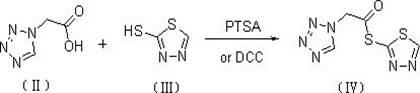The preparation method of ceftezole sodium
A technology of ceftezole sodium and ceftezole acid, which is applied in the field of synthesis of ceftezole sodium, can solve the problems of expensive hospital preparations, strong pollution, and economic burden, and meet the needs of industrial production and simplify operation steps , The effect of simple and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Synthesis of 1H-tetrazolium acetate-1,3,4-thiadiazole-2-thioester
[0029] 134.4 g (1.05 mol) of 1H-tetrazolium acetic acid and 118.0 g (1.00 mol) of 2-mercapto-1,3,4-thiadiazole were dissolved in 1000 mL of tetrahydrofuran, cooled to 0°C to 4°C, and then added A solution of 17.2g (0.10mol) of p-toluenesulfonic acid in 50mL of tetrahydrofuran was kept at the temperature for 2h, the solvent was recovered by distillation under reduced pressure, cooled to 0°C to 4°C, and filtered to obtain 220.8g of 1H-tetrazolium acetic acid-1 , 3,4-thiadiazole-2-thioester, yield 96.8%.
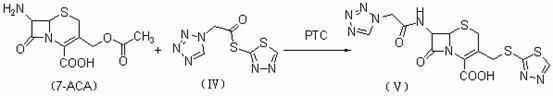
[0030] 2. Synthesis of ceftizole acid
[0031] Add 227.0 g (0.83 mol) of 7-aminocephalosporanic acid (7-ACA) and 112.9 g (0.83 mol) of crystalline sodium acetate to 1500 mL of water and 1500 mL of ethanol, stir to dissolve, and cool down to 0°C to 4°C in an ice bath. Then, 1H-tetrazolium acetate-1,3,4-thiadiazole-2-thioester prepared above was added in a molar ratio of 1.0:1.2, and the reaction w...
Embodiment 2
[0035] 1. Synthesis of 1H-tetrazolium acetate-1,3,4-thiadiazole-2-thioester
[0036]134.4 g (1.05 mol) of 1H-tetrazolium acetic acid and 118.0 g (1.00 mol) of 2-mercapto-1,3,4-thiadiazole were dissolved in 1200 mL of ethyl acetate, cooled to 0°C to 4°C, and then Then add 226.6g (1.10mol) of dicyclohexylcarbodiimide (DCC) and 500mL of ethyl acetate solution, keep the temperature for reaction for 2h, recover the solvent by vacuum distillation, cool down to 0℃~4℃, filter to obtain 213.8g of 1H-tetrazolium acetate-1,3,4-thiadiazole-2-thioester, yield 93.7%.
[0037] 2. Synthesis of ceftizole acid
[0038] As in Example 1, the phase transfer catalyst adopts tetrabutylammonium bromide; the organic solvent used is toluene.
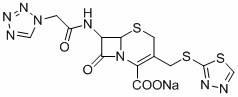
[0039] 3. Synthesis and purification of ceftizole sodium
[0040] Same as Example 1. 135.6g of ceftizole sodium was obtained, the yield was 86.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com