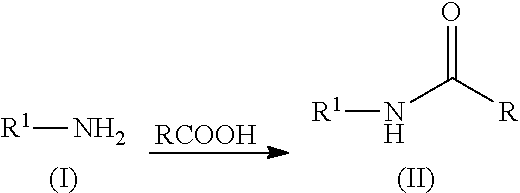
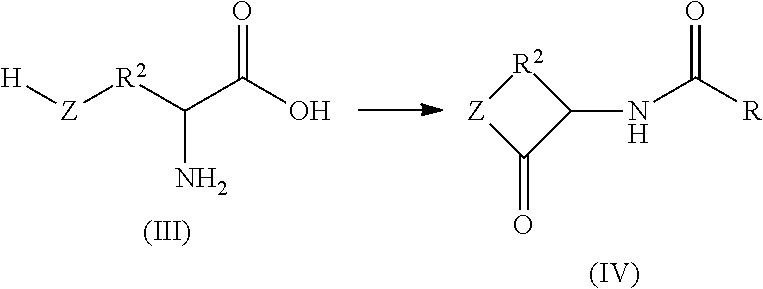
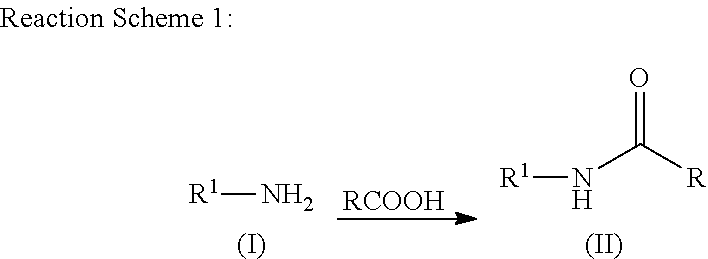
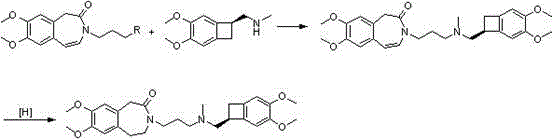
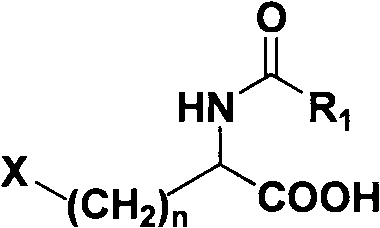
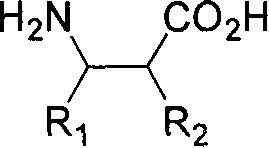
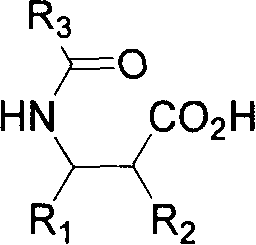
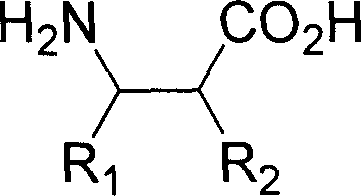
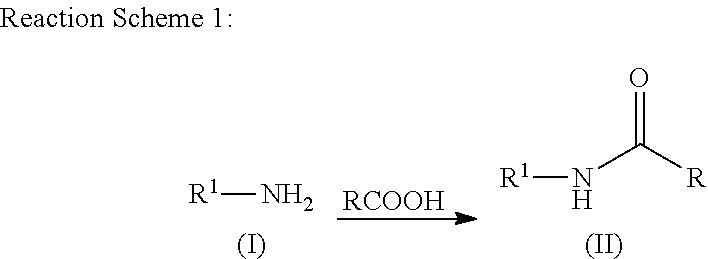
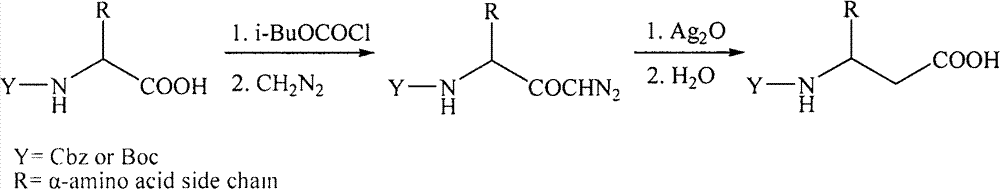
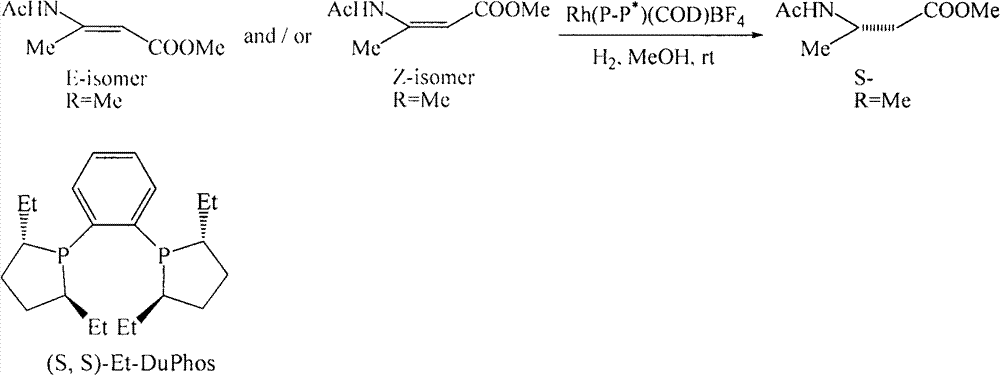
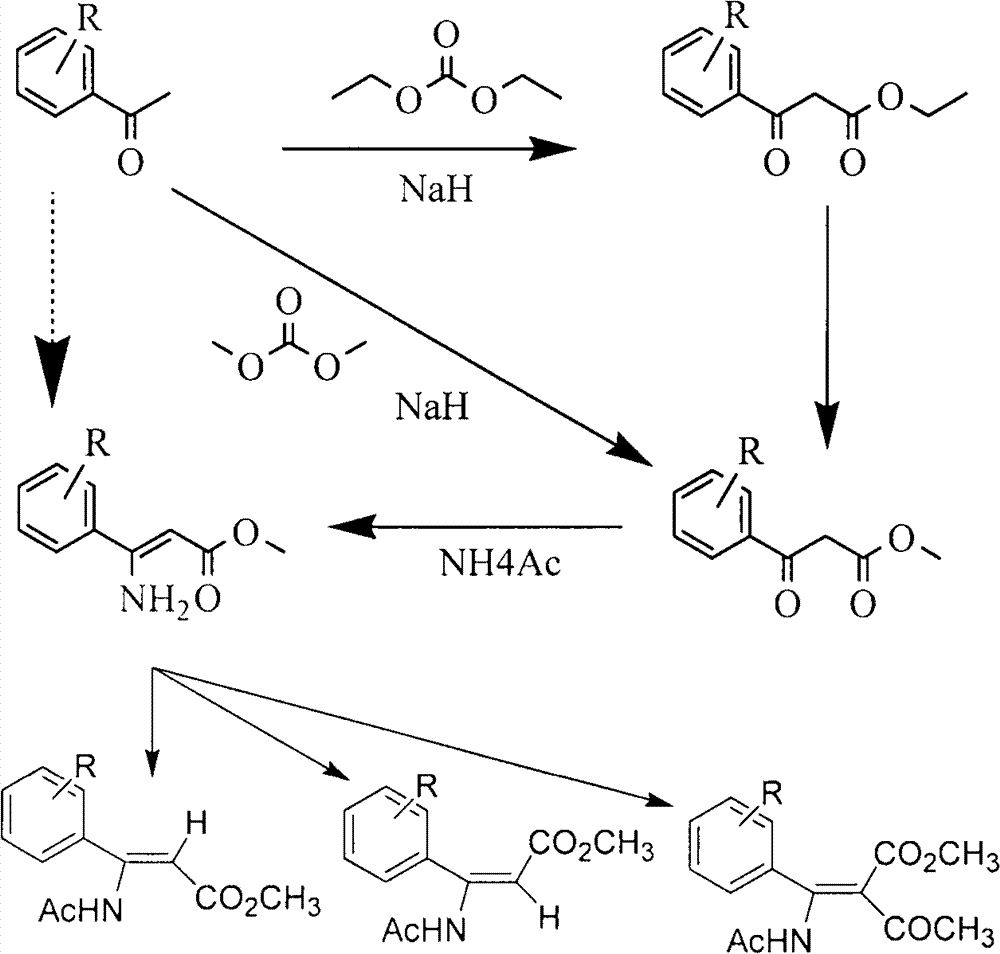
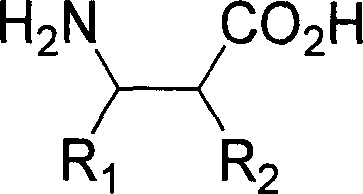
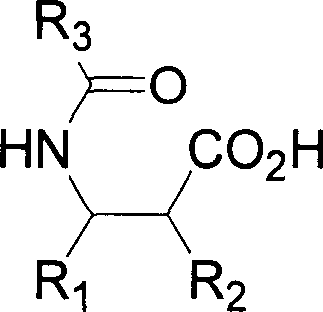
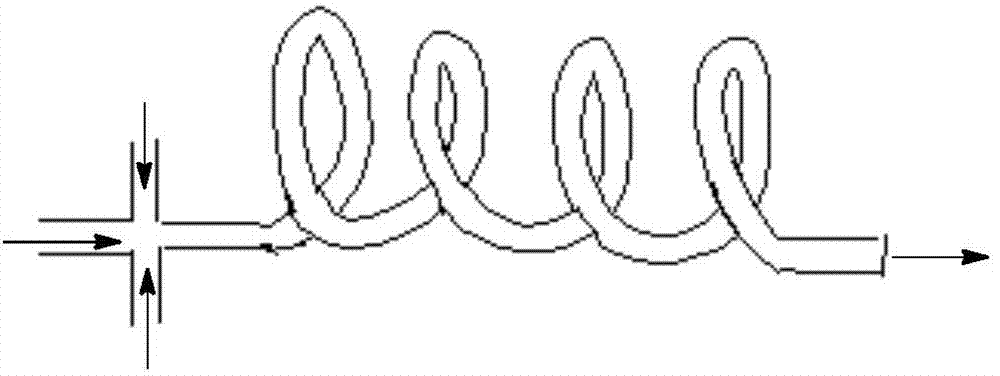Patents
Literature
40 results about "N acylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of high-purity cefuroxime acid
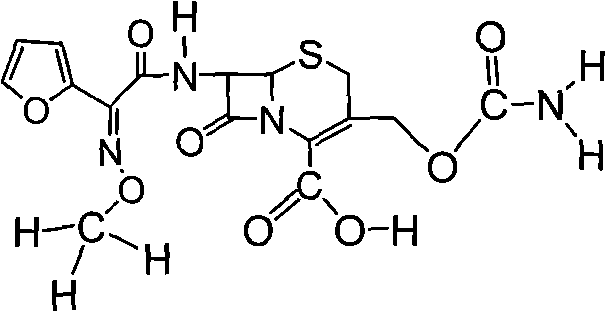
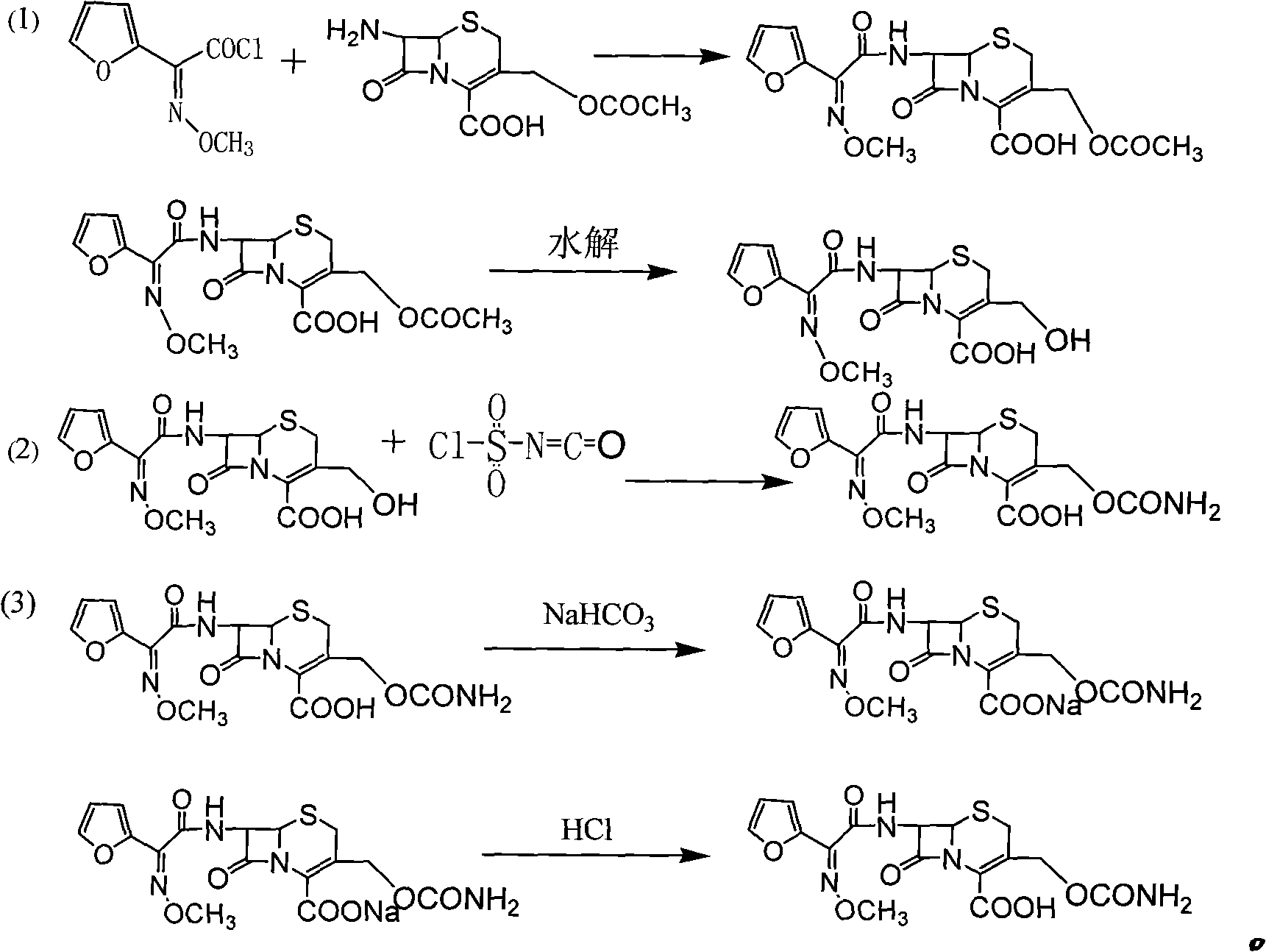
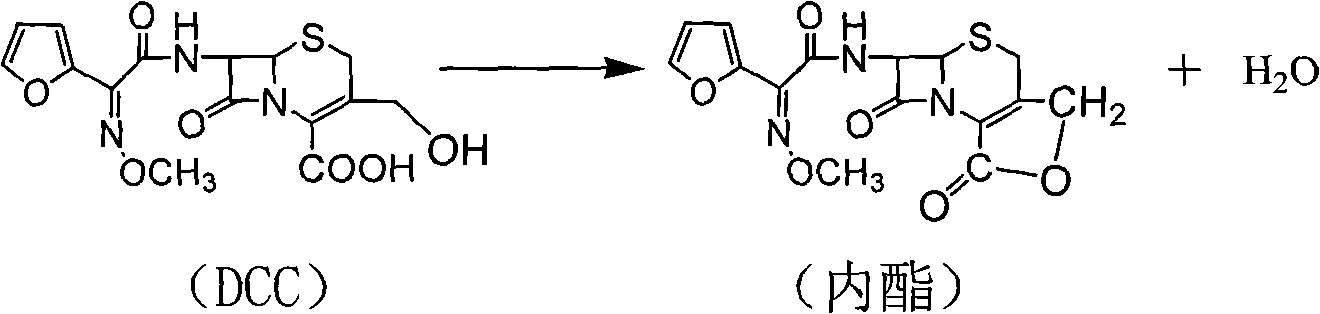
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
Methods for one-pot n-demethylation/n-acylation of morphine and tropane alkaloids
The present invention provides a method for the N-demethylation and / or N-acylation of an N-methylated heterocycle such as morphine alkaloids or tropane alkaloids. The method comprises reacting the heterocycle with an acylating agent in the presence of a metal catalyst.
Owner:BROCK UNIVERSITY
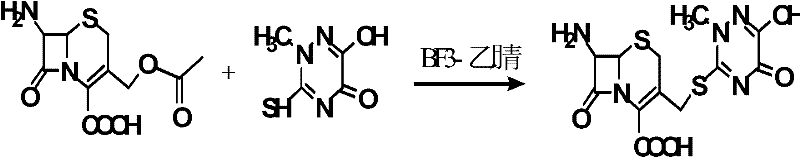
Synthetic method of ceftriaxone sodium crude salt
