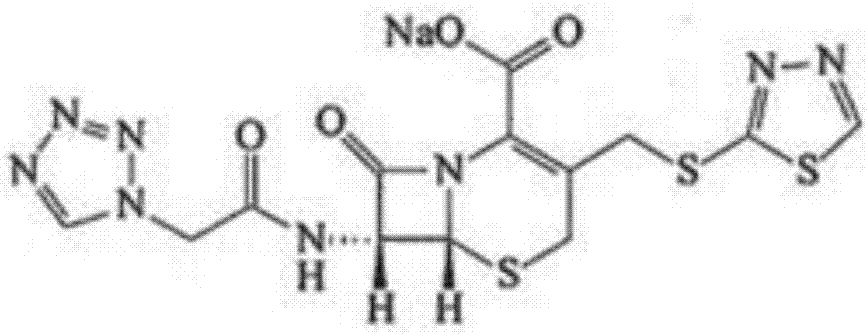
Preparation process of ceftezole sodium for injection sterilize powder-injection for injection
A technology for ceftazole sodium and sterile powder injection, which is applied in the field of preparation of ceftazole sodium sterile powder injection for injection, can solve problems such as uncontrollable clarity, and achieves the advantages of enhancing injection safety and improving product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] The preparation process of Ceftezole Sodium Sterile Powder for Injection of the present invention, the specific steps are as follows:
[0026] (1) Raw material preparation process: first use drinking water to clean the surface of the ceftezole sodium powder barrel / bag, then wipe and disinfect with 0.2% bromogeramine or 2% lysol; 35m / s, filter purification efficiency ≥ 0.6μm dust ≥ 99.99%; enter the C-class clean area and use 1% peracetic acid for aerosol spray disinfection for 15min, the mist particles include 90% of the particles with a diameter of 15μm; again in the transfer window Perform air shower treatment for 10 minutes, the nozzle wind speed is 50m / s, the filter purification efficiency is ≥ 0.2μm dust ≥ 99.99%, and finally enters the material temporary storage room in the B-level clean area for temporary storage.
[0027] (2) Cleaning process of soda-lime glass molded injection bottles: firstly, the qualified injection bottles are sprayed and cleaned with circul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com