezole sodium crystal with the advantages of high purity, high thermal stability, homogeneous particles and good inter-batch repeatability can be obtained, and important significance for improving the stability and the safety of the ceftezole sodium for injection is realized; in addition, the preparation method has the advantages of simple operation, easiness in control for conditions and suitability for batch production.
Owner:SHENYANG SANJIU PHARMA +1
Process for preparing ceftezole sodium
ActiveCN104262361ALarge batchSimple and fast operationOrganic chemistryCeftezole SodiumActivated carbon filtration
The invention relates to a process for preparing ceftezole sodium. The process comprises the following steps: (1) adding N,N-dimethylacetamide or N,N-dimethylformamide and a first part of acetone, adding ceftezole while stirring, and adding purified water or cooled water for injection until ceftezole is completely dissolved; (2) adding medicinal activated carbon, filtering and washing the activated carbon to obtain a reaction solution A; (3) adding a third part of acetone and adding a sodium-forming agent until the solid is completely dissolved; (4) adding medicinal activated carbon, filtering and washing the activated carbon with a fourth part of acetone to obtain a reaction solution B; (5) cooling the reaction solution B to 8-12 DEG C, dropwise adding the reaction solution A into the reaction solution B for carrying out salt formation and crystallization; (6) after the dropwise addition of the reaction solution A is completed, continuously carrying out heat preservation at 8-12 DEG C and stirring for 60-90 minutes; and (7) washing filter cakes with sterilized and filtered acetone and drying at 40-50 DEG C to obtain the sterile ceftezole sodium solid. The process has the advantages of larger batches, simplicity in operation, high yield, good quality and low processing cost, the solvent is simply recovered and the process is suitable for industrial production.
Owner:ZHEJIANG DONGYING PHARMA
Preparation method of ceftezole sodium
The invention discloses a preparation method of ceftezole sodium, and belongs to the technical fields of pharmaceutical synthesis and refining. According to the method, 2-mercapto-1,3,4-thiadiazole and 7-aminocephalosporanic acid(ACA) are adopted to react and prepare an intermediate 1; and the obtained intermediate 1 reacts with tetrazolium anhydride(an intermediate 2) for acidation, salifying andrefining to prepare and obtain the ceftezole sodium. The preparation method provided by the invention is simple in operation, mild in reaction conditions, relatively high in yield and purity, low incontents of maximum single impurities and moisture and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
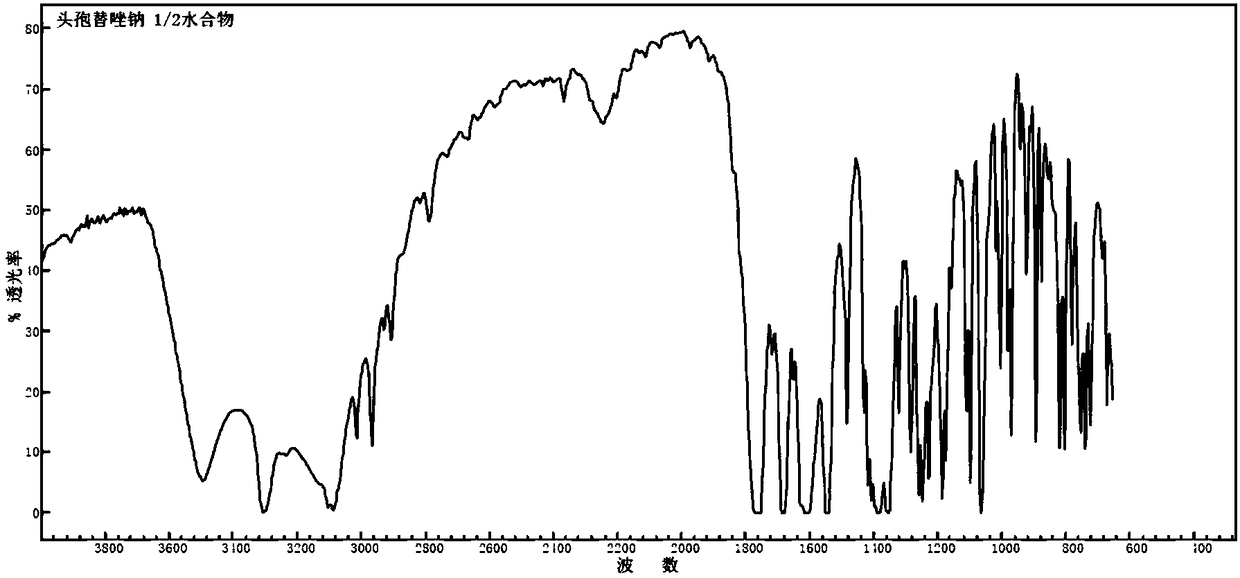
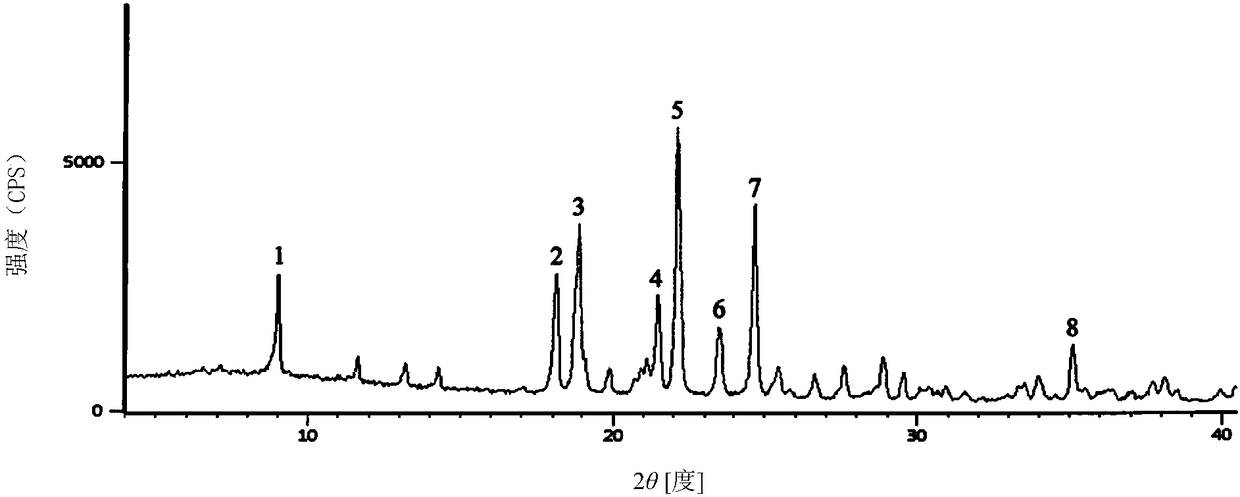
1/2 water ceftezole sodium compound
The invention discloses a 1 / 2 water ceftezole sodium compound and a preparation method of the 1 / 2 water ceftezole sodium compound, and each molar of ceftezole sodium contains 1 / 2 molar of water. The low-hygroscopicity high-purity 1 / 2 water ceftezole sodium compound having good thermodynamic stability is finally prepared by adsorbing by using an activated carbon, controlling the pH and the crystallization temperature and using a specific solvent. The defects of high hygroscopicity, poor purity and poor thermodynamic stability of the raw material produced in the prior art are overcome, and the 1 / 2 water ceftezole sodium compound has a broader application prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Medicinal composition containing ceftezole sodium compound and preparation method for medicinal composition
InactiveCN102872021AWell formedGood resolubilityAntibacterial agentsOrganic active ingredientsCeftezole SodiumAdditive ingredient
The invention provides a medicinal composition containing a ceftezole sodium compound and a preparation method for the medicinal composition. The medicinal composition contains ceftezole sodium and sodium carbonate cosolvent, and the weight ratio of the ceftezole sodium to the sodium carbonate is (5-10): 1. By reasonably proportioning the components of the medicinal composition, storage stability is realized by using a small quantity of accessories, and medication safety is ensured. Ceftezole sodium freeze-dried powder injection prepared by a secondary freeze-drying method is good in formation and re-dissolution property and high in stability and safety.