InactiveCN102559829AHigh purityHigh yieldOrganic chemistryFermentationChemical synthesisEnzymatic hydrolysis
The invention belongs to the field of chemical synthesis, and particularly relates to a synthetic method of a ceftriaxone sodium crude salt. The method comprises the following steps of: (1) performing electrophilic substitution on 7-ACA and a triazine ring by taking a BF3-acetonitrile solution as a catalyst to obtain 7-ACT at last; and (2) undergoing an N-acylation reaction on 7-ACT and AE-active ester in an organic phase, adding sodium iso-octoate, and undergoing a salt forming reaction to obtain the ceftriaxone sodium crude salt. The method is characterized in that: the 7-ACT is prepared with an enzymatic hydrolysis method after electrophilic substitution in the step (1); and a flocculating agent is added after the N-acylation reaction is completed in the step (2). The method has the advantages that: the product yield and purity are raised; the 7-ACT is prepared with the enzymatic hydrolysis method in the first step, and the enzymatic hydrolysis method has the characteristics of specificity and high efficiency, so that side reactions are avoided, the yield is increased by over 8 percent, and can be up to 88 percent, and the product purity is raised; and the flocculating agent is added in the second step, so that insoluble matters in a reaction liquid are removed, and a high-purity ceftriaxone sodium crude salt is obtained finally.
