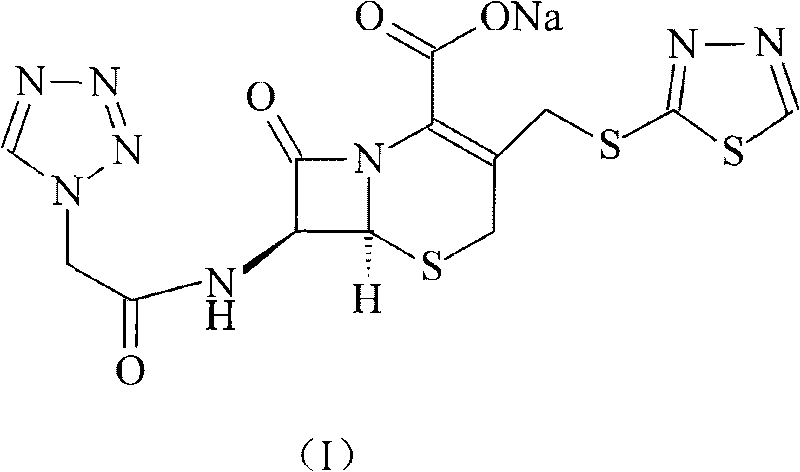
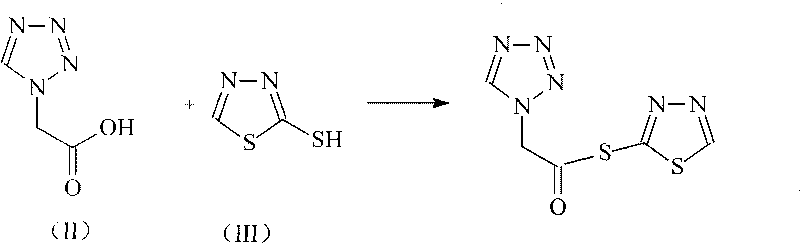
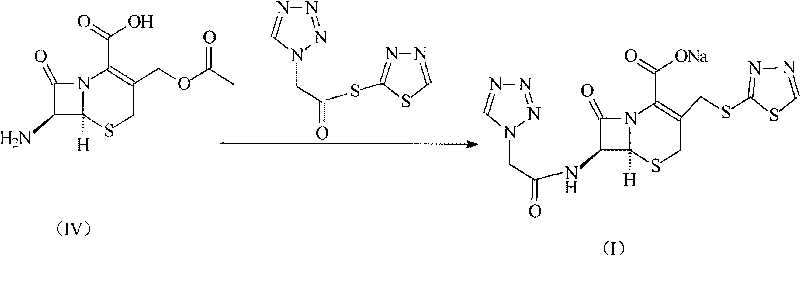
Ceftezole sodium compound with novel route
A technology of ceftezole sodium and compounds, applied in the direction of organic chemistry, etc., can solve the problems of low overall yield, unfavorable industrial production, lengthy synthesis steps, etc., and achieve the effects of improving yield and purity, simplifying operation steps, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Synthesis of 2-mercapto-1,3,4-thiadiazole ester of tetrazoleacetic acid
[0026] Add 275ml of isopropyl chloroformate to 1 liter of tetrahydrofuran solution containing 256 grams (2mol) of tetrazoleacetic acid, cool to 10°C, add 243ml of N-methylpiperidine, keep the reaction temperature not exceeding 15°C, Stir, add 236 grams of 2-mercapto-1,3,4-thiadiazole in 700ml of tetrahydrofuran solution, react at 15°C for 2 hours, then concentrate the reaction solution to a viscous state, add 2 liters of petroleum ether Vigorously stirred for 2 hours, a solid was formed. After continuing to stir for 2 hours, it was filtered to obtain 433 g of a yellow solid product, with a yield of 95%.
Embodiment 2
[0027] Embodiment 2: the synthesis of ceftezole sodium
[0028] Under the protection of nitrogen, 272 grams of 7-ACA and 84 grams of sodium bicarbonate were added to 1 liter of water to form a clear solution, and then 228 grams of tetrazoleacetic acid 2-mercapto-1,3,4-thiadiazole ester was added and 2 liters of acetone, then the mixture was reacted at 60°C for 4 hours, while adjusting the pH to 7.5 with 3% aqueous sodium bicarbonate solution, after the reaction, add 4L isopropanol, control the reaction temperature to 15°C, and stir After 2 hours, crystals were precipitated, filtered, the filter cake was washed with 500 ml of ethanol, and vacuum-dried at 40° C. to obtain 430 g of ceftezole sodium product, with a yield of 93%.
[0029] Elemental Analysis Molecular Formula: C 13 h 11 N 8 NaO 4 S 3
[0030] Theoretical value C: 33.76%, H: 2.39%, N: 24.23%, O: 13.83%, S: 20.79%,
[0031] Experimental values C: 33.82%, H: 2.42%, N: 24.32%, O: 13.85%, S: 20.87%.
[0032] 1...
Embodiment 3 4
[0033] Synthesis of embodiment 3 tetrazolium acetic acid 2-mercapto-1,3,4-thiadiazole ester
[0034] Add 275ml of isopropyl chloroformate to 1 liter of tetrahydrofuran solution containing 256 grams (2mol) of tetrazoleacetic acid, cool to 15°C, add 245ml of N-methylpiperidine, keep the reaction temperature not exceeding 15°C, Stir, add 236 grams of 2-mercapto-1,3,4-thiadiazole in 700ml of tetrahydrofuran solution, react at 15°C for 2.5 hours, then concentrate the reaction solution to a viscous state, add 2 liters of petroleum ether Vigorously stirred for 2.5 hours, a solid was formed. After continuing to stir for 2 hours, it was filtered to obtain 427.5 g of a yellow solid product, with a yield of 93.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com