Owner:罗诚
Ceftezole sodium compound and medicinal preparation including same
ActiveCN104961751AUniform particle size distributionGood dispersionAntibacterial agentsPowder deliveryCeftezole SodiumSterile water
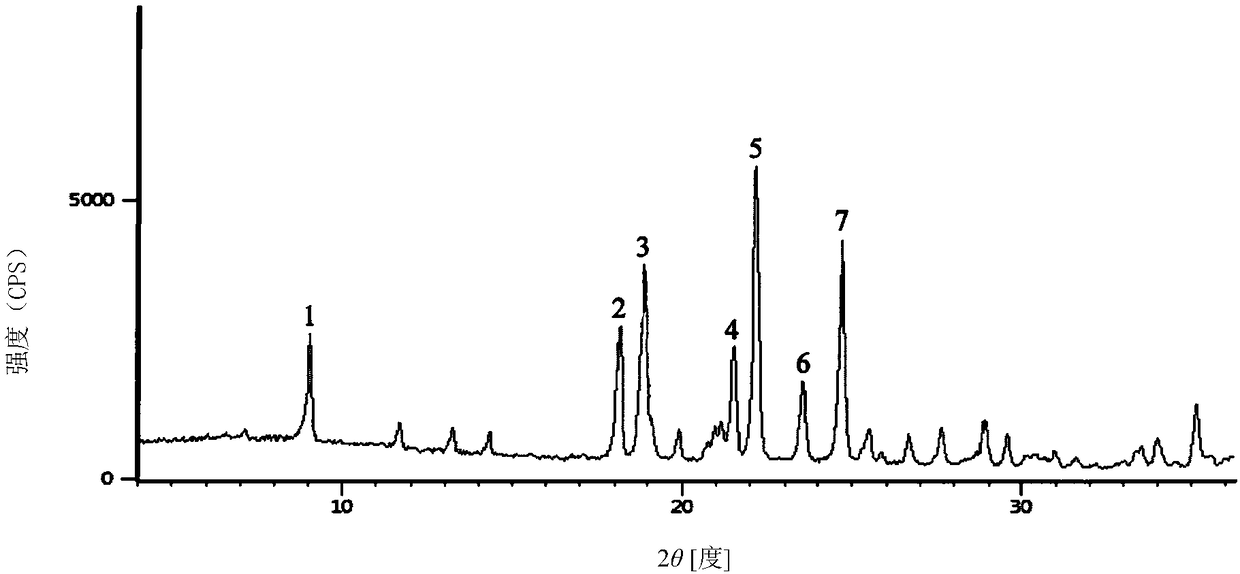
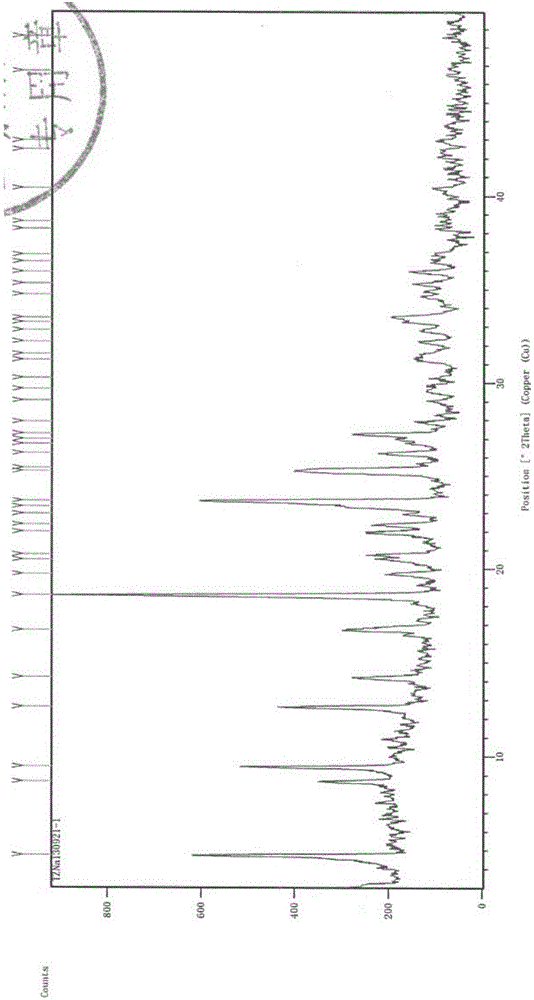
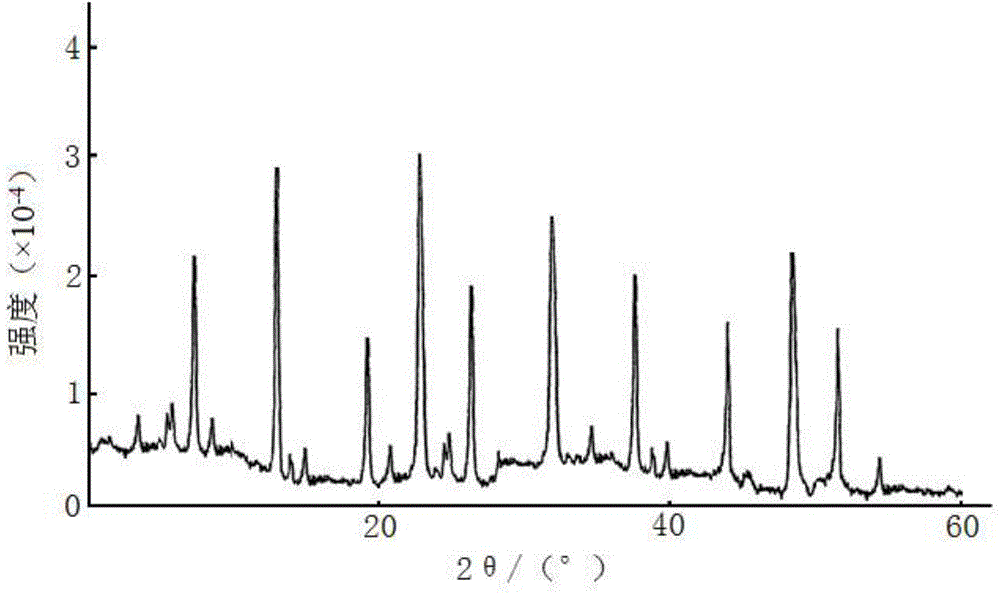
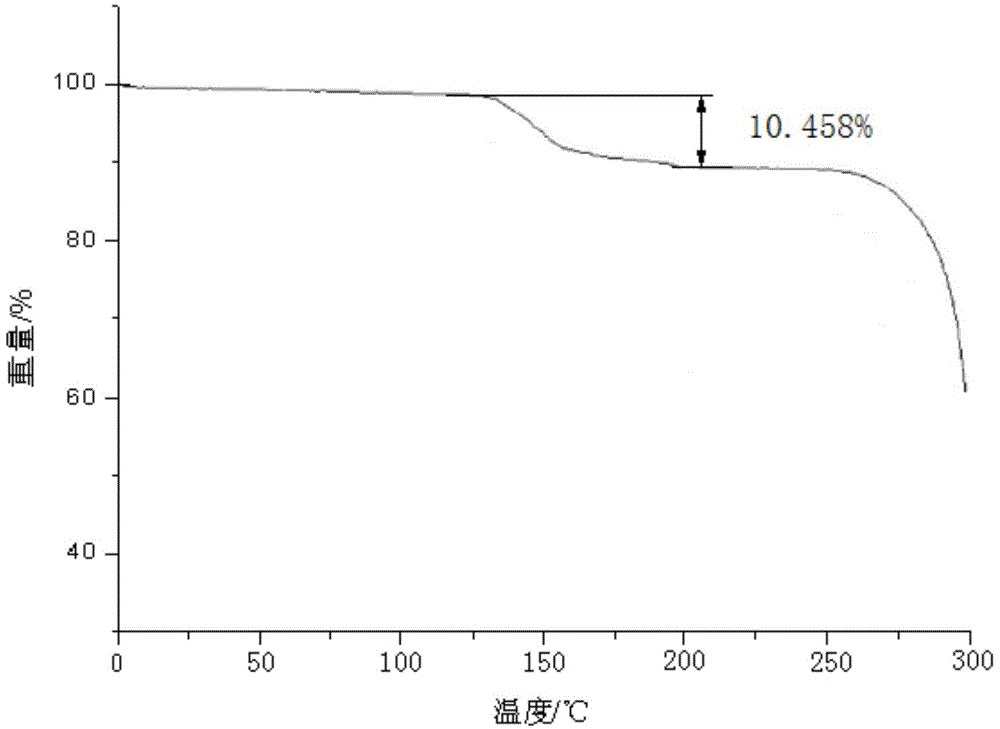
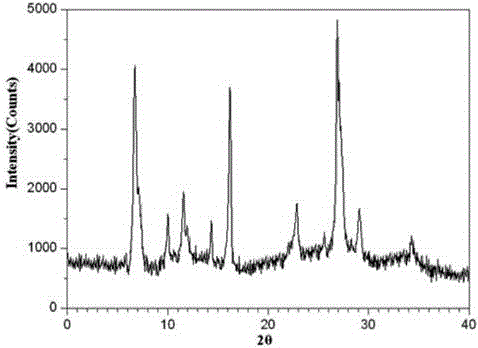
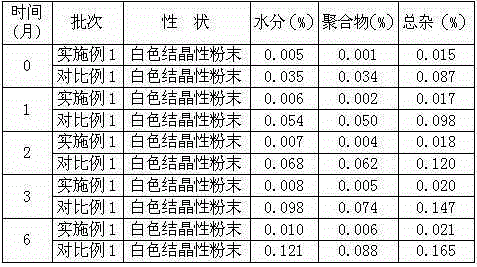
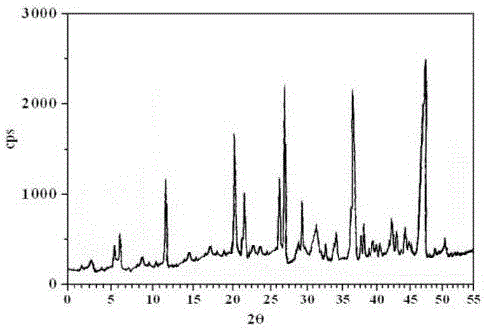
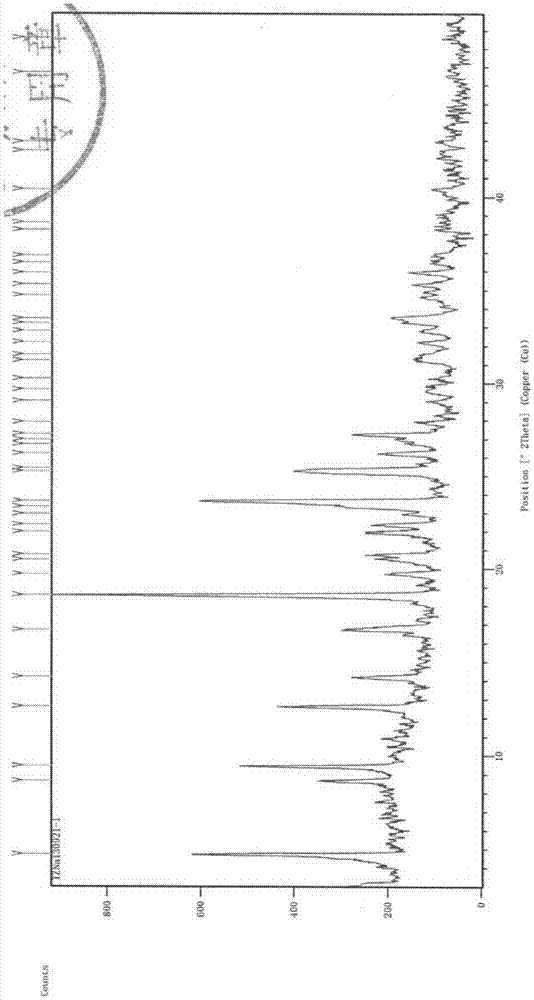
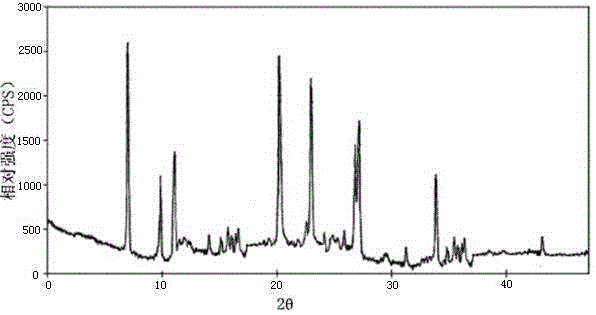
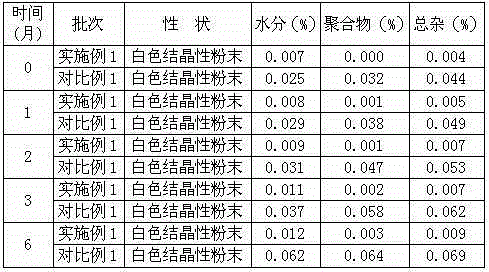
The invention belongs to the technical field of medicine, and particularly relates to a ceftezole sodium compound and a medicinal preparation including the same. The ceftezole sodium compound is ceftezole sodium trihydrate, and the structural formula is shown in the specification. An X-ray powder diffraction pattern, obtained through Cu-K alpha ray measurement, of the ceftezole sodium compound is shown as picture 1. A sterile powder-injection is adopted as the preparation including the ceftezole sodium compound. The content of polymer in the ceftezole sodium compound is low, and is almost not changed during an acceleration test and a long-term test. After a ceftezole sodium sterile power needle manufactured through the ceftezole sodium compound is combined with the five-percent glucose injection, the relative content of ceftezole sodium is hardly changed, and compatible stability is good.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Preparation method of ceftezole sodium lyophilized powder for injection