Owner:YIYUAN XINQUAN CHEM
High-purity ranolazine and preparation method thereof
InactiveCN101560196AGood lookingHigh purityOrganic chemistryCardiovascular disorderAlkyl transferDimethylaniline N-oxide
The invention discloses a preparation technology and a refining method of ranolazine, which are more suitable for industrial production. The method comprises the following concrete steps: using 2, 6-dimethylaniline and methylpyrocatechine as original materials; sequentially carrying out four reaction processes of N-acylation, O-alkylation, N-alkylation and N-alkylation to synthesize ranolazine; and then, recrystallizing to obtain a refined product. The refined product obtained by the method has high purity, and the method also has the advantages of simple operation, low production cost and high yield and is more suitable for industrial production.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
One-pot synthesis method of N-hydrocarbon acyl cyclic lactam derivative
ActiveCN103819364AAvoid rearrangement reactionsAvoid decompositionOrganic compound preparationCarboxylic acid amides preparationHydroxylamineN acylation
The invention discloses a one-pot synthesis method of an N-hydrocarbon acyl cyclic lactam derivative. The method comprises the following steps: adding lactam and acyl chloride into a reaction kettle, stirring, performing N-acylation reaction, slowly adding a weak alkali compound into the reaction kettle to neutralize acid generated in the N-acylation reaction, reacting for a while, further adding hydroxylamine and a weak alkali compound or only adding the weak alkali compound into the reaction kettle, and performing open loop reaction to obtain a N-hydrocarbon acylamino hydroximic acid salt or N-hydrocarbon acylamino carboxylate. The method adopts a one-pot synthesis method, is gentle in reaction condition, high in yield, low in cost, easy to operate and short in production period, and the industrial production is met.
Owner:CENT SOUTH UNIV
Method utilizing micro-reactor to continuously synthesize amide herbicide
InactiveCN104262188ARealize continuous productionSimple production equipmentOrganic compound preparationCarboxylic acid amides preparationAlkyl transferMicroreactor
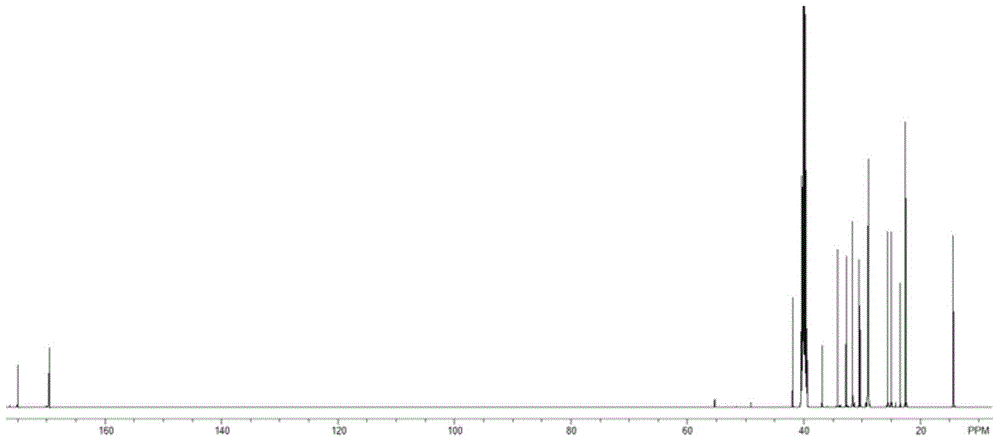
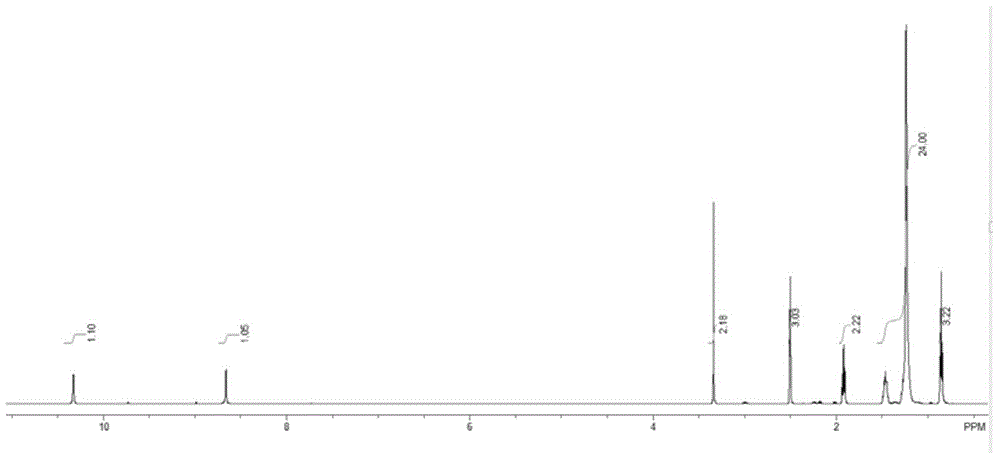
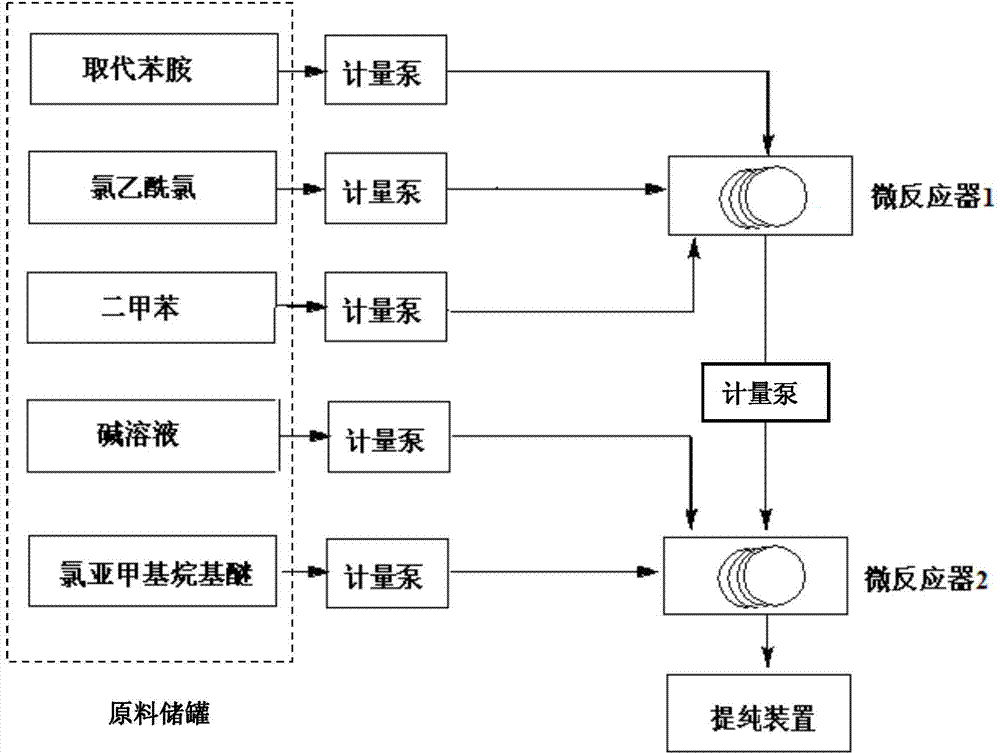
The invention discloses a method utilizing a micro-reactor to continuously synthesize amide herbicide, and belongs to the technical field of organic synthesis. The method takes substituted aniline as the raw material, and then the substituted aniline is subjected to continuous two steps of N-acylation and heterogeneous N-alkylation in a micro-reactor so as to obtain the amide herbicide; wherein the conversion rate of substituted aniline can reach 98% or more, the total yield of the reactions can exceed 95%, and the product purity is more than 96%. The intermediate purifying step is saved, thus the production procedure is simplified, the reaction time is reduced, the alkali solution consumption is reduced, the production equipment is simple, the production efficiency is high, and the product yield and purity are high.
