Preparation method for type I ceftezole sodium crystal
A technology of ceftezole sodium and crystals, which is applied in the field of preparation of type I ceftezole sodium crystals, can solve problems such as inability to obtain ceftezole sodium crystals, difficulty in ensuring uniform products, poor repeatability of the preparation process, etc., and achieve improved stability Sex and safety, low cost, good effect of crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
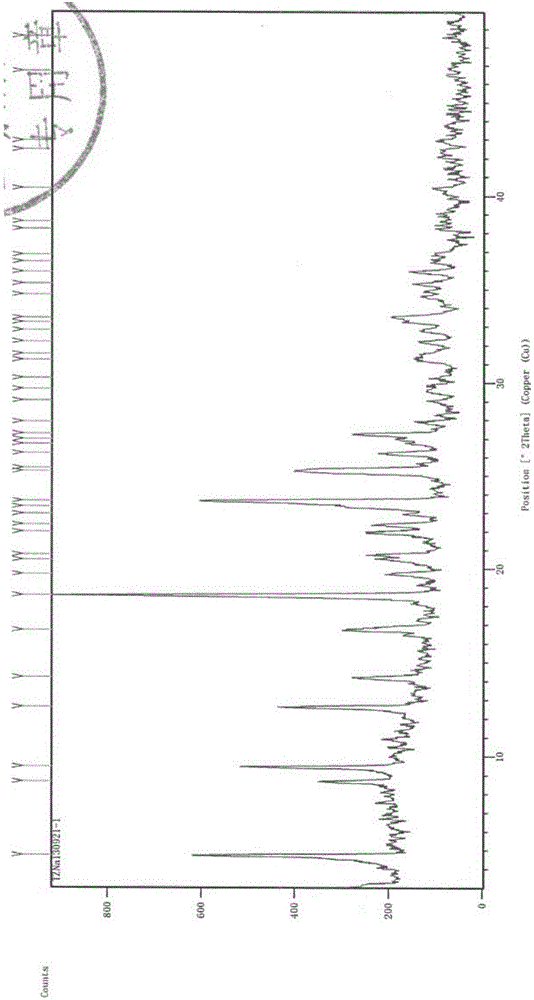
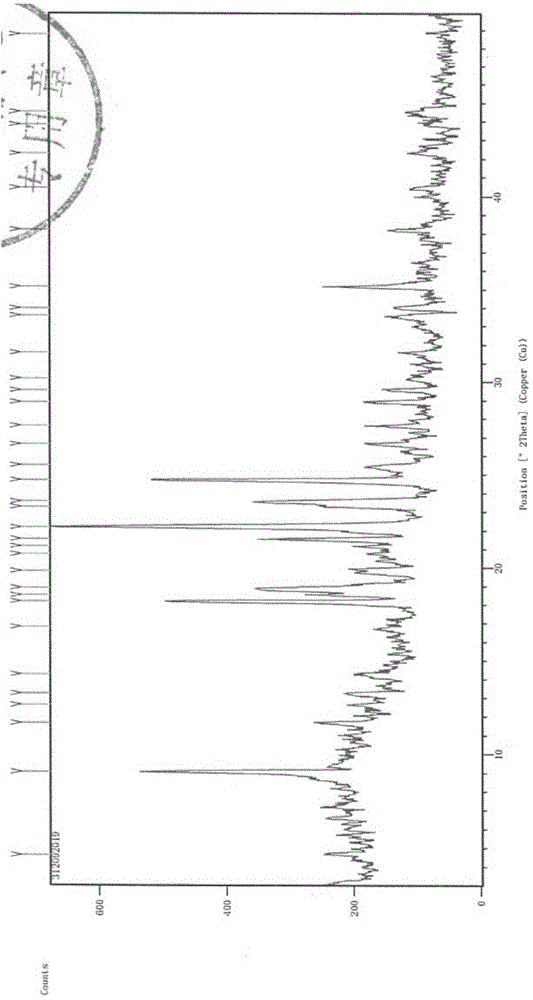
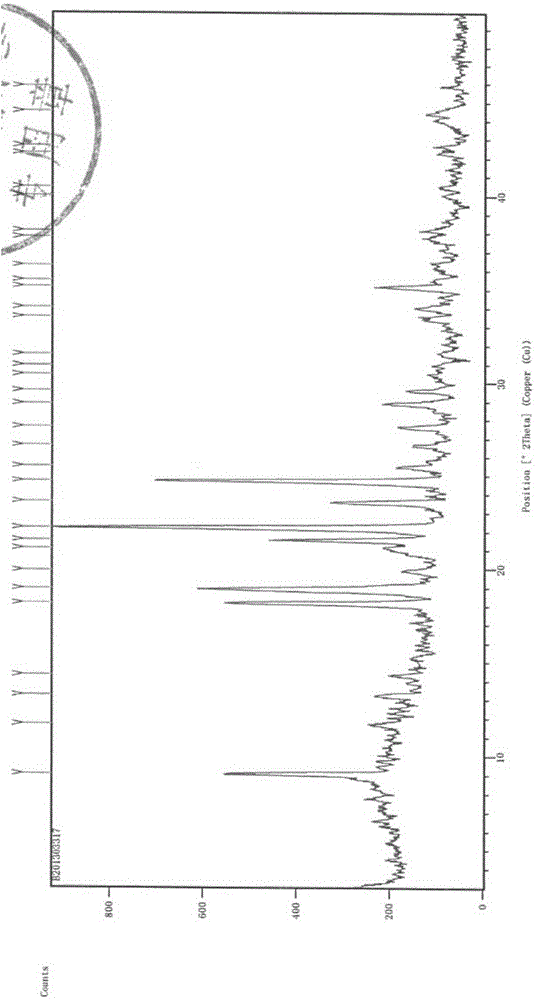
[0060] Experimental Example 1 X-ray Diffraction (XRD) Experiment
[0061] Testing instrument: Empyrean X-ray diffractometer;
[0062] Detection conditions: Cu target Kα 1 Ray, voltage 40kV, current 40mA, divergence slit 1 / 32°, anti-scatter slit 1 / 16°, anti-scatter slit 7.5mm, 2θ range: 3°~50°, step size 0.02°, dwell time per step 40s;
[0063] Detection basis: The Pharmacopoeia of the People's Republic of China (2010 Edition II) Appendix IXFX-ray powder diffraction method.
experiment example 2
[0064] Experimental Example 2 Thermogravimetric Analysis Experiment
[0065] Testing instrument: TG209 thermogravimetric analyzer from NETZSCH, Germany;
[0066] Detection conditions: Atmosphere: air, 20Ml / min; Scanning program: room temperature ~ 350°C, heating rate: 10°C / min;
[0067] Detection basis: General Principles of Thermal Analysis Methods JY / T 014-1996.
experiment example 3
[0068] Experimental example 3 Polarizing microscope test
[0069] Testing instrument: Chongqing Aote Optical Instrument Co., Ltd. SMART-POL polarizing microscope;
[0070] Detection conditions: eyepiece 10 times, objective lens 10 / 0.25 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com