InactiveCN104666252AImprove stabilityHigh clinical safetyAntibacterial agentsPowder deliveryCeftezole SodiumVitamin
The invention discloses a preparation method of ceftezole sodium lyophilized powder for injection, and relates to a ceftezole sodium injection. The ceftezole sodium lyophilized powder is prepared from the following raw materials: 42 g of ceftezole sodium, 4.3 g of citric acid, 8.5 g of vitamin B1, 48 g of polysorbate 40, and 200 ml of water for injection; in the preparation method, the pH value is adjusted to be 6.4-6.5.
Owner:成都创屹远景科技有限公司
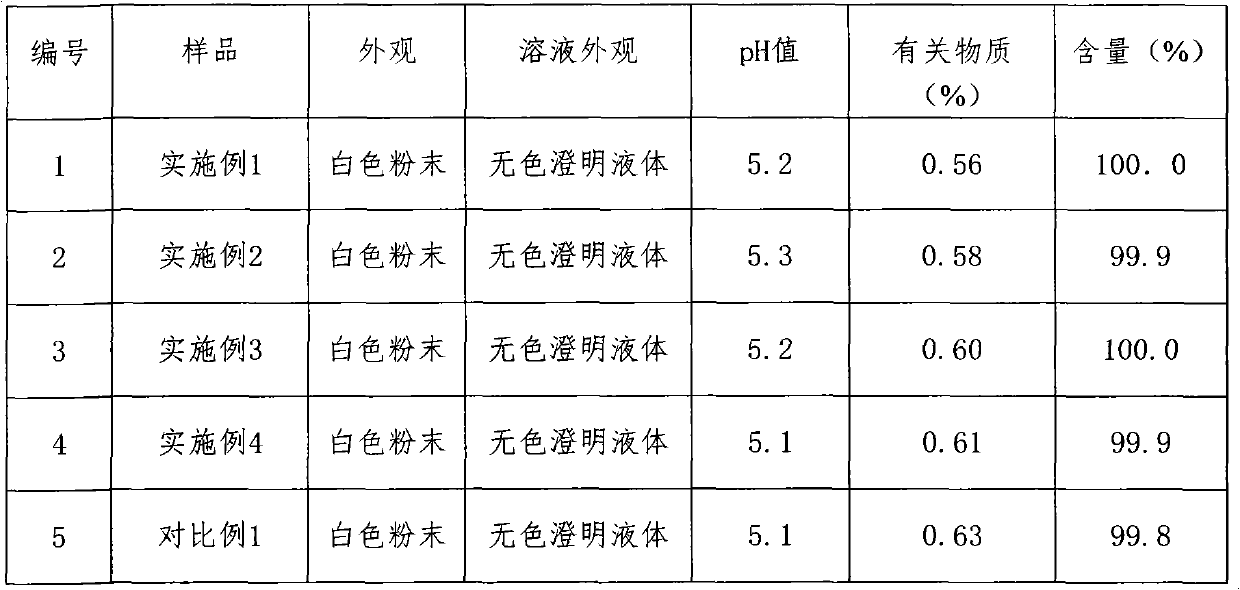
Method for controlling related substances in ceftezole sodium
InactiveCN104224793ASimple and easy quality control methodImprove product qualityOrganic active ingredientsCeftezole SodiumChemistry
The invention provides a process for preparing ceftezole sodium for injection and a method for controlling related substances in the process of preparing ceftezole sodium for injection. The method for controlling the quality of ceftezole sodium for injection is simple and feasible, is capable of guaranteeing stable product quality, enables the validity to be 24 months, and is suitable for large-scale industrial production.