Owner:JILIN UNIV
Method for analyzing c-terminal amino acid sequence of peptide using mass spectrometry
InactiveUS20060134720A1High strengthEasy to identifyPeptide/protein ingredientsMicrobiological testing/measurementChemical treatmentArginine
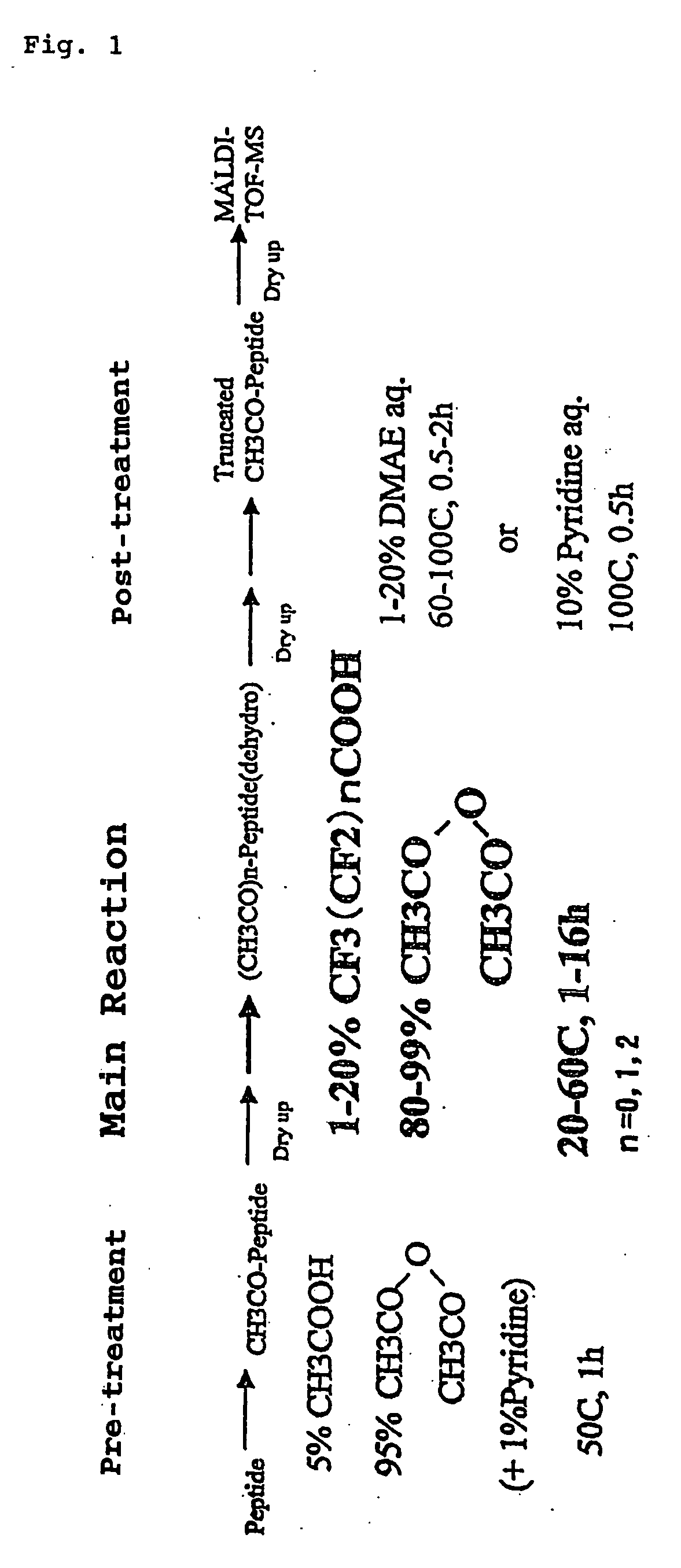
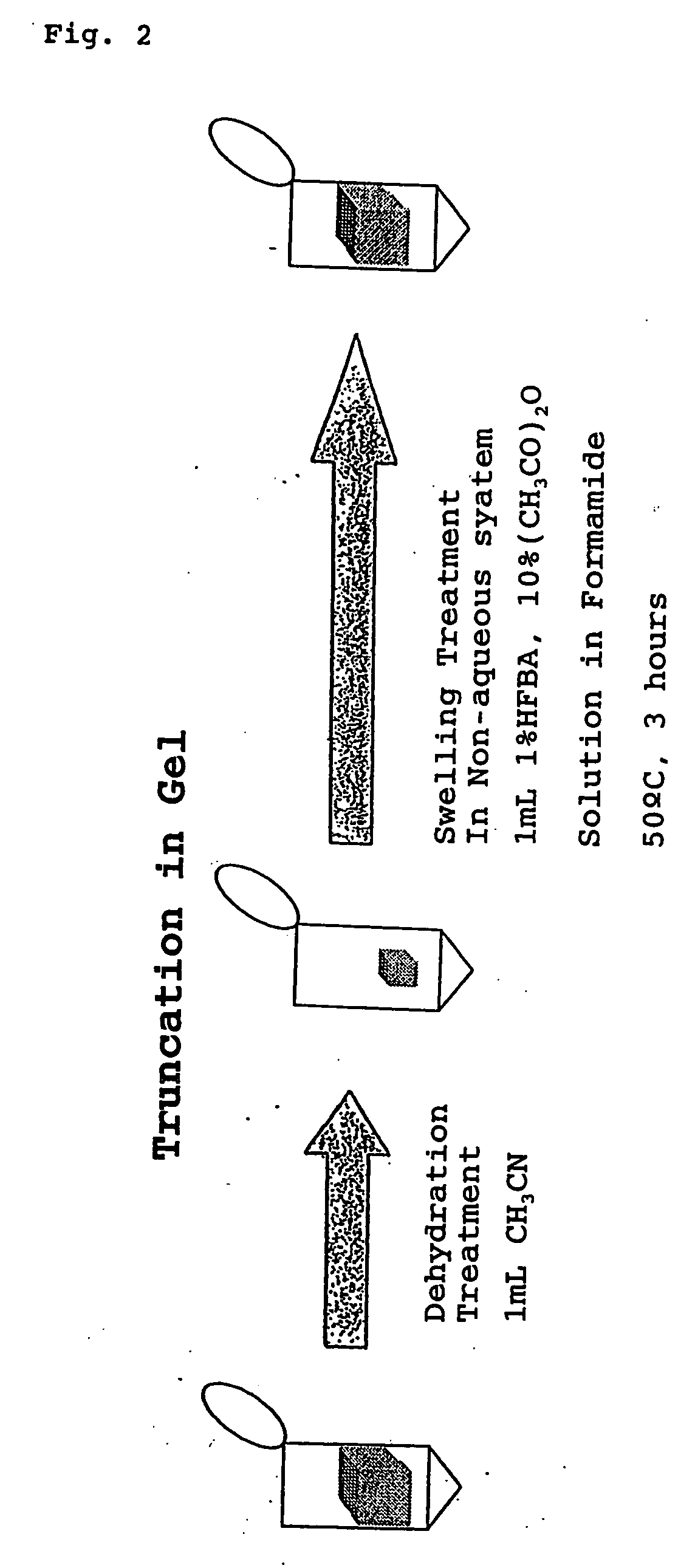
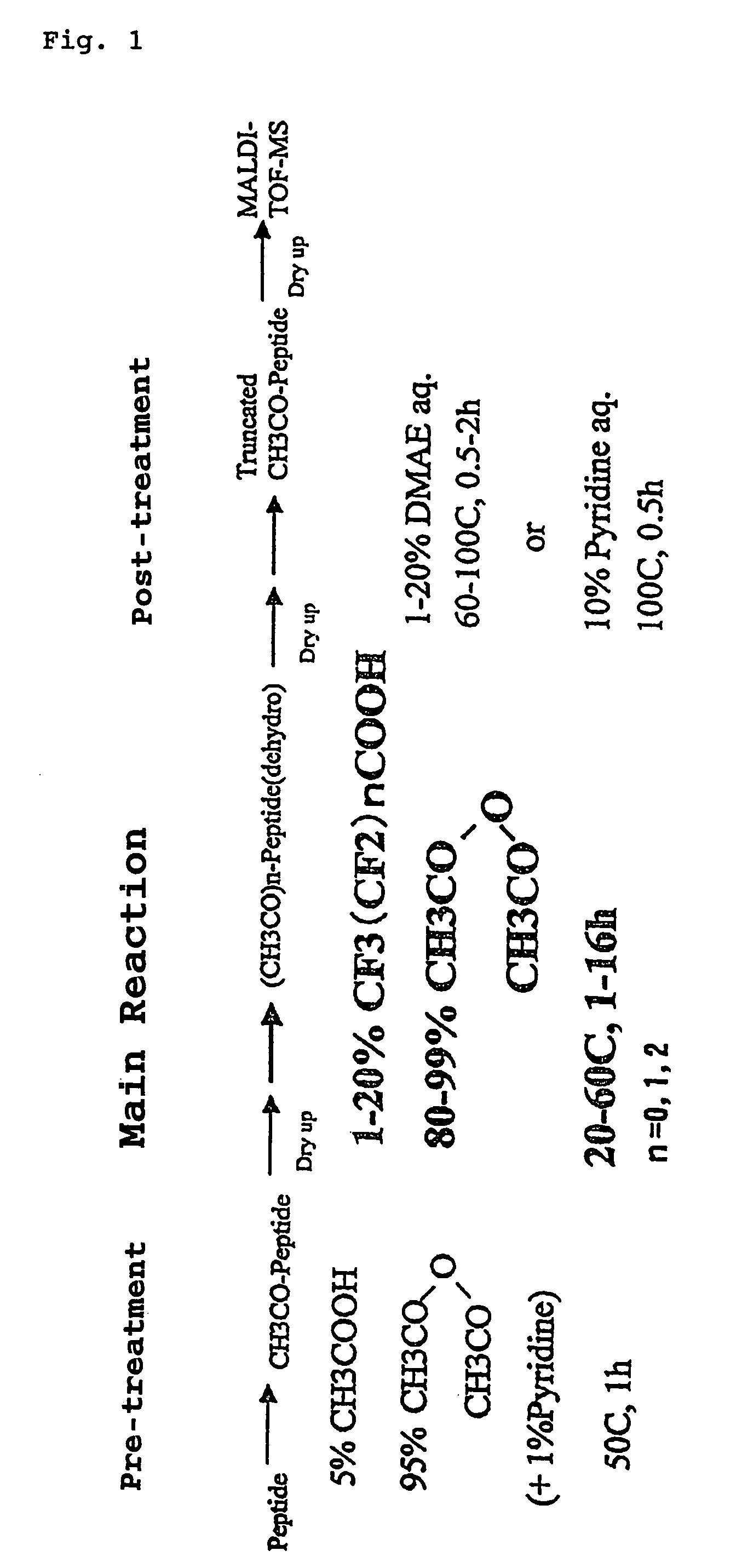
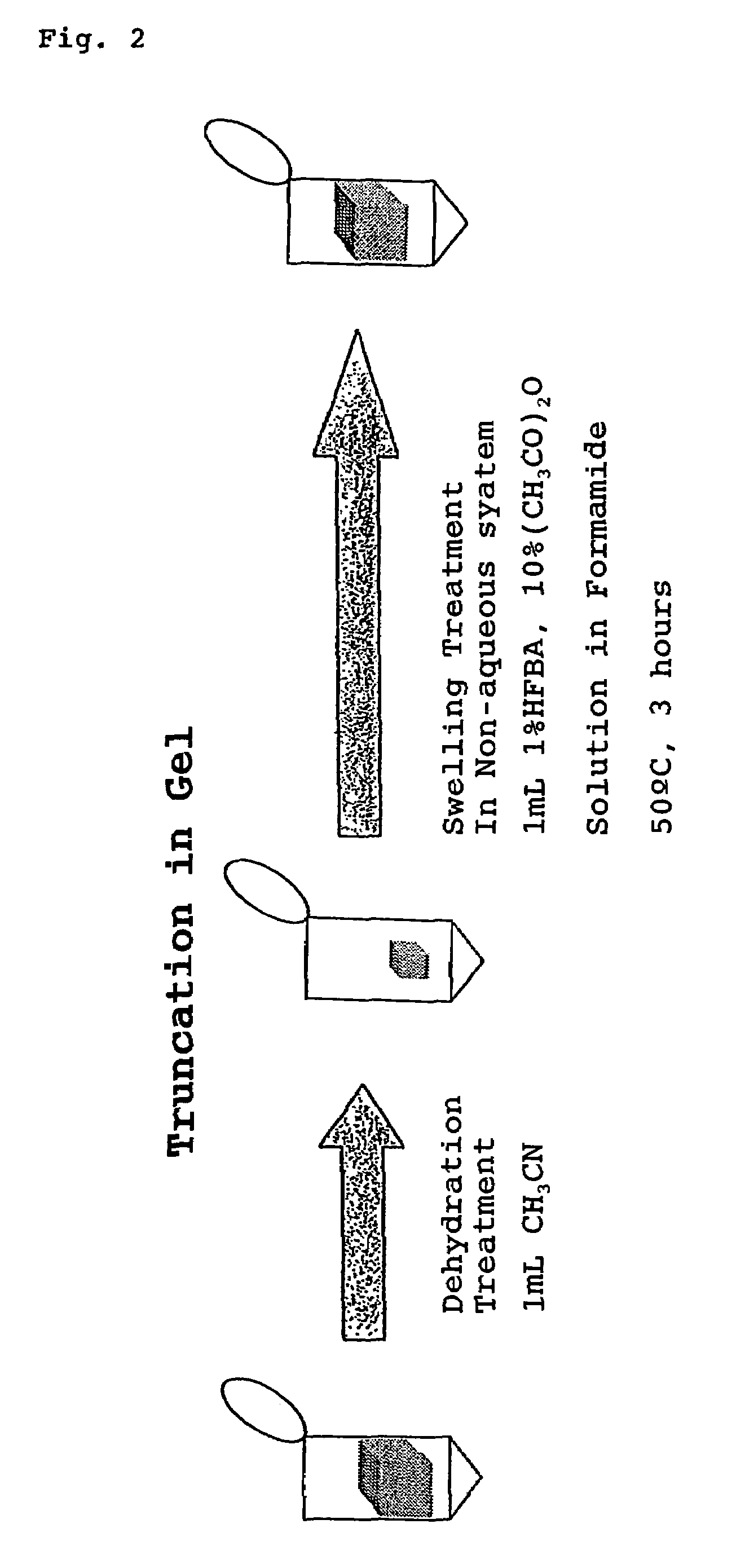
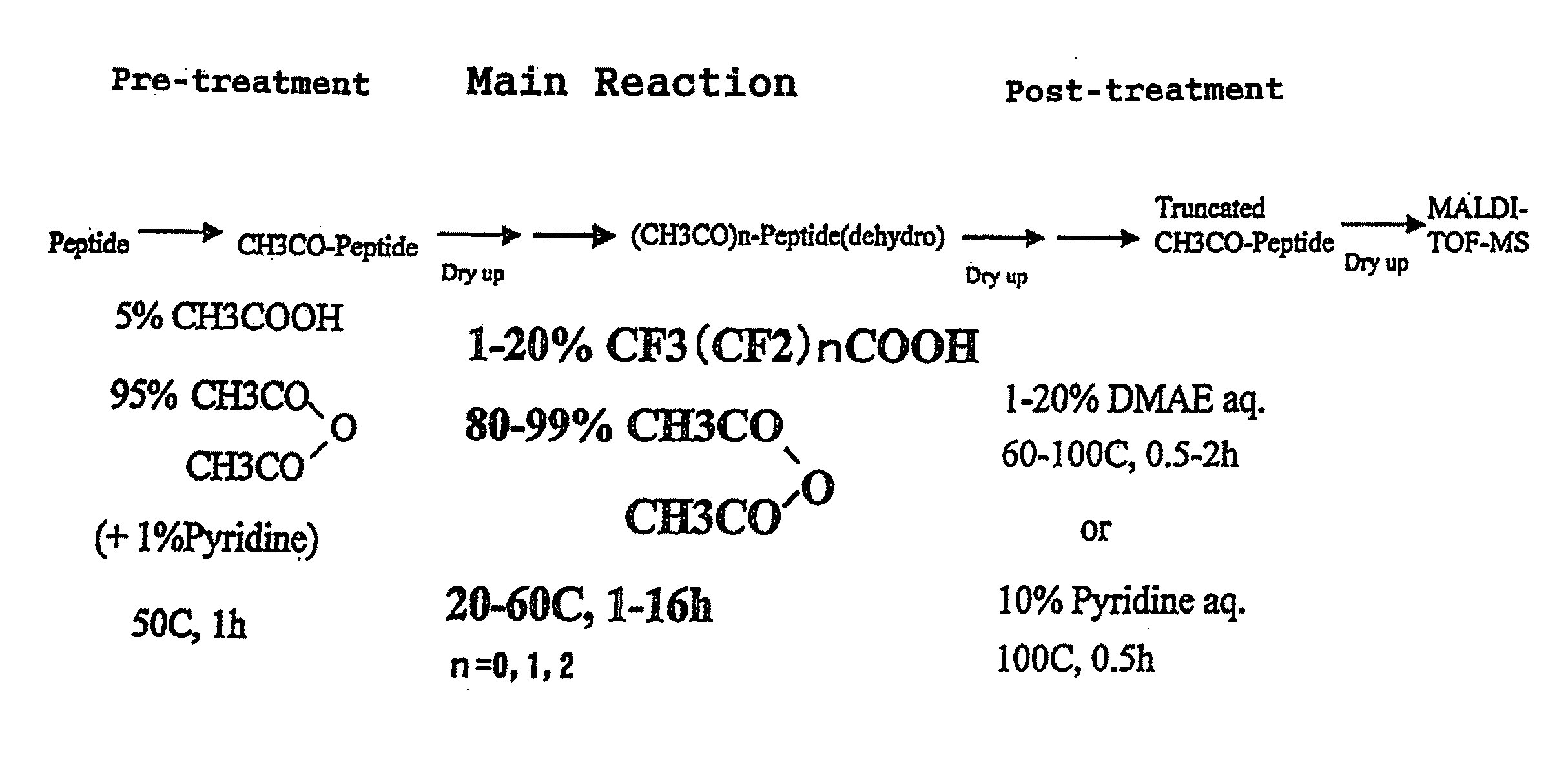
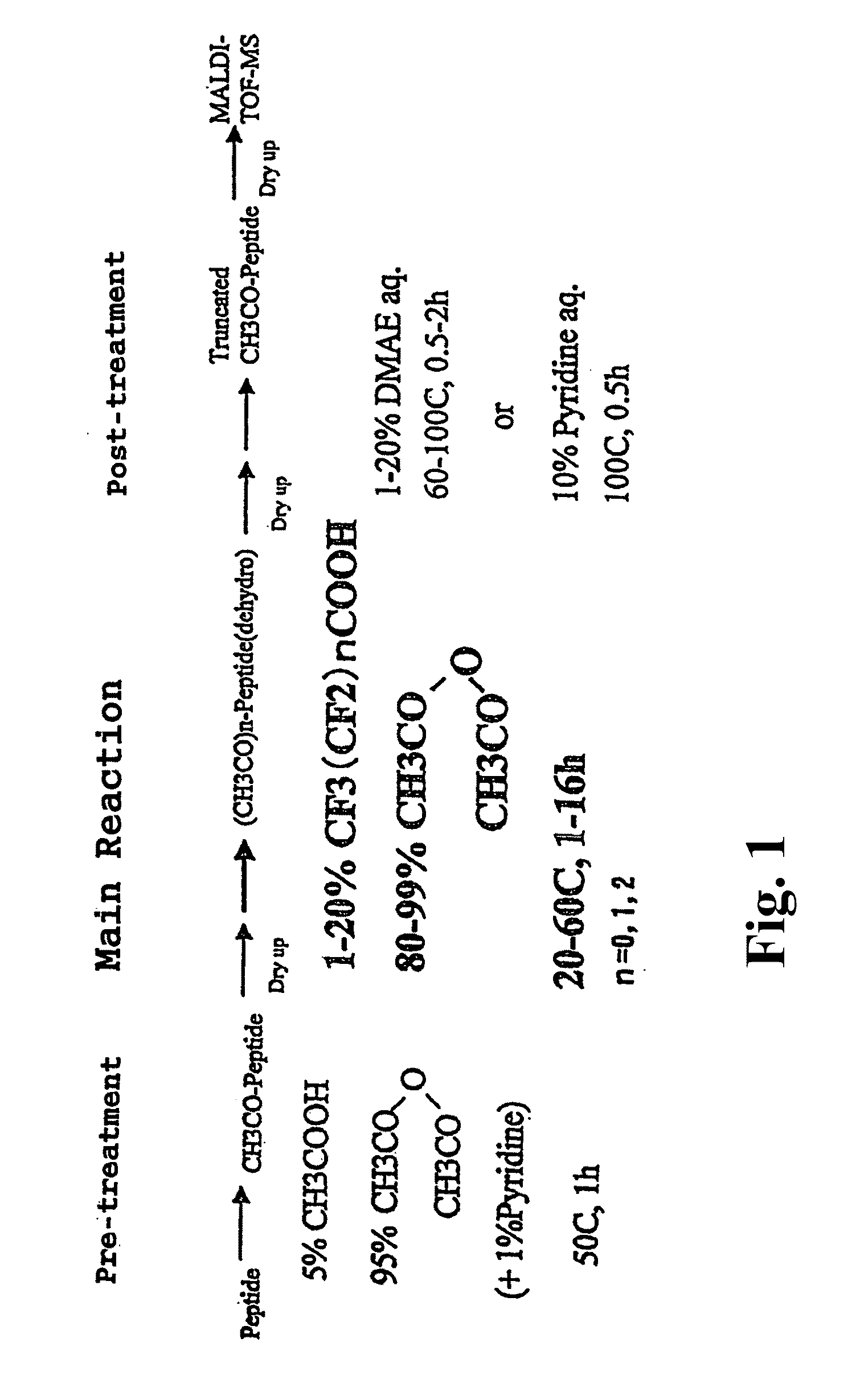
The present invention provides a method for analyzing the C-terminal amino acid sequence of a peptide by using a reaction for successively releasing the C-terminal amino acids of the peptide, which method can suppress, when successively releasing the C-terminal amino acids of a peptide of long amino acid length, such a undesirable side reaction as cleavage of peptide bond in the intermediate position of the peptide and can carry out the chemical treatment thereof under widely applicable conditions; In the method, a dry sample of a peptide with long amino acid length is beforehand subjected to an N-acylation treatment; by using a reaction reagent where an alkanoic acid anhydride is combined with a small amount of a perfluoroalkanoic acid, successive release of C-terminal amino acids is conducted under mild conditions; a hydrolysis treatment is applied; then, selective fragmentization at site of arginine residue is performed by digestion by trypsin; thereafter, decreases in molecular weight are measured for the C-terminal side fragments derived from a series of reaction products with use of a MALDI-TOF-MS apparatus; thereby, the C-terminal amino acid sequence of the peptide sample is identified.
Owner:NEC CORP
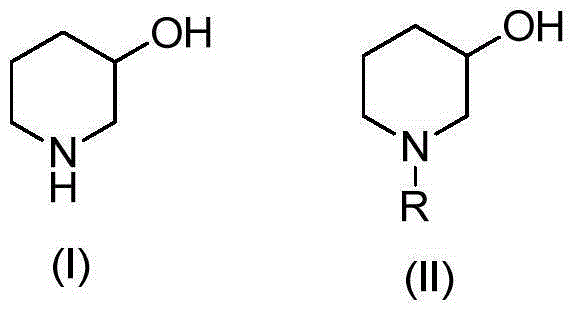
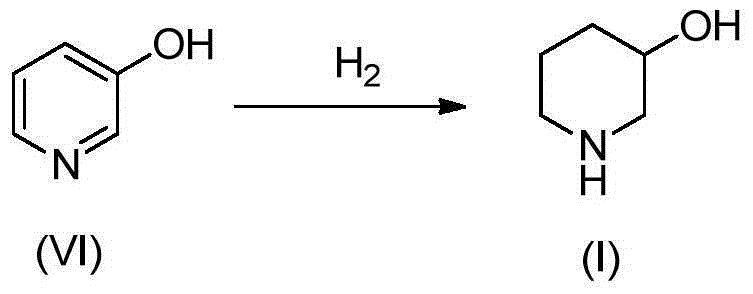
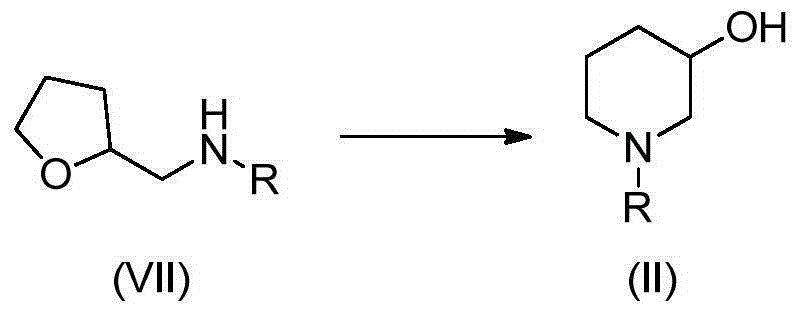
