Medicinal composition containing ceftezole sodium compound and preparation method for medicinal composition
A technology of ceftazole sodium and composition, which is applied in the field of pharmaceutical compositions containing ceftazole sodium compounds and their preparation, and can solve the problems of pH value change, hidden safety hazards, temperature and light instability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
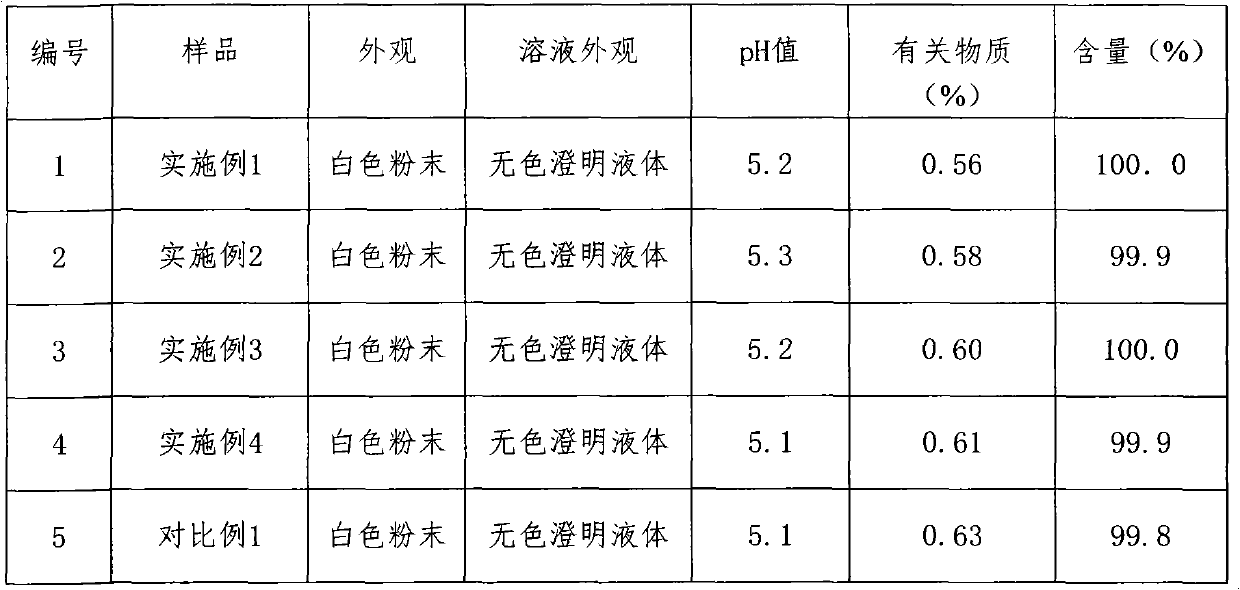
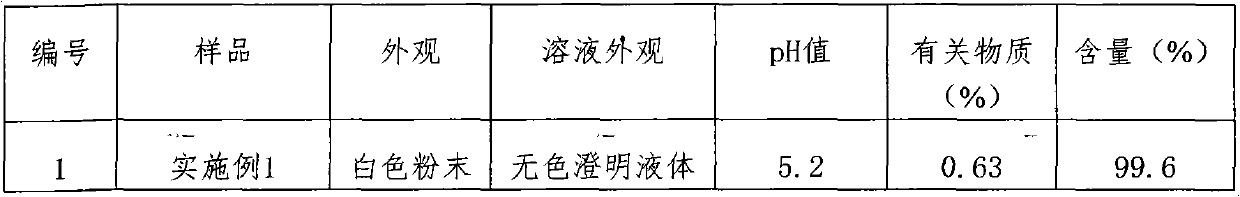
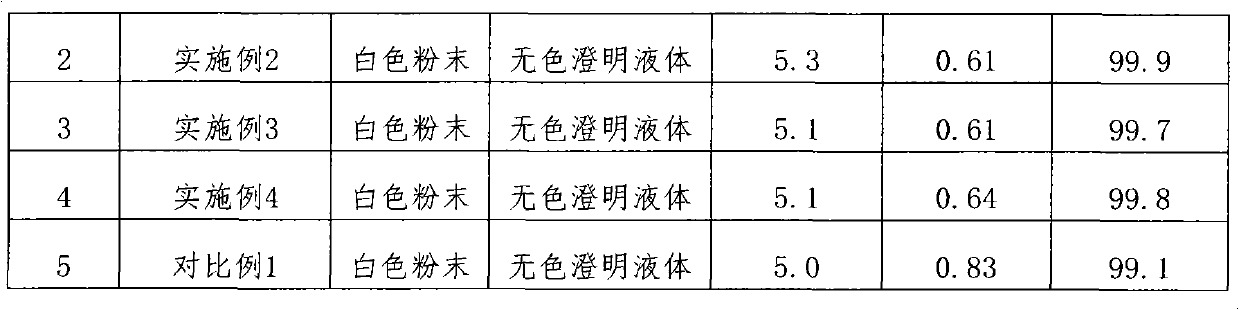
Embodiment 1
[0020] Prescription: the pharmaceutical composition of the present invention comprises the following ingredients: ceftezole sodium 20mg, sodium carbonate 2mg.