Owner:SICHUAN PHARMA
Method for preparing ceftezole sodium for injection
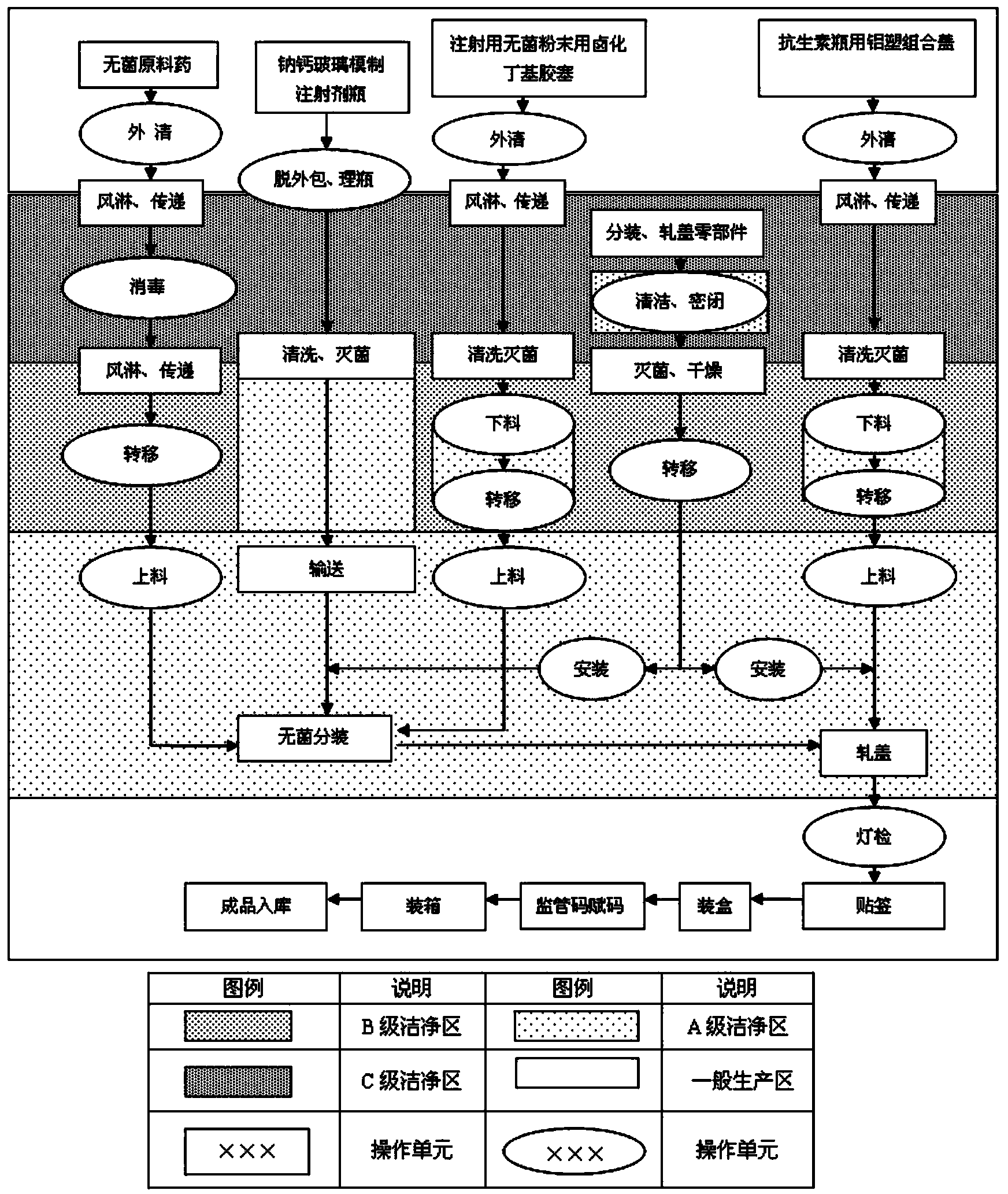
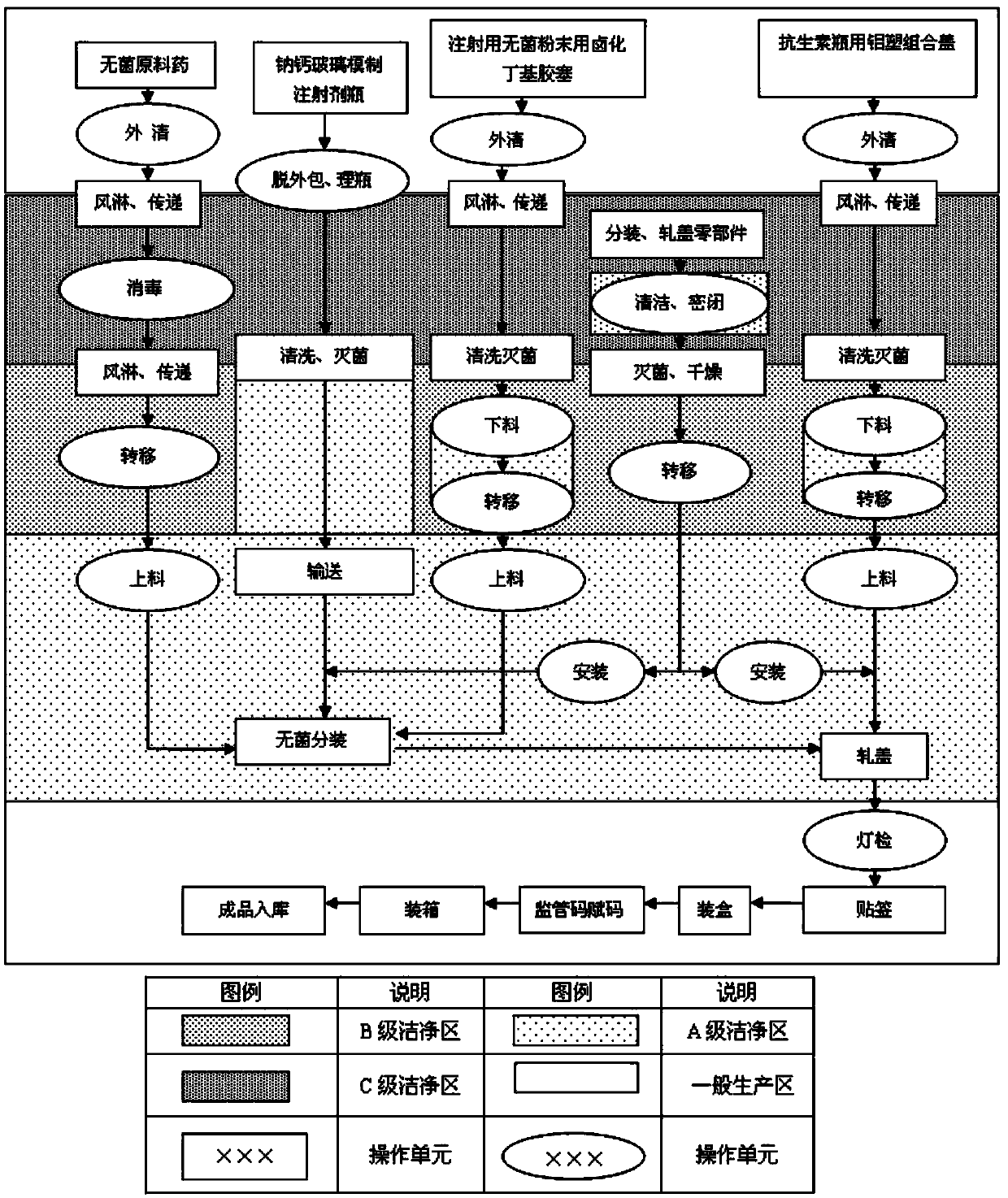
InactiveCN104188915ASimple production processImprove product qualityAntibacterial agentsPowder deliveryCeftezole SodiumBottle
The invention provides a ceftezole sodium preparation for injection. Ceftezole sodium asepsis powder is put in an injection bottle molded by soda-lime glass, a halogenated butyl rubber stopper is used for packaging, and an aluminum plastic combined cap for an antibiotic bottle is rolled at the opening of the bottle. The invention further provides a method for preparing the ceftezole sodium preparation for injection. The ceftezole sodium preparation is simple in production process, stable in product quality and convenient to store, has validity up to 24 months, and is suitable for large-scale industrial production.