Preparation method of 3-hydroxypiperidine, preparation method of derivative of 3-hydroxypiperidine, and intermediate of 3-hydroxypiperidine
InactiveCN106432059AMild reaction conditionsReduce manufacturing costOrganic compound preparationBulk chemical productionOrganic solventHalogen
The invention discloses a preparation method of 3-hydroxypiperidine, a preparation method of a derivative of 3-hydroxypiperidine, and an intermediate of 3-hydroxypiperidine. The preparation method of 3-hydroxypiperidine (I) is characterized in that 5-halo-2-hydroxypentylamine halogen acid salt (III) undergoes a ring closure reaction in water under the action of an inorganic alkali to obtain the 3-hydroxypiperidine (I). The preparation method of N-protected 3-hydroxypiperidine (II) comprises the following steps: 1, 5-halo-2-hydroxypentylamine halogen acid salt (III) undergoes the ring closure reaction in water under the action of the inorganic alkali to obtain the 3-hydroxypiperidine (I); and 2, the 3-hydroxypiperidine (I) and a nitrogen protection reagent undergo an N-acylation reaction in an organic solvent under the action of the inorganic alkali to obtain the N-protected 3-hydroxypiperidine (II). The preparation methods have the advantages of simple operation, no expensive catalysts, low production cost, easily available raw materials, simplicity in operation, high reaction conversion rate, high selectivity, simple process, and suitableness for industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
Bisamide type ultra-long-life room-temperature phosphorescent compound as well as preparation method and application thereof
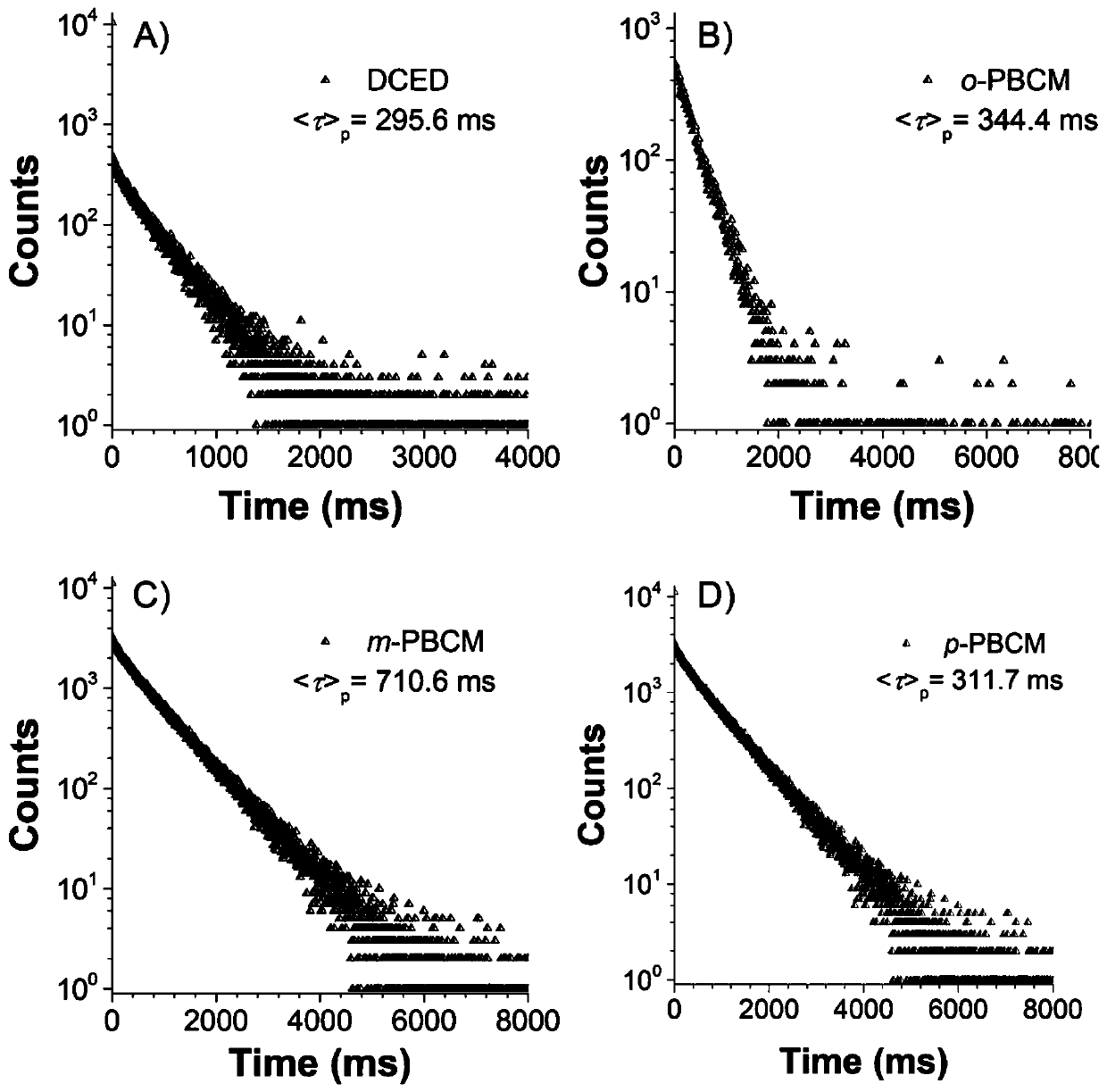
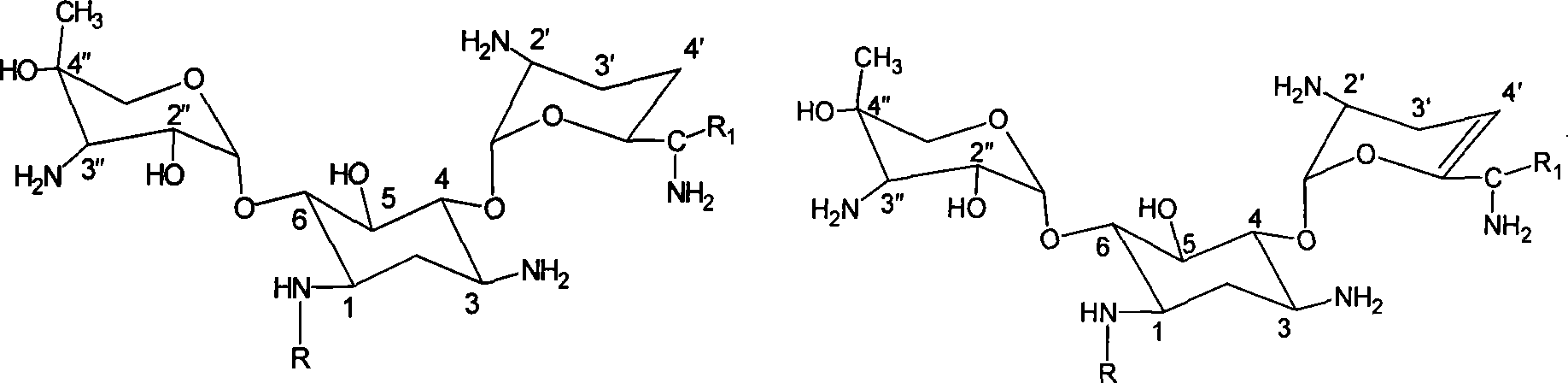
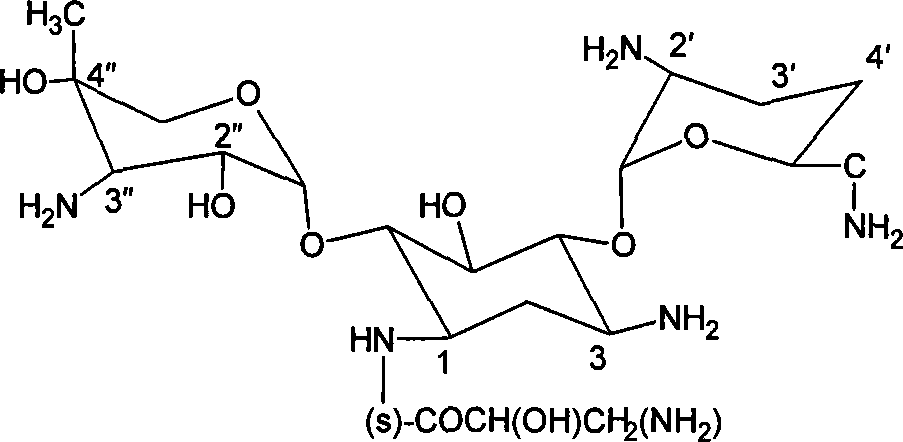
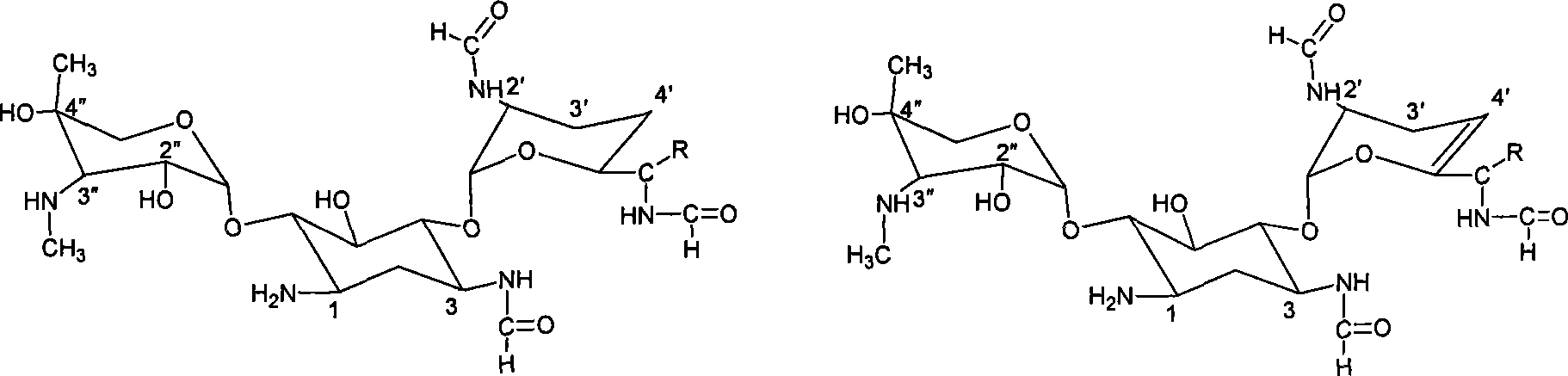
