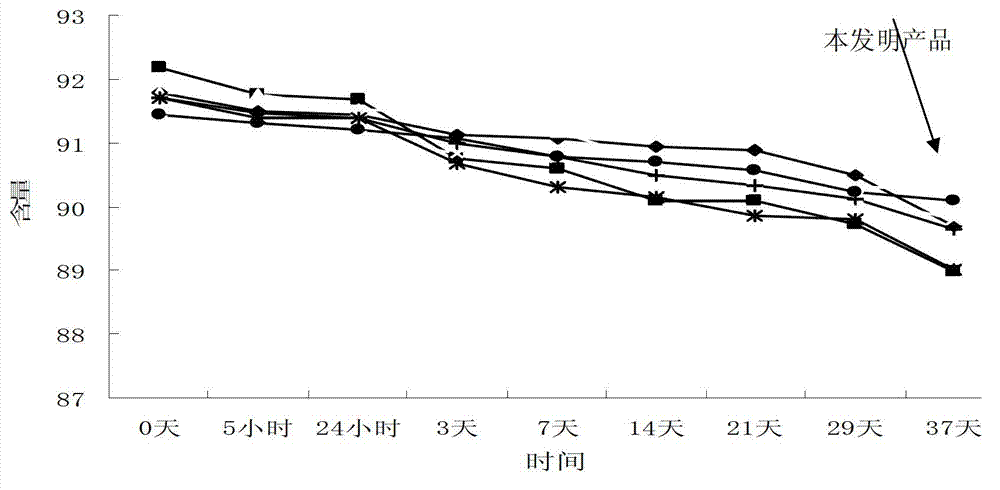
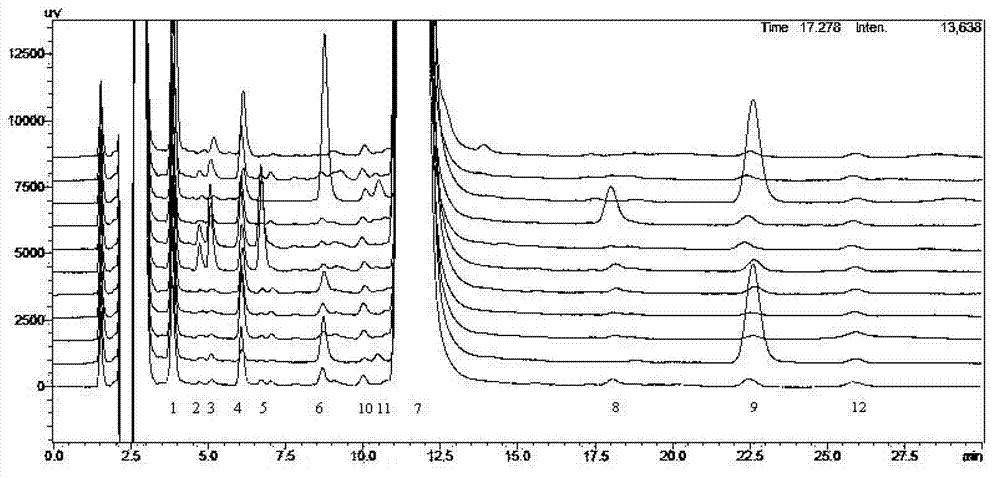
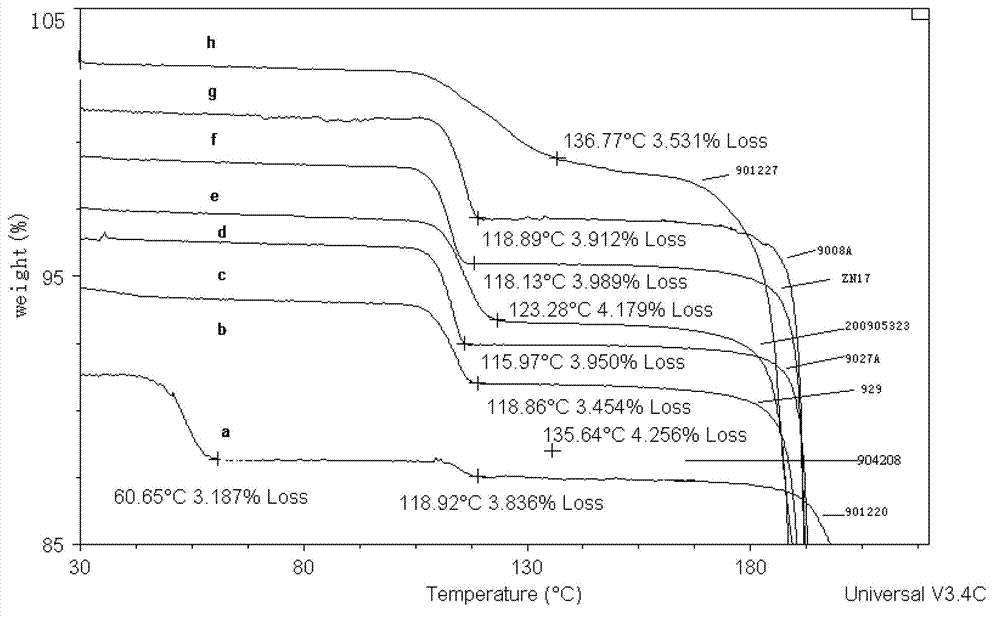
Crystallization method of ceftezole sodium
A technology of ceftizole sodium and ceftizole acid, applied in the field of medicine, can solve problems such as affecting the quality of finished products, high difficulty, small product particle size, etc., so as to reduce impurities and polymer content, improve stability and safety, and improve crystal The effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A crystallization method of Ceftezole Sodium, the steps are as follows:
[0045] ⑴ Dosing: Aseptically treat the reaction kettle, filter, and utensils used. Add 1950g (4.63mol) of ceftezole acid, 390g (4.63mol) of sodium bicarbonate and 20L of purified water into a 50L reactor, stir at room temperature to completely dissolve, flush into nitrogen protection, and adjust to 7.5 according to the actual pH value;
[0046] (2) Decolorization: Add 60g of activated carbon to the above solution, stir and absorb for 30 minutes, then decarbonize through titanium rod filtration, and obtain the filtrate through two-stage filtration with 0.22μm microporous membrane;
[0047] (3) Impurity removal: add 19.5 g each of sodium bisulfite and EDTA to the filtrate obtained in step (2);
[0048] (4) ultrafiltration: adopt step (3) gained solution to adopt the ultrafiltration membrane ultrafiltration that cut-off is 1000;
[0049] (5) Crystallization: Transfer the filtrate to a 50L crystalli...
Embodiment 2
[0053] A crystallization method of Ceftezole Sodium, the steps are as follows:
[0054] (1) Dosing: carry out aseptic treatment to the reaction kettle, filter and utensils used; add 2000g (4.54mol) ceftezole acid, 382g (4.54mol) sodium bicarbonate and 20L purified water into the 50L reaction kettle, and stir at room temperature Make it completely dissolved, rush into nitrogen protection, and adjust to 7.5 according to the actual pH value;
[0055] (2) Decolorization: Then add 62.4g of activated carbon, stir and absorb for 30 minutes, decarbonize by titanium rod filtration, and filter in two stages with 0.22μm microporous membrane;
[0056] (3) Impurity removal: add 20 g each of sodium bisulfite and EDTA to the filtrate obtained in step (2);
[0057] (4) ultrafiltration: step (3) gained solution is adopted to cut off the ultrafiltration membrane ultrafiltration of 1000;
[0058] (5) Crystallization: transfer the filtrate to a 50L crystallization tank in a local 10000-class cl...
Embodiment 3
[0060] A crystallization method of Ceftezole Sodium, the steps are as follows:
[0061] (1) Dosing: Aseptically treat the reaction kettle, filter and utensils used, add 2120g (4.81mol) ceftezole acid, 405g (4.81mol) sodium bicarbonate and 21L purified water into the 50L reaction kettle, and stir at room temperature Make it completely dissolved, rush into nitrogen protection, and adjust to 7.5 according to the actual pH value;
[0062] ⑵ Decolorization: Then add 62g of activated carbon, stir and absorb for 30 minutes. Titanium rod filtration decarbonization, 0.22μm microporous membrane two-stage filtration;
[0063] (3) Impurity removal: Add 21.2 g of sodium bisulfite and EDTA to the filtrate obtained in step (2);
[0064] (4) ultrafiltration: step (3) gained solution is adopted to cut off the ultrafiltration membrane ultrafiltration of 1000;
[0065] (5) Crystallization: transfer the filtrate to a 50L crystallization tank in a local 10000-class clean area, add 12L of asepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com