Owner:SICHUAN PHARMA
Preparation method of ceftezole acid and sodium salt thereof
InactiveCN112174984AImprove final yieldGuaranteed final yieldOrganic chemistrySodium bicarbonateCeftezole Sodium
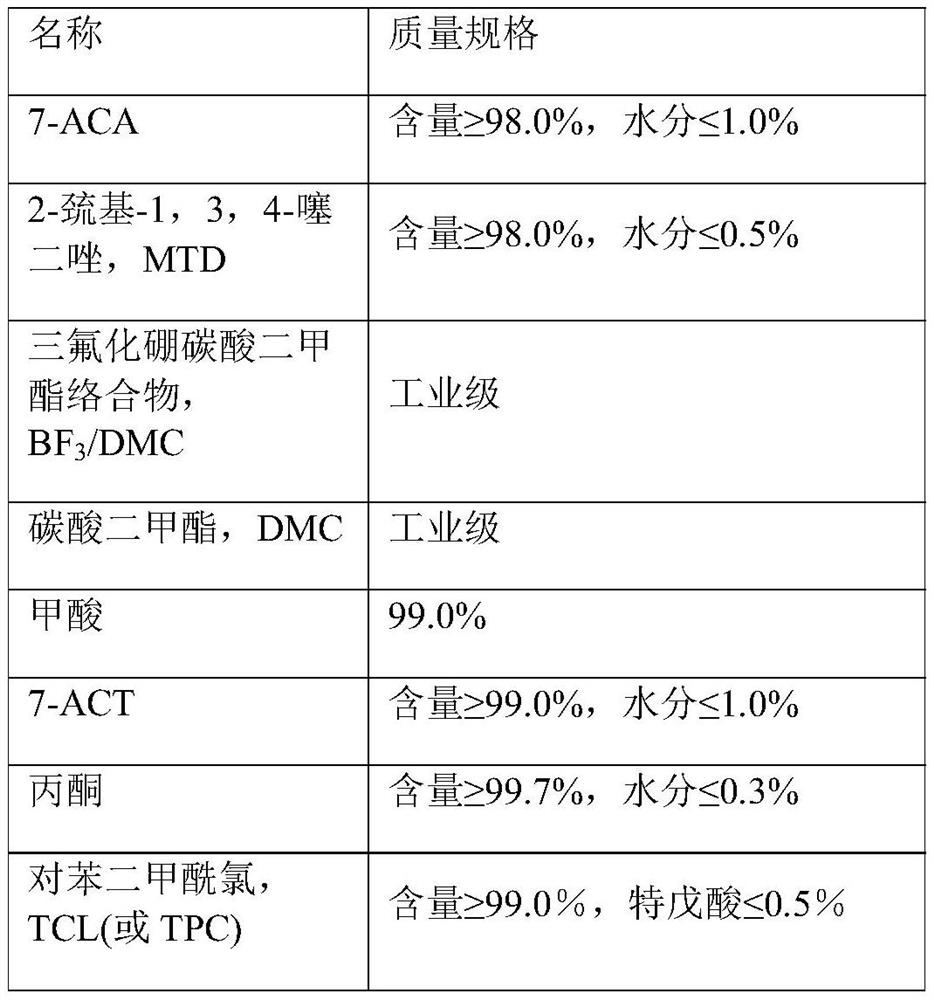
The invention relates to the technical field of medicines, and particularly discloses a preparation method of ceftezole acid and sodium salt thereof. The preparation method comprises the following steps: adding MTD and 7-ACA into a dimethyl carbonate, boron trifluoride dimethyl carbonate complex and formic acid system, and reacting at 18-22 DEG C to obtain an intermediate 7-ACT; and reacting the 7-ACT with a mixed anhydride solution prepared from TAA and TCL at 11-14 DEG C, and carrying out decolorizing, crystallizing, centrifuging and drying operations to obtain the ceftezole acid. The methodcomprises the following steps: adding ceftezole acid into water, regulating the pH value to 4.9-5.5 by using sodium bicarbonate, primarily growing crystals in a methanol-ethanol mixed solvent, and supplementing ethanol to further grow crystals, thereby obtaining ceftezole sodium. The preparation method provided by the invention has the advantages of simple process, mild reaction conditions and small pollution, the cost can be reduced, the yield of the ceftezole acid can be effectively improved, the yield of the ceftezole sodium prepared from the ceftezole acid is more than or equal to 98 percent, and the purity of the obtained ceftezole sodium is more than 99.8 percent.
Owner:湖北凌晟药业股份有限公司
Preparation process of ceftezole sodium for injection sterilize powder-injection for injection
InactiveCN107095851AImprove injection safetyImprove product qualityAntibacterial agentsPowder deliveryCeftezole SodiumBiochemical engineering
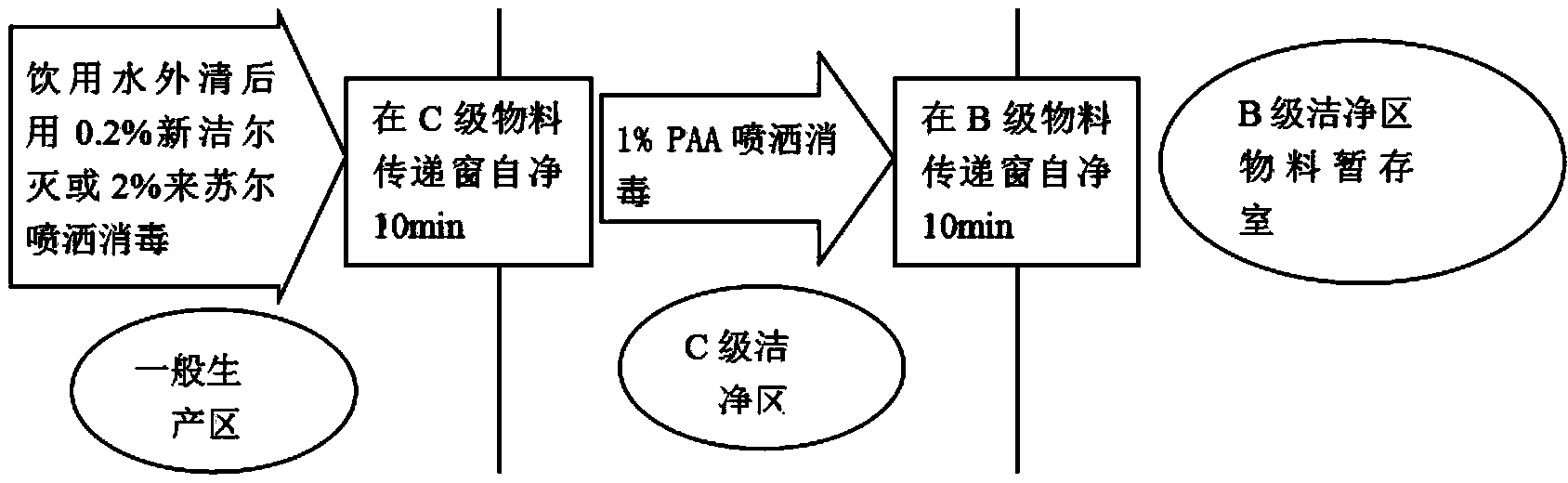
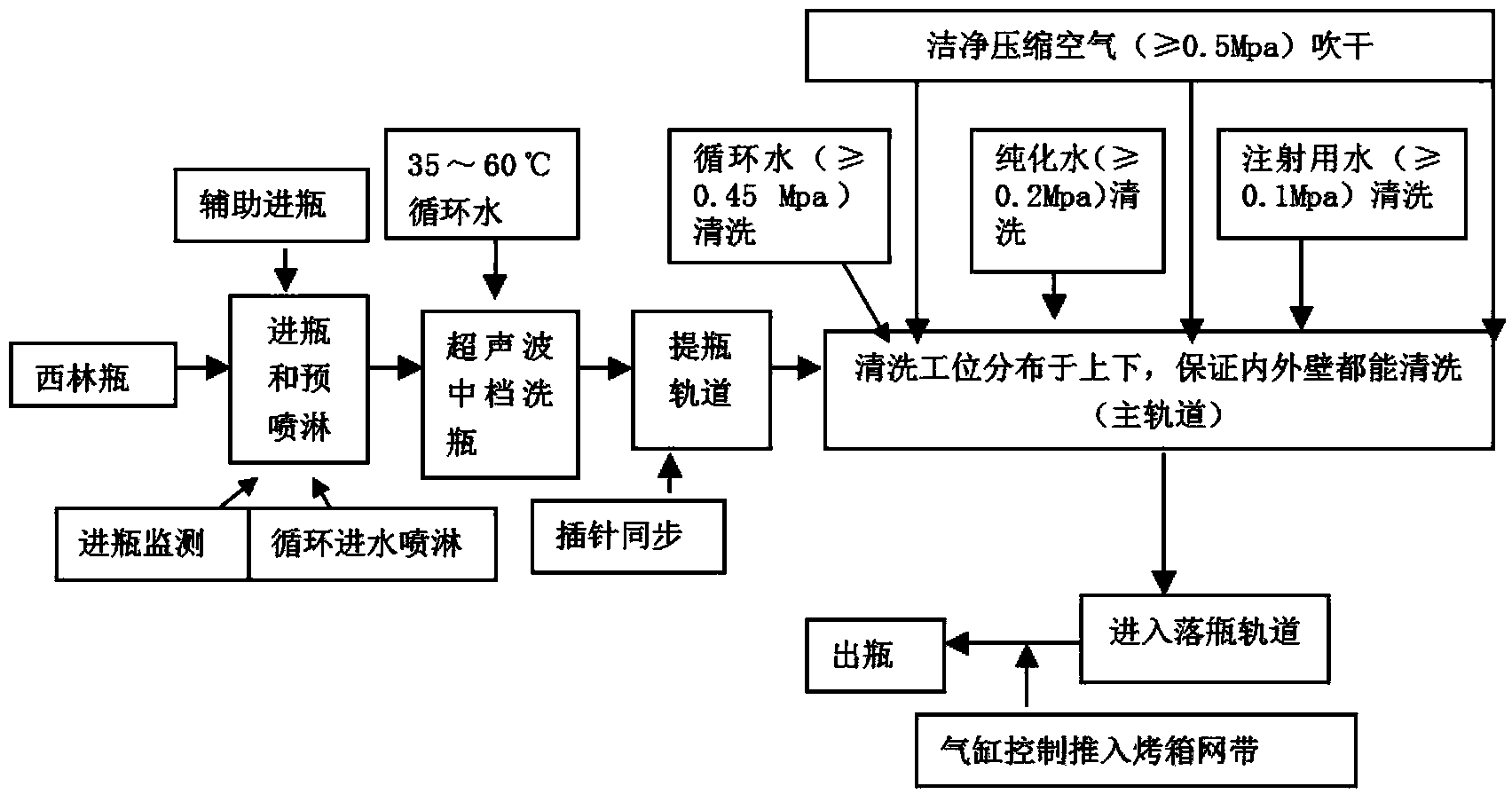
The invention discloses a preparation process of a ceftezole sodium for injection sterilize powder-injection for injection. The preparation process successively comprises the following steps of: a raw material preparation step, a soda-lime glass moulded injection bottle cleaning step, a rubber plug cleanings step, an aluminum cover cleaning step and a sterile packaging step. By reasonably designing the step processes and reasonably controlling the parameters in the processes, the parameters which are tested meet the demand, and the quality of a product is further up-regulated, and the injection safety of the ceftezole sodium for injection is enhanced.