ActiveCN109879795AEnhanced spin-orbit couplingThe synthesis steps are simpleOrganic chemistryFluorescence/phosphorescenceN acylationSingle crystal
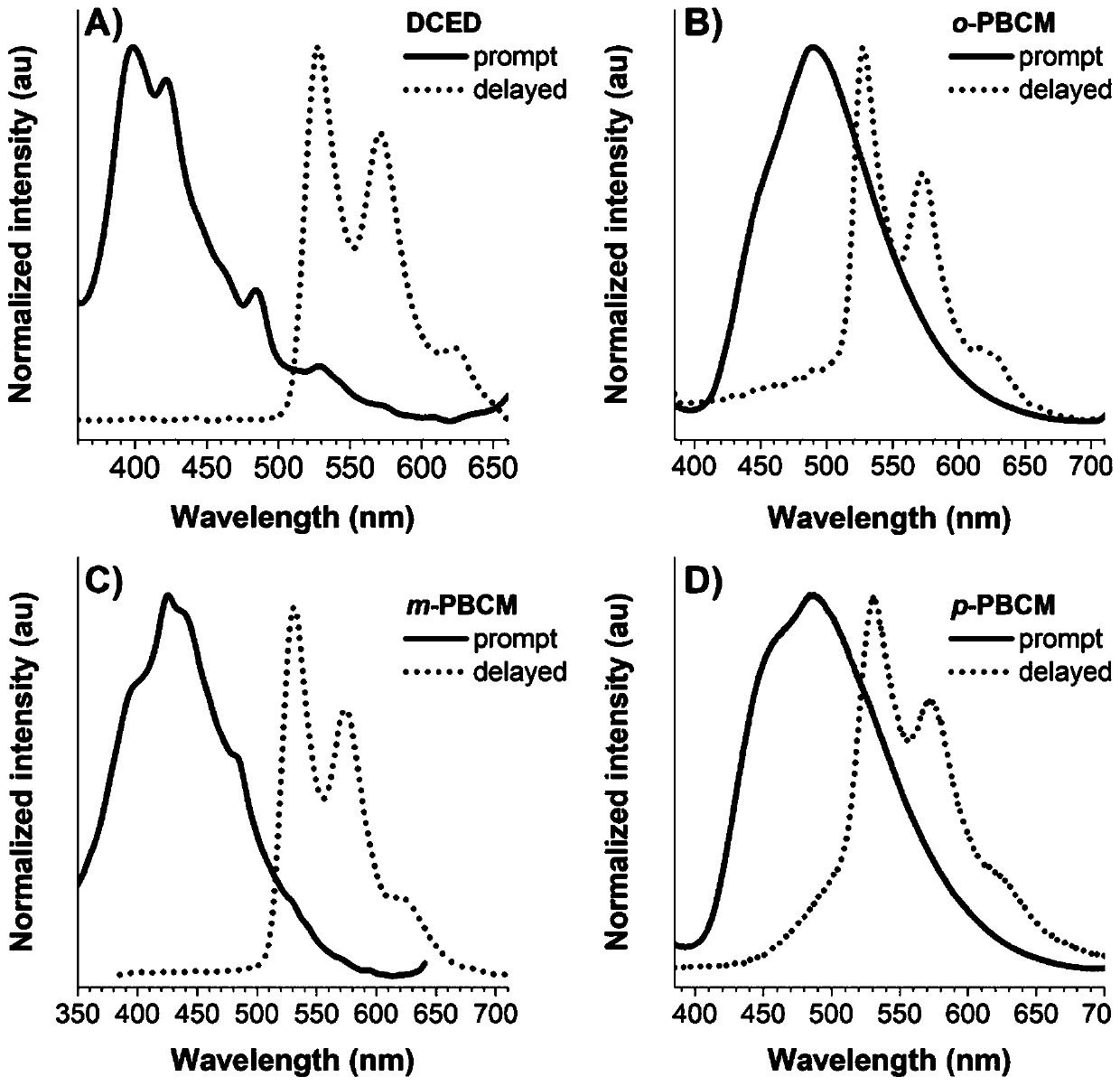
The invention relates to a bisamide type ultra-long-life room-temperature phosphorescent compound and a preparation method and application thereof. Four pure organic room-temperature phosphorescent molecules with ultra-long service life are prepared by one-step reaction of N acylation of carbazole and acyl chloride, and thus four compounds, namely, DCED, o-PBCM, m-PBCM and p-PBCM are obtained, andsingle crystals of three compounds DCED, o-PBCM and p-PBCM and floccules of m-PBCM after recrystallization are obtained by using a recrystallization method in an ethanol solution. Compared with the prior art, the four substances obtained by the preparation method have ultra-long service life and room-temperature phosphorescent properties, and particularly, the phosphorescent service life of the compound m-PBCM obtained by the preparation method at room temperature is up to 710.6 ms. When the compound m-PBCM is applied to the field of biology, an m-PBCM nanoparticle solution is injected to theright back of a nude mouse for afterglow imaging, and the signal-to-noise ratio of the m-PBCM nanoparticle solution reaches up to 428; after being injected into a forepaw of a nude mouse, the m-PBCMnanoparticle solution can also be used as an effective afterglow contrast agent to accurately illuminate axillary lymph nodes with excellent sensitivity and signal-to-noise ratio.
Owner:SHANGHAI JIAO TONG UNIV +1
Process for the synthesis of highly pure cationic surfactant products
