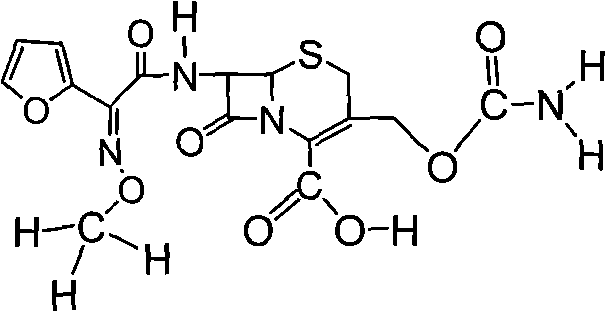
Preparation method of high-purity cefuroxime acid
A technology of cefuroxime acid and cefuroxime, applied in the field of preparation of intermediates, can solve the problems of low product purity, high drying temperature, long time and the like, and achieve the effects of high product purity, high product yield and short drying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
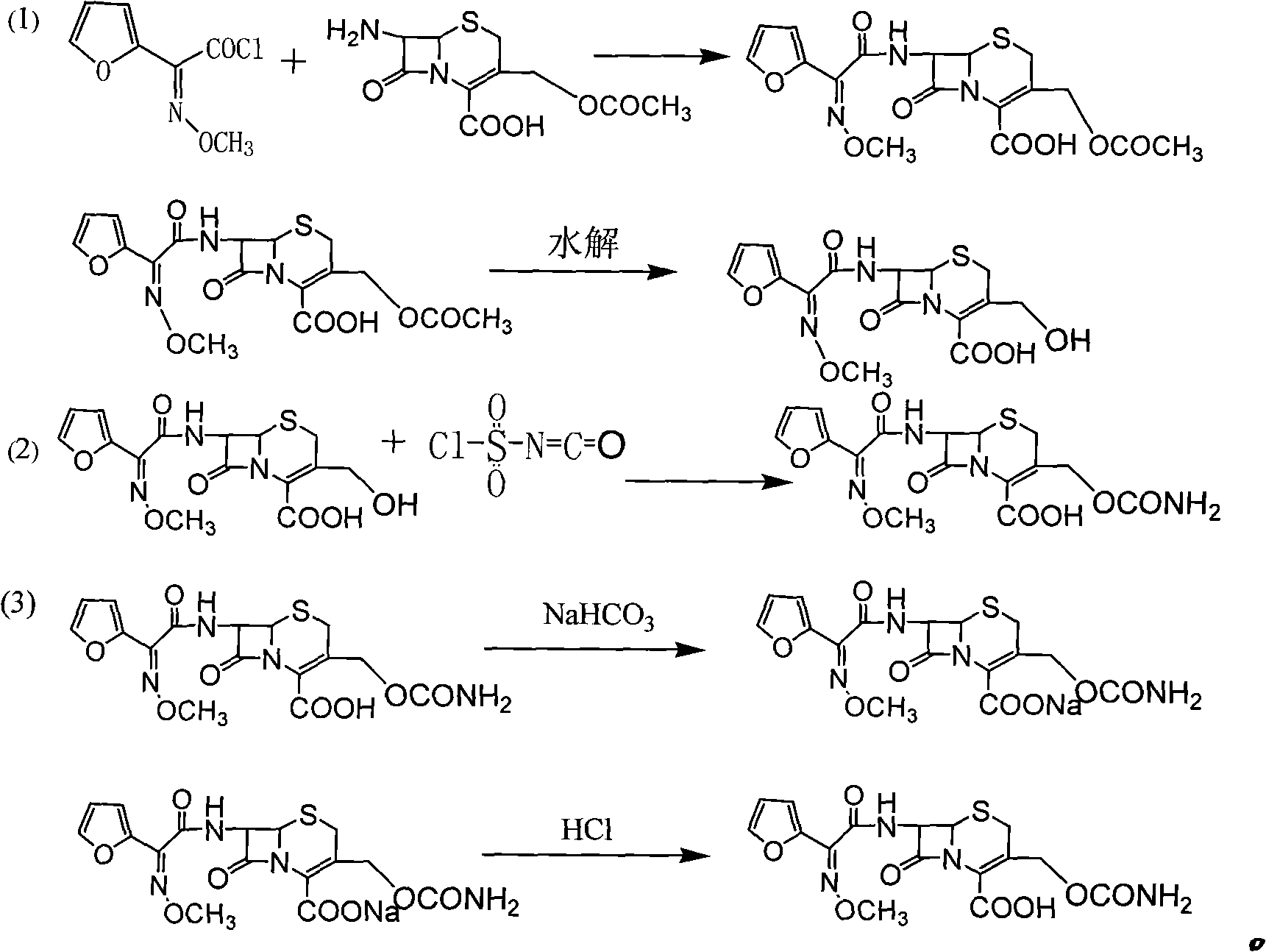
[0035] (1), the preparation of 3-deformylamino cefuroxime (DCC):
[0036] Add 380ml of purified water and 200g of 7-ACA to the reaction flask, stir and dissolve with 200ml of 15% sodium hydroxide, then add 1500g of furoacetyl chloride dichloromethane solution with a prepared content of 12% into the above solution, and control the reaction temperature 0-15°C, stirred for 2 hours, and kept the pH of the solution at 5.5-7.5 during the reaction. Stand still for 10-30 minutes after the reaction, separate the water phase, extract the organic phase once with 50ml of purified water, combine the water phase, add 80ml of methanol, cool to -25~-30°C, add 15% (mass) sodium hydroxide 200g, stirred and reacted for 30 minutes, added 80ml of glacial acetic acid, then adjusted the pH of the feed solution to 1.0-2.0 with 25% hydrochloric acid, filtered after stirring for 15 minutes, washed the filter cake DCC with 180ml of purified water, and vacuum-dried at 40°C until the water content Less t...
