Method for controlling quality of Ceftezole sodium used for injection
A technology for ceftizole sodium and injection, which is applied in the field of medicine, can solve the problems of not exceeding 5.5%, affecting the difference in filling amount, affecting the stability of preparations, etc., and achieves accurate filling amount, good powder fluidity and good clarity. Effect
Inactive Publication Date: 2010-08-11
天津新丰制药有限公司
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] The stability of ceftezole sodium for injection is closely related to the water content. High water content will affect the stability of the preparation. Too low water content will lead to static electricity of ceftezole sodium powder and affect the difference in loading capacity.
In the existing ceftezole sodium drug standard, the moisture content shall not exceed 5.5%. In the standard control, the ceftezole content is 90.0% to 110.0% of the labeled amount. There is no more accurate water content for ceftezole sodium at present. Control Method
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0021] A quality control method of ceftezole sodium for injection includes the following steps:
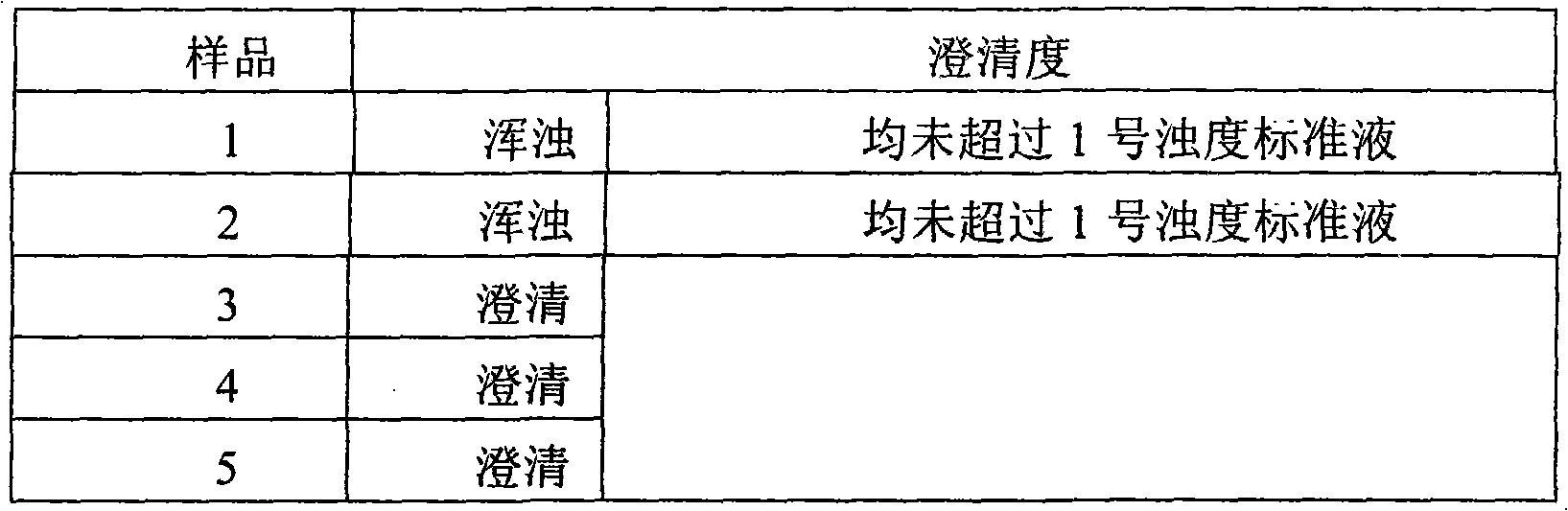
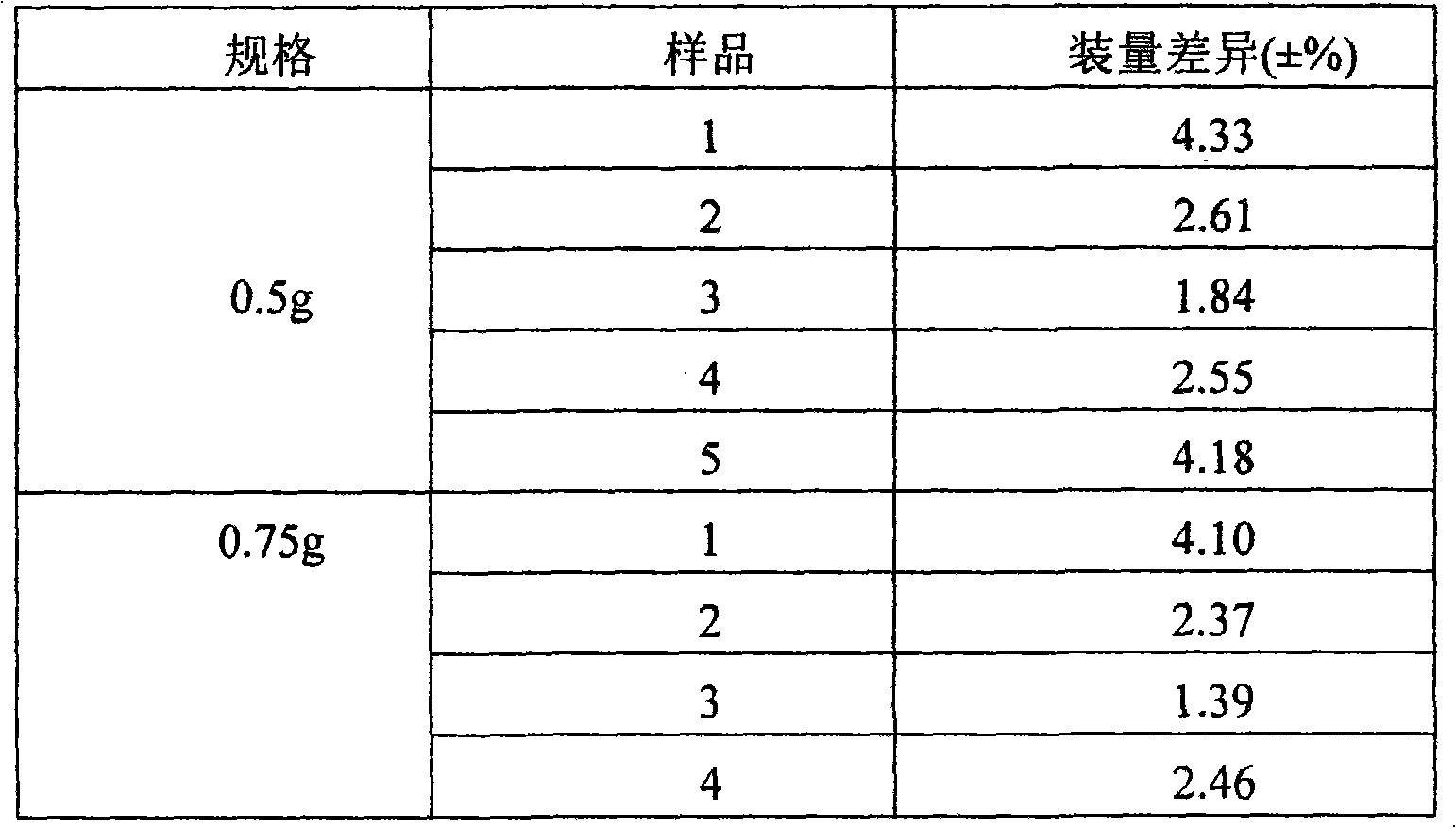
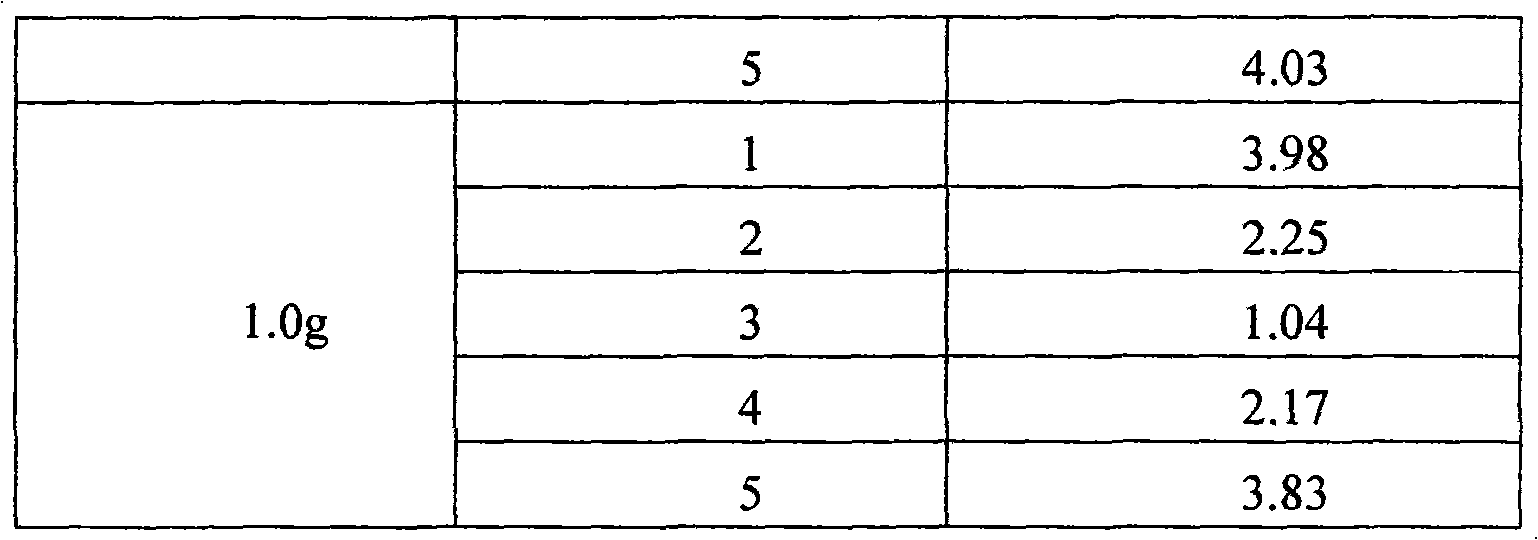
[0022] (1) Control the moisture content of cefotiazole sodium in the range of 2% to 4%;
[0023] (2) Control the humidity of the packaging environment at 40%-45%.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for controlling the quality of Ceftezole sodium used for injection, comprising the steps as follows: (1) controlling the moisture content of a Ceftezole sodium raw material to be 2-4%; and (2) controlling the humidity of the encapsulation environment to be 40-45%. By controlling the raw material moisture and the humidity of the encapsulation environment in the invention, the moisture content of a drug preparation can be controlled better so as to obtain a preparation with the characteristics of good clarity, favorable powder mobility and more accurate charging amount, thus being more applicable to clinic use.
Description
Technical field [0001] The invention belongs to the technical field of medicine, and relates to a method for quality control of cephalosporin drugs, in particular to a method for controlling the quality of cefotizole sodium for injection. Background technique [0002] Ceftezole Sodium (Ceftezole Sodium, molecular weight 462.47, molecular formula C 13 H 11 N 8 NaO 4 S 3 ), the chemical name is (6R,7R)-3-([1,3,4-thiadiazol-2-yl)thio]methyl)-7-((1H-tetrazol-1-yl)acetamido )-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate sodium salt, white to light yellow powder or crystalline powder, odorless, hygroscopic. [0003] Cefotizole sodium is the first generation of cephalosporin antibiotics for injection. It is a semi-synthetic cephalosporin derivative. Its mechanism of action is to inhibit the synthesis of bacterial cell walls to exert antibacterial activity. It has antibacterial activity against gram-positive bacteria, especially cocci. For example, Staphylococcus aureus, Strept...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/14A61K31/546A61P31/04
Inventor 刘文玖王春玲李红梅李相君王原闫晓楠
Owner 天津新丰制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com