The present invention is related to synthesis of highly pure cationic surfactant products by eliminating or reducing impurities generation that has beset prior art. This is achieved through the N-acylation of ester of amino acid and its inorganic salts or its organic salts (e.g. amino acid or hydrochloride of amino acid or sulfate of amino acid or acetate of amino acid etc.) in non-hydrolytic or nearly non-hydrolytic reaction conditions involving mono or biphasic reaction system with fatty acid halide (C4 to C20), under moderate uniform basic condition yielding high purity N-acyl substituted amino acid ester, particularly ethyl lauroyl arginate. The present process achieves pH control through process strategy rather than the measurement and control steps. This ambient temperature process is stable through a range of temperature variation eliminating rigid low temperature control.
Owner:ORGANISTRY
Aminoglycoside antibiotics
InactiveCN101139371ASame structural featuresSignificant anti-MRSA activityAminosugarsMedicineResistant virus
The present invention relate4s to an aminoglycoside antibiotic which belongs to the application technology filed of aminoglycoside antibiotic. The present invention provides the derivative of the structure of 2'-NH 2 -3 ', 4'-deoxy-3'-N demethylase 1-N acylation as well as the application of the derivative in the preparation of medicinal combination in the treatment of drug-resistant virus infections.
Owner:CHANGZHOU FANGYUAN PHARMA
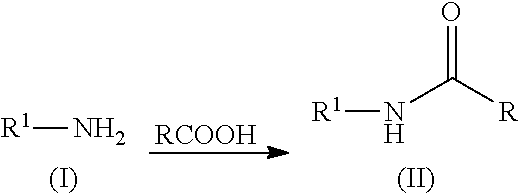
N-acylation of amines
ActiveUS8865920B2Organic compound preparationCarboxylic acid amides preparationN acylationCarboxylic acid
Provided herein are processes for the preparation of N-acylated amines. In particular, the processes comprise contacting an amine with an acid comprising a carboxylic acid group to form the N-acylated amine.
Owner:NOVUS INT INC
Set of intermediate compounds used for synthesis of Ivabradine, and applications thereof