Owner:SICHUAN PHARMA
Medicine ceftezole sodium composition for curing infectious diseases
InactiveCN105055423AHigh purityEasy to prepareAntibacterial agentsOrganic active ingredientsCeftezole SodiumInfective disorder
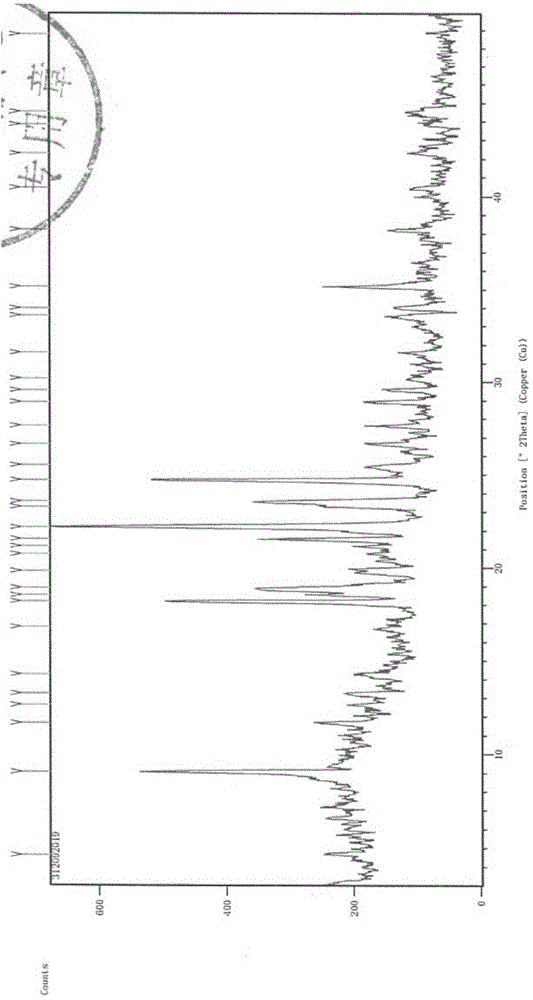
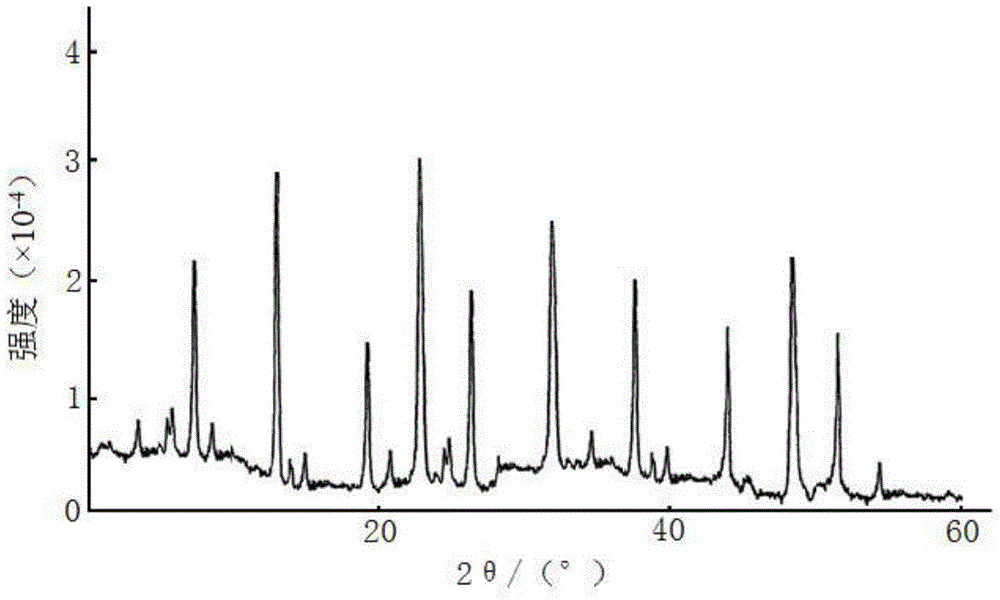
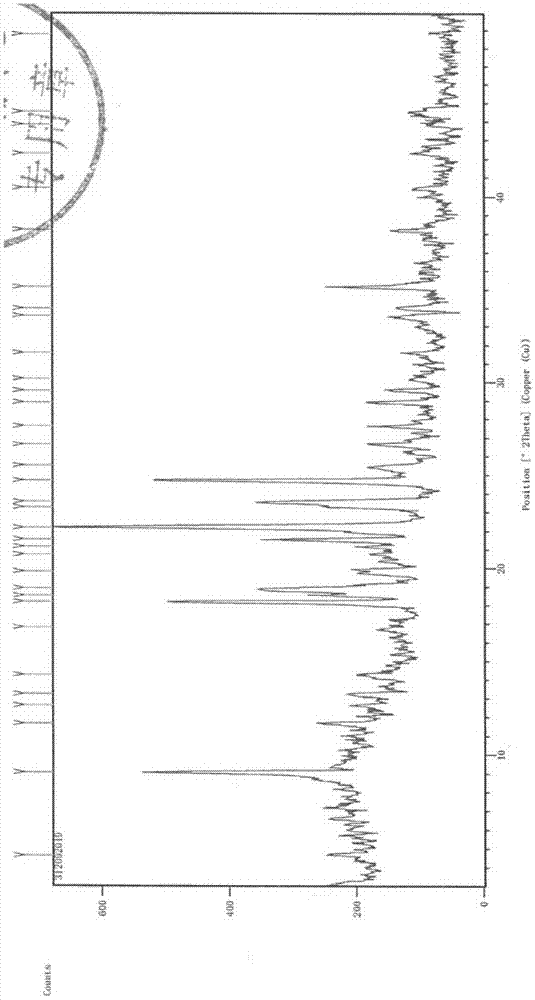
The invention discloses a medicine ceftezole sodium composition for curing infectious diseases, belonging to the technical field of medicines. The composition is prepared from ceftezole sodium and anhydrous sodium carbonate; the ceftezole sodium is a crystal, and an X-ray powder diffraction pattern obtained by Cu-Kalpha ray measurement is shown as picture 1. The new crystal form of the ceftezole sodium provided by the invention is different from crystal form structures in the prior art, by means of experimental verification, the new crystal form compound is surprisedly found to be high in purity, good in mobility and stability, low in polymer content, free of hygroscopicity and safe and reliable in clinic application, and a powder-injection prepared by using the new crystal form compound is good in stability, good in stability after being matched with a solvent, extremely low in insoluble particle content and very suitable for clinic application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
A kind of preparation technology of ceftezole sodium
ActiveCN104262361BLarge batchSimple and fast operationOrganic chemistryCeftezole SodiumActivated carbon
The invention relates to a process for preparing ceftezole sodium. The process comprises the following steps: (1) adding N,N-dimethylacetamide or N,N-dimethylformamide and a first part of acetone, adding ceftezole while stirring, and adding purified water or cooled water for injection until ceftezole is completely dissolved; (2) adding medicinal activated carbon, filtering and washing the activated carbon to obtain a reaction solution A; (3) adding a third part of acetone and adding a sodium-forming agent until the solid is completely dissolved; (4) adding medicinal activated carbon, filtering and washing the activated carbon with a fourth part of acetone to obtain a reaction solution B; (5) cooling the reaction solution B to 8-12 DEG C, dropwise adding the reaction solution A into the reaction solution B for carrying out salt formation and crystallization; (6) after the dropwise addition of the reaction solution A is completed, continuously carrying out heat preservation at 8-12 DEG C and stirring for 60-90 minutes; and (7) washing filter cakes with sterilized and filtered acetone and drying at 40-50 DEG C to obtain the sterile ceftezole sodium solid. The process has the advantages of larger batches, simplicity in operation, high yield, good quality and low processing cost, the solvent is simply recovered and the process is suitable for industrial production.
