Sterilization medicine ceftezole sodium compound and preparation method thereof
A technology of ceftezole sodium and its compound, which is applied in the field of bactericidal drug ceftezole sodium compound and its preparation, can solve the problems of unfavorable stability, great harm to patients, unstable β-lactam ring, etc., and is suitable for clinical application , The preparation method is simple, the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
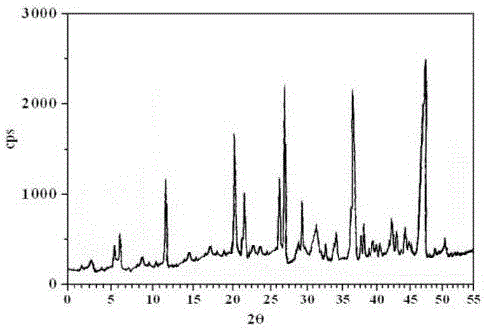
[0022] Example 1: Preparation of Ceftezole Sodium Crystalline Compound
[0023] (1) Add the crude product of ceftezole sodium into the mixed solution of water and acetonitrile whose volume is 5 times the weight of ceftezole sodium, the volume ratio of water and acetonitrile is 2:1, heat up to 25°C, stir until completely dissolved ;
[0024] (2) In a sound field with a frequency of 30KHz and an output power of 45W, add a mixed solution of ethanol, chloroform, and cyclohexane whose volume is 10 times the weight of ceftezole sodium while stirring, ethanol, chloroform, and cyclohexane The volume ratio of hexane is 2:1:1, the stirring speed is 85 rev / min, and the adding speed is 80 ml / min;
[0025] (3) After adding the mixed solution of ethanol, chloroform and cyclohexane, under a sound field with a frequency of 25KHz and an output power of 40W, cool down to -5°C at 10°C / hour, grow crystals for 3 hours, and wash. Vacuum-dried to obtain ceftezole sodium compound.
[0026] The X...
experiment example 1
[0027] Experimental Example 1: Accelerated Test
[0028] Take the ceftezole sodium crystalline compound prepared in Example 1 and the commercially available ceftezole sodium raw material, simulate the marketing package, put them in a sealed clean container, and place 6 ceftezole sodium under the conditions of 25°C ± 2°C and humidity 60% ± 5%. During the test period, samples were taken at the end of 1, 2, 3, and 6 months to inspect the key stability inspection items. The results are shown in Table 1.
[0029] Table 1 Acceleration experiment results
[0030]
experiment example 2
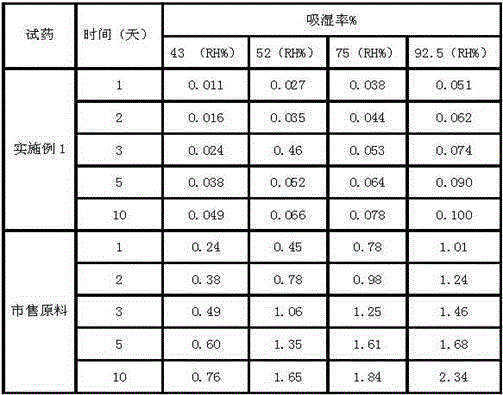
[0031] Experimental Example 2: Hygroscopicity Test
[0032] 1 instrument
[0033] PL203 electronic balance, LRH-250-S constant temperature and humidity incubator, HH-400SD drug stability test box;
[0034] 2 methods
[0035] Take a glass desiccator with a supersaturated solution of salt at the bottom (in order to ensure the saturation of the salt solution, there should be excess salt at the bottom of the desiccator), with a built-in weighing bottle in the desiccator, and place it in a constant temperature box for 48 hours to constant humidity. Take about 2g of the sample, put it in a weighing bottle, weigh it accurately, open the bottle cap, put it into the upper part of the desiccator, store it in a constant temperature and humidity incubator at 25°C or a stability test box at 20°C according to different temperature requirements, and operate in parallel 3 parts were weighed at different times to calculate the moisture absorption rate at different times.
[0036] Calculat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com