Embodiment 2
[0040] Preparation of cefuroxime acid:
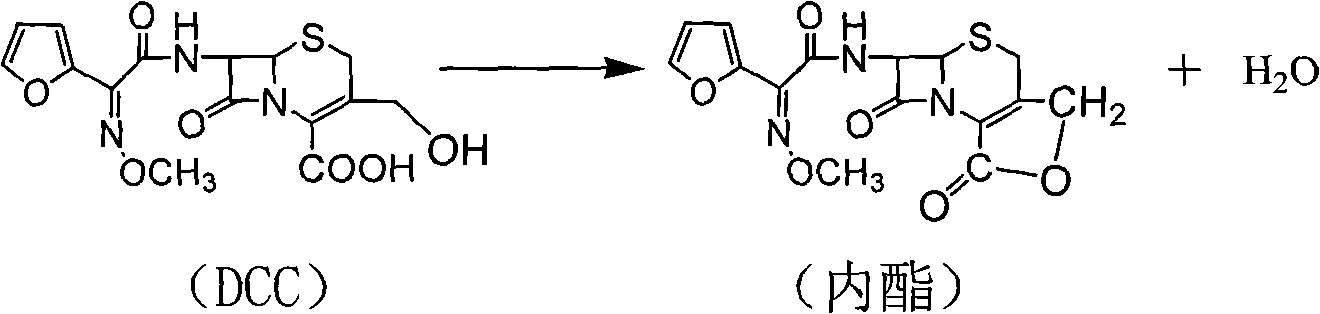
[0041] The preparation of 3-deformamidocefuroxime (DCC) is the same as in Example 1, and DCC 100g is prepared according to (1) in Example 1, and 300ml of tetrahydrofuran is added for dissolution, and cefuroxime acid is prepared according to the method in (2) in Example 1 To the reaction solution, add 220ml of ethyl acetate and 360ml of dichloromethane, then add sodium bicarbonate solution, adjust the pH to 7.5, stir for 10 minutes, let it stand for layers, extract lactone and unsaponifiable matter from the organic phase, and add Add 660ml of ternary composite solvent, then add 31% hydrochloric acid to adjust PH=2.0, let stand for 25 minutes, then separate the liquid, carry out vacuum distillation on the organic phase, stop distillation when the solution distillate is 300ml, cool to 5~10°C, Stir for 2 hours. After vacuum filtration, the filter cake was vacuum-dried at 40° C. for 2 hours to obtain 102.18 g, with a purity of 99.31%, a wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com