Owner:ZHEJIANG DONGYING PHARMA
Method for controlling quality of Ceftezole sodium used for injection
InactiveCN101797231AControl moisture contentHigh clarityAntibacterial agentsPowder deliveryCeftezole SodiumCLARITY
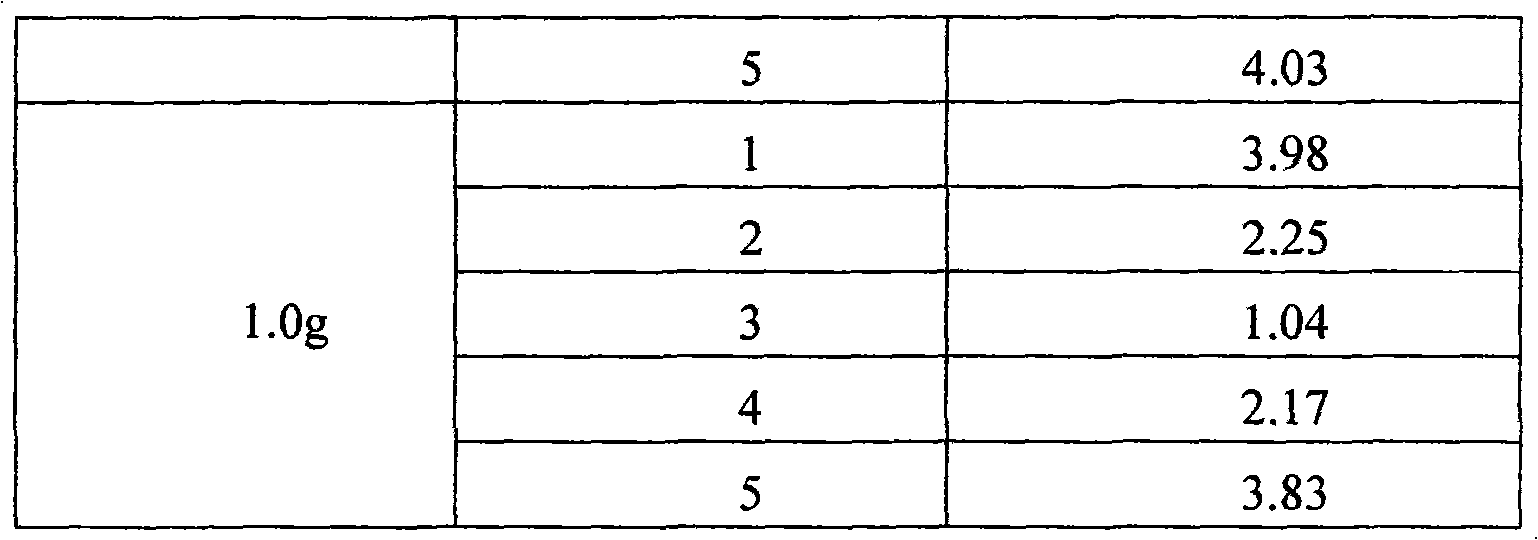
The invention discloses a method for controlling the quality of Ceftezole sodium used for injection, comprising the steps as follows: (1) controlling the moisture content of a Ceftezole sodium raw material to be 2-4%; and (2) controlling the humidity of the encapsulation environment to be 40-45%. By controlling the raw material moisture and the humidity of the encapsulation environment in the invention, the moisture content of a drug preparation can be controlled better so as to obtain a preparation with the characteristics of good clarity, favorable powder mobility and more accurate charging amount, thus being more applicable to clinic use.
Owner:天津新丰制药有限公司
Sterilization medicine ceftezole sodium compound and preparation method thereof
InactiveCN105061473AImprove stabilitySuitable for clinical applicationAntibacterial agentsOrganic chemistry methodsCeftezole SodiumPharmaceutical drug
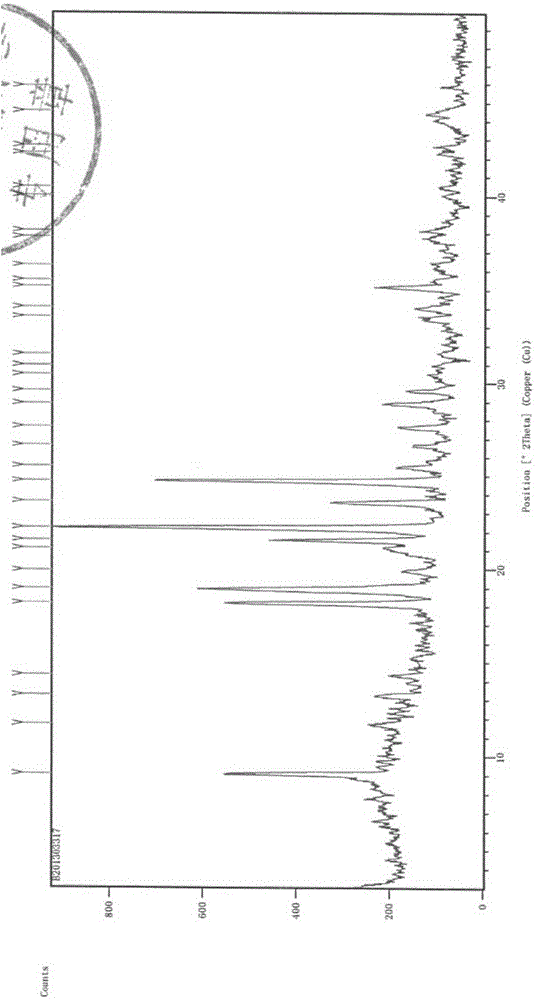
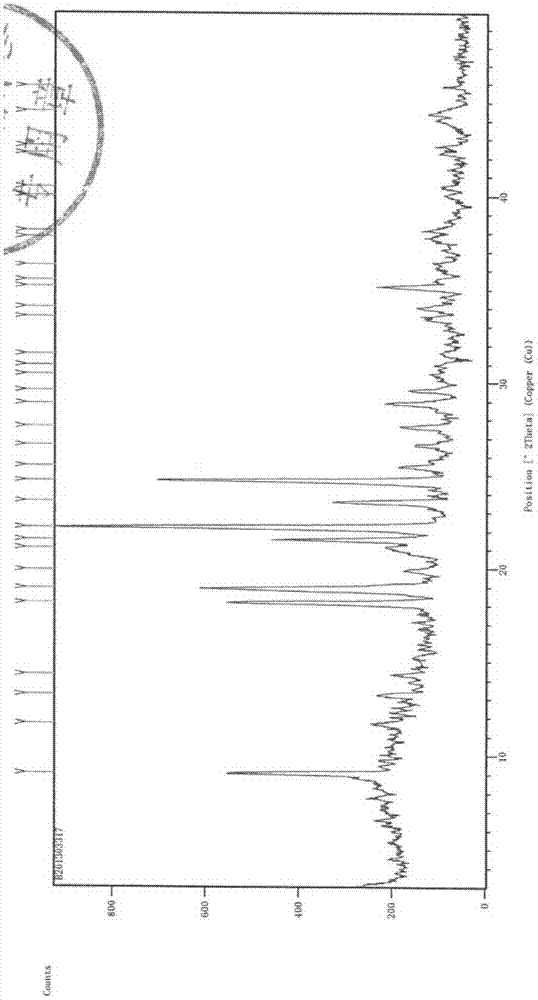
The invention relates to a sterilization medicine ceftezole sodium compound and a preparation method thereof, and belongs to the technical field of medicine. An X-ray powder diffraction pattern, obtained by measuring by using a Cu-K alpha ray, of the ceftezole sodium compound is shown in the Figure I of the description. Through a research of the hygroscopicity of the ceftezole sodium compound, the inventor surprisingly discovers that the hygroscopicity of the compound provided by the invention is greatly lower than that of an existing crystalline compound, and that the purity is high, the liquidity is good, and the content of a polymer is low, thus, the ceftezole sodium crystalline compound provided by the invention is more stable, and is more suitable for the preparation of preparations, and clinical application is safe and reliable.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
A kind of ceftezole sodium compound and the pharmaceutical preparation containing the compound
ActiveCN104961751BLow polymer contentReduce drug riskAntibacterial agentsPowder deliveryCeftezole SodiumSterile water
The invention belongs to the technical field of medicine, and particularly relates to a ceftezole sodium compound and a medicinal preparation including the same. The ceftezole sodium compound is ceftezole sodium trihydrate, and the structural formula is shown in the specification. An X-ray powder diffraction pattern, obtained through Cu-K alpha ray measurement, of the ceftezole sodium compound is shown as picture 1. A sterile powder-injection is adopted as the preparation including the ceftezole sodium compound. The content of polymer in the ceftezole sodium compound is low, and is almost not changed during an acceleration test and a long-term test. After a ceftezole sodium sterile power needle manufactured through the ceftezole sodium compound is combined with the five-percent glucose injection, the relative content of ceftezole sodium is hardly changed, and compatible stability is good.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Preparations containing ceftezole sodium
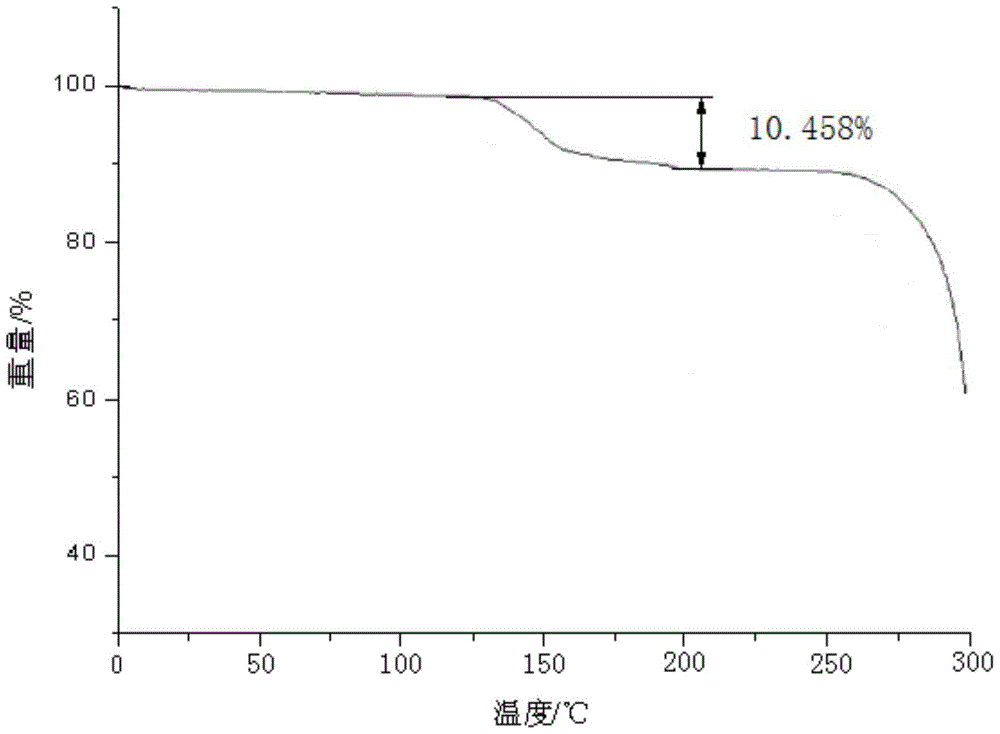
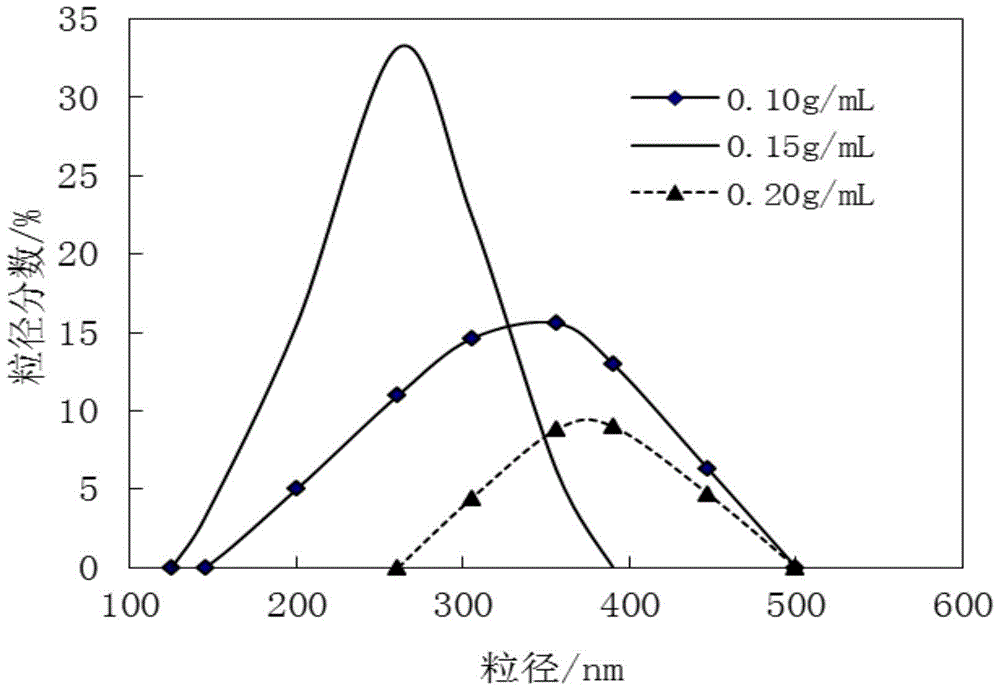
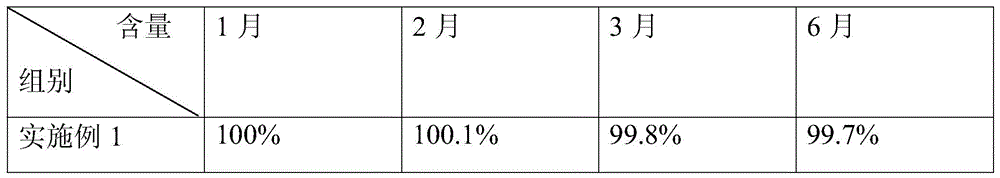