[0021] Preparation:
[0022] 1) Preparation of liquid medicine: weigh the prescribed amount of ceftezole sodium and sodium carbonate, and dissolve them with water for injection; add 0.1% activated carbon, mix well; shake and let stand for 30 minutes, first coarsely filter, and then use a 0.22 μm filter membrane to fine filter;
[0023] 2) freeze drying:
[0024] After cooling from room temperature to -60°C in 60 minutes, keep it for 120 minutes;
[0025] It takes 60 minutes to raise the temperature to -10°C and keep it for 240 minutes, and the vacuum degree is controlled at 14Pa;
[0026] It took 120 minutes to raise the temperature to 10°C and keep it for 180 minutes; then, it took 60 minutes to raise the temperature to 30°C and keep it for 120 minutes, and the vacuum degree was controlled at 15Pa;
[0027] 3) Enc...
Embodiment 2
[0029] Prescription: the pharmaceutical composition of the present invention comprises the following ingredients: ceftezole sodium 24mg, sodium carbonate 3mg.
[0030] Preparation:
[0031] 1) Preparation of liquid medicine: weigh the prescribed amount of ceftezole sodium and sodium carbonate, and dissolve them with water for injection; add 0.1% activated carbon, mix well; shake and let stand for 30 minutes, first coarsely filter, and then use a 0.22 μm filter membrane to fine filter;
[0032] 2) freeze drying:
[0033] After cooling from room temperature to -45°C in 30 minutes, keep it for 180 minutes;
[0034] It takes 90 minutes to raise the temperature to -10°C and keep it for 240 minutes, and the vacuum degree is controlled at 14Pa;
[0035] It took 180 minutes to raise the temperature to 10°C and keep it for 180 minutes; then, it took 30 minutes to raise the temperature to 20°C and keep it for 180 minutes, and the vacuum degree was controlled at 15Pa;
[0036] 3) Enc...
Embodiment 3
[0038] Prescription: the pharmaceutical composition of the present invention comprises the following ingredients: ceftezole sodium 20mg, sodium carbonate 4mg.
[0039] 1) Preparation of liquid medicine: weigh the prescribed amount of ceftezole sodium and sodium carbonate, and dissolve them with water for injection; add 0.1% activated carbon, mix well; shake and let stand for 30 minutes, first coarsely filter, and then use a 0.22 μm filter membrane to fine filter;
[0040] 2) freeze drying:
[0041] After cooling from room temperature to -45°C in 50 minutes, keep it for 120 minutes;
[0042] It takes 60 minutes to raise the temperature to -10°C and keep it for 180 minutes, and the vacuum degree is controlled at 10Pa;
[0043] It took 180 minutes to raise the temperature to 10°C and keep it for 180 minutes; then, it took 60 minutes to raise the temperature to 30°C and keep it for 120 minutes, and the vacuum degree was controlled at 15Pa;
[0044] 3) Encapsulation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com