Ceftezole sodium powder injection and synthesizing method thereof
A technology of ceftezole sodium and its synthesis method, which is applied in the field of medicine, can solve the problems of high price, low yield, and large environmental damage, and achieve the effects of easy treatment of three wastes, simple and easy reaction, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
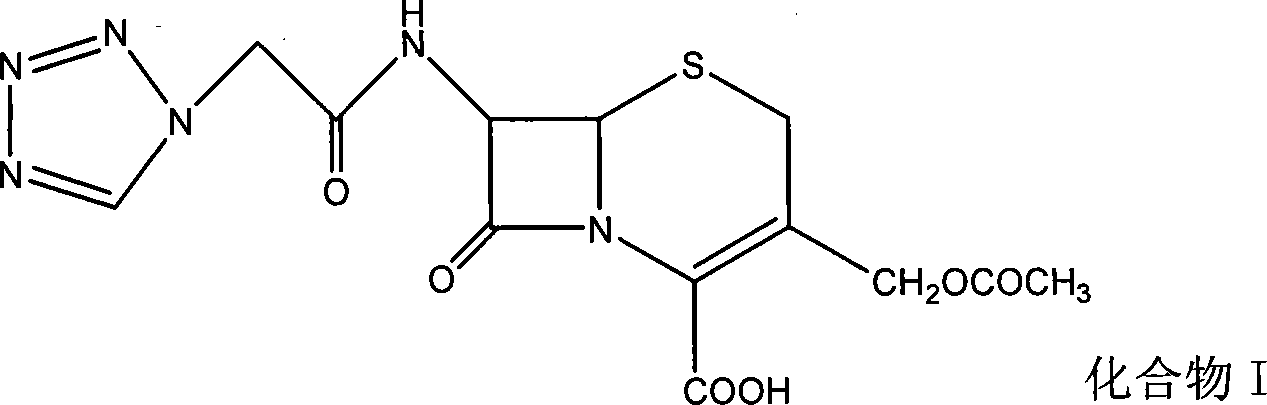
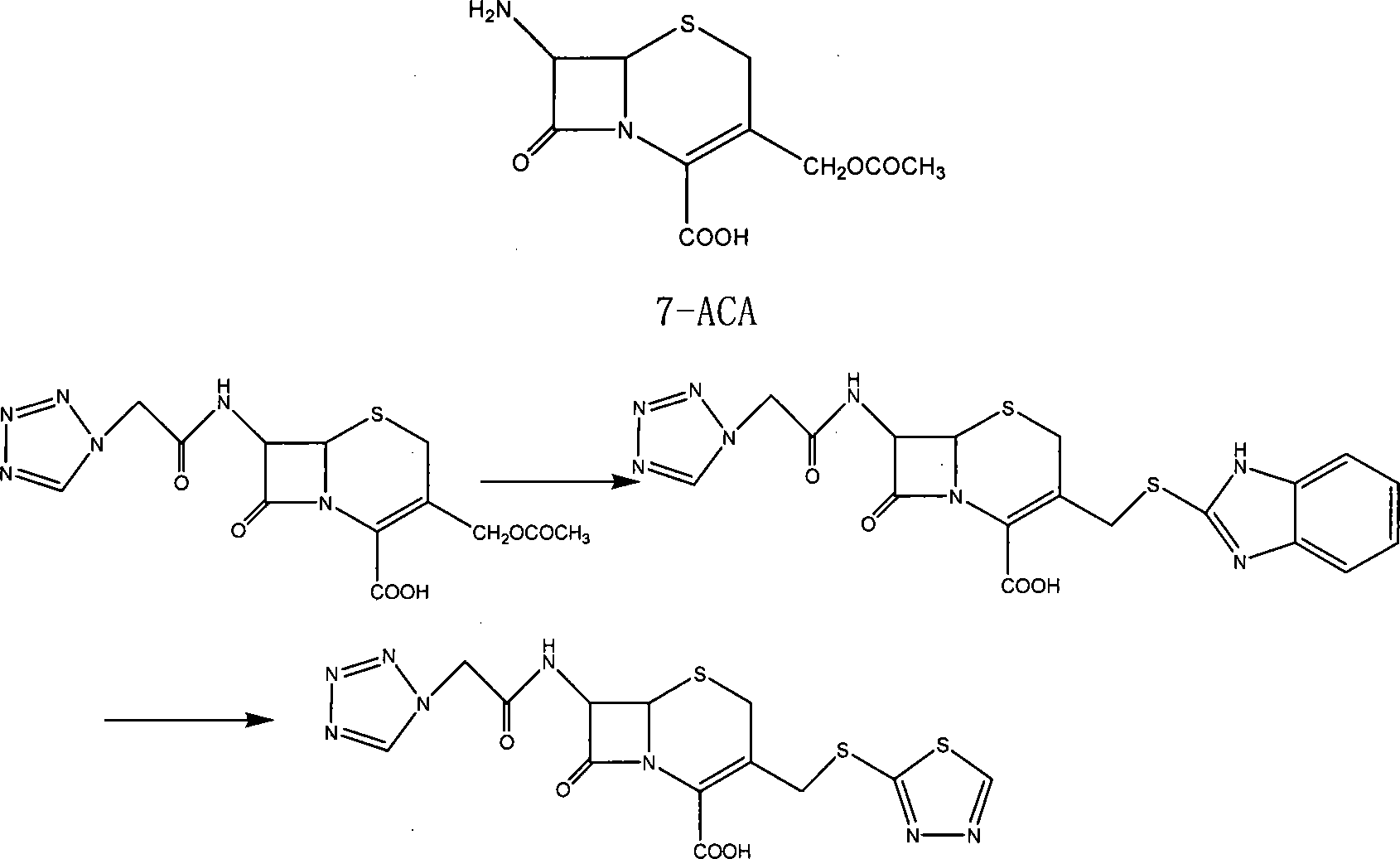
[0068] Embodiment 1: the synthesis of 7-(1H-1-tetrazolyl) acetamide-cephalosporanic acid (I)
[0069] Add 1412g (11.03mol) tetrazoleacetic acid, 1609g (8.75mol) N, N'-dicyclohexylcarbodiimide (DCC), and 50L dimethyl sulfoxide into a 100L reactor, and stir to dissolve completely. Mix and dissolve 2000g (7.35mol) of 7-amino-cephalosporanic acid, 2227g (22.05mol) of triethylamine, and 30L of ethyl acetate. After dissolving, add dropwise to the above tetrazoleacetic acid solution, and control the reaction temperature at -5 to 0 Between ℃, 1.5h added. After the dropwise addition, react at -5~0°C for 1h, at 0~5°C for 1h, and at room temperature for 3h. The precipitate was filtered off, 70L of water was added to the filtrate, and the water layer was separated. The aqueous layer was washed twice with 10 L of ethyl acetate. Use 10% hydrochloric acid to adjust the pH<2, and a large amount of precipitate precipitates out. Add 45L of ethyl acetate, and extract twice. The extracts wer...
