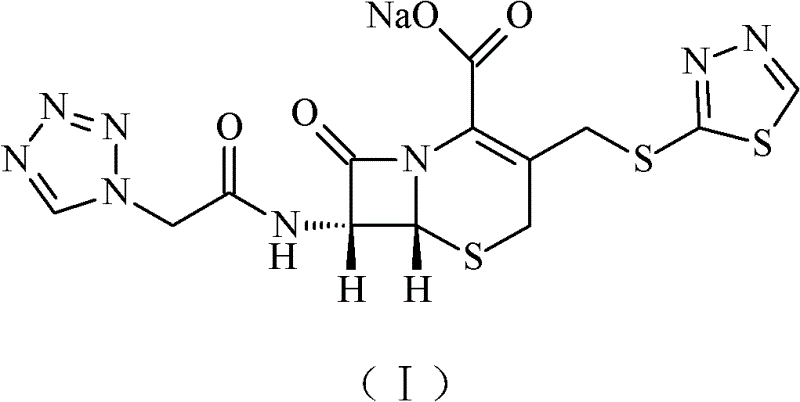
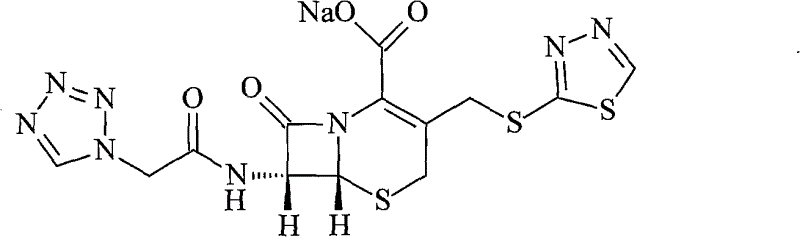
Ceftezole sodium compound and novel method thereof
A technology of ceftezole sodium and ceftezole, which is applied in the medical field, can solve the problems of affecting the curative effect of clinical medication and the purity of ceftezole sodium is not very high, and achieve the effects of low cost, reduced toxic and side effects, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The purification of embodiment 1 ceftizole sodium
[0028] (1) 100g of ceftizole sodium crude product was dissolved in 1000ml of water, then slowly adding 0.1mol / L hydrochloric acid, stirring and reacting until the pH of the solution was 1.5, i.e., ceftizole precipitation was produced, and suction filtration obtained ceftizole 83.9g;
[0029] (2) 83.9g of ceftiazole obtained in the previous step was dissolved in 400g of methanol, the activated carbon of the total solution volume 4.0g was added, the temperature was kept at 60°C and stirred for 30min, and the filtrate was collected by filtration and decarburization;
[0030] (3) The filtrate obtained in step (2) is treated with an anion / cation exchange membrane electrodialysis device: the anion exchange membrane uses the AHA anion exchange membrane provided by the Japanese Tokuyama Caoda company, and the cation exchange membrane is provided by the Japan Tokuyama Caoda company The CMB cation-exchange membrane, the solutio...
Embodiment 2
[0035] The purification of embodiment 2 ceftizole sodium
[0036] (1) 100g of ceftizole sodium crude product was dissolved in 1000ml of water, then slowly adding 0.5mol / L phosphoric acid, stirring and reacting until the pH of the solution was 1.8, i.e., ceftazole precipitation was produced, and suction filtration obtained ceftizole 82.1g;
[0037] (2) the 82.1g ceftiazole obtained in the previous step was dissolved in 350g methylene chloride, the gac of 3.5g was added, and the temperature was kept at 60°C and stirred for 20min, filtered and decarburized, and the filtrate was collected;
[0038] (3) The filtrate obtained in step (2) was treated with an anion / cation exchange membrane electrodialysis device: NEOSEPTA ACS anion exchange membrane produced by ASTOM Co., Ltd. was used for the anion exchange membrane, and NEOSEPTA CMX cation exchange membrane produced by ASTOM Co., Ltd. was used for the cation exchange membrane. Membrane, feeding the solution obtained in the step (2)...
Embodiment 3
[0044] Embodiment 3 Refining of ceftizole sodium
[0045] (1) 100g of ceftizole sodium crude product was dissolved in 1000ml of water, then slowly adding 0.5mol / L phosphoric acid, stirring and reacting until the pH of the solution was 2.5, i.e., ceftazole precipitation was produced, and suction filtration obtained ceftizole 80.9g;
[0046] (2) 80.9g of ceftiazole obtained in the previous step was dissolved in 300g of isopropanol, the activated carbon of 3.0g was added, the temperature was kept at 60°C and stirred for 30min, and the filtrate was collected by filtration and decarburization;
[0047] (3) The filtrate obtained in step (2) is treated with an anion / cation exchange membrane electrodialysis device: the anion exchange membrane uses the AHA anion exchange membrane provided by the Japanese Tokuyama Caoda company, and the cation exchange membrane is provided by the Japan Tokuyama Caoda company The CMB cation-exchange membrane, the solution obtained in step (2) is fed int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com