The invention relates to ceftezole sodium spray. The ceftezole sodium spray is prepared from the following components in parts by weight: 5 parts of ceftezole sodium, 450 parts of ethanol, 408 parts of polysorbate, 5 parts of laurinol, and 50 parts of F125.
Owner:XINXIANG MEDICAL UNIV
A kind of preparation method of I-type ceftezole sodium crystal
The invention discloses a type I ceftezole sodium crystal and a preparation method thereof. The preparation method is characterized by comprising the following steps of mixing sodium salt solution with ceftezole acid to obtain an aqueous solution of ceftezole sodium, decolorizing with activated carbon and filtering; dropping the aqueous solution of ceftezole sodium in mixed solution of organic solvent I and water by stirring; when needle-like precipitates are found, reducing the stirring rotating speed and growing the crystal at room temperature; continuously dropping the organic solvent I and organic solvent II, growing the crystal at low temperature and raising the stirring rotating speed; filtering, flushing the crystal with the mixed solution of the organic solvent I and the water and performing vacuum drying to obtain the type I ceftezole sodium crystal. According to the preparation method for the type I ceftezole sodium crystal, provided by the invention, the single type I ceftezole sodium crystal with the advantages of high purity, high thermal stability, homogeneous particles and good inter-batch repeatability can be obtained, and important significance for improving the stability and the safety of the ceftezole sodium for injection is realized; in addition, the preparation method has the advantages of simple operation, easiness in control for conditions and suitability for batch production.
Owner:SHENYANG SANJIU PHARMA +1
Ceftezole sodium compound and novel method thereof
The invention relates to a ceftezole sodium compound and a novel method thereof. The method provided by the invention achieves the refining aim by acidification reaction, activated carbon adsorption, electroosmosis device processing and preparation of chromatographic columns, so as to finally obtain a high-purity ceftezole sodium compound, overcomes the defects of low purity of the existing raw materials, improves the product quality of the preparation, reduces the toxic and side effects, and ensures the clinical safety of the medicine at the same time.
Owner:HAINAN LINGKANG PHARMA CO LTD
Anti-infective drug ceftezole sodium composition
InactiveCN105106219AImprove stabilityLow insoluble particulate contentAntibacterial agentsPowder deliveryCeftezole SodiumPharmaceutical drug
The invention discloses an anti-infective drug ceftezole sodium composition, which belongs to the field of medical technology, and comprises ceftezole sodium and sodium chloride, wherein the ceftezole sodium is crystals, and an X-ray powder diffraction diagram which is obtained by using Cu-Ka rays to measure is shown in a picture 1. A novel crystal of the ceftezole sodium is different form an existing crystal structure, the anti-infective drug ceftezole sodium composition is high in purity through experimental verification, is excellent in mobility, excellent in stability, low in polymer content, not easy to wet and reliable in clinical application. Power injections which are prepared through the anti-infective drug ceftezole sodium composition is excellent in stability, excellent in stability after being combined with solvent, extremely low in content of insoluble particles, and is extremely suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com