Embodiment 2
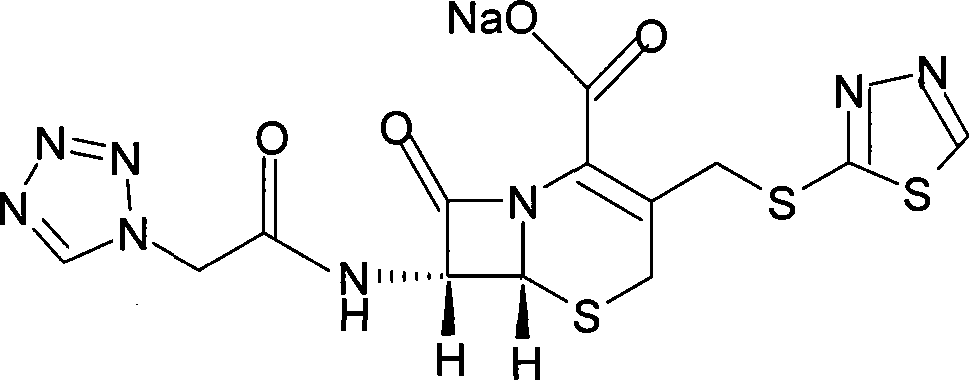
[0070] Example 2: 7-(1H-1-tetrazolyl)acetamido-3-[2-(1,3,4-thiadiazolyl)]thiomethyl-3-cephem-4-carboxylic acid ( Synthesis of Ceftezole)
[0071] 2043g (5.35mol) compound (III) was dissolved in an appropriate amount of 10% NaHCO 3 The solution was dissolved (about 4300ml), and then this solution and 755g (6.40mol) 1,3,4-thiadiazole-2-thiol (IV) were added to 80L of phosphate buffer solution with a pH value of 6.4, and heated to 60°C , Stir the reaction for 6h. It was then washed with ether until the solution became clear. The aqueous layer was acidified with 10% hydrochloric acid to pH<2.0, and a large amount of precipitate was deposited. Extracted twice with 70L ethyl acetate, combined extracts, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated under reduced pressure, crystallized, and suction filtered to obtain 2028 g of crude product.
Embodiment 3
[0072] Example 3: 7-(1H-1-tetrazolyl)acetamido-3-[2-(1,3,4-thiadiazolyl)]thiomethyl-3-cephem-4-carboxylic acid ( Refining of ceftezole)
[0073] 2028g crude ceftezole was heated to 60°C with 6 times of acetone to dissolve completely. Then let cool and crystallize for 2 hours, filter with suction, wash with a little ethanol, and dry under reduced pressure at 40°C for 4 hours to obtain 1834 g of product, yield: 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com