ActiveCN104829470ALow priceReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationBiochemical engineeringN acylation
The invention provides a set of intermediate compounds used for synthesis of Ivabradine and a preparation method thereof, and also provides a method used for synthesizing Ivabradine from the intermediate compounds. According to the method, the set of intermediate compounds are subjected to a plurality of N alkylation or N acylation so as to obtain a compound IV; and Ivabradine is directly synthesized from the compound IV. The method is short in synthesis route; raw materials are simple and easily available; reaction sequence is reasonable; operation is simple and high in efficiency; the method is green and friendly to the environment; synthesis difficulty of Ivabradine is reduced greatly; cost is low; product yield is high; and the method is suitable for industrialization production.
Owner:江苏宇田医药有限公司
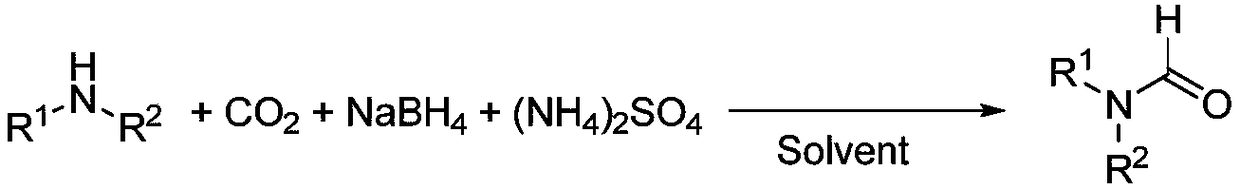
N-formylation synthesis method taking CO2 as carbon source under mild condition
ActiveCN108623493AStable in natureReduce usageOrganic compound preparationCarboxylic acid amide separation/purificationSynthesis methodsFormylation reaction
The invention provides an N-formylation synthesis method taking CO2 as a carbon source under a mild condition and belongs to the technical field of chemistry and chemical engineering. Under normal-temperature and normal-pressure conditions and in a solvent with a low boiling point, CO2 is used as a carbon source to realize N-formylation reaction of various amine type substrates. The N-formylationsynthesis method provided by the invention has the advantages that a reaction system takes sodium borohydride and ammonium sulfate as reaction reagents, and the CO2 is subjected to reduction under normal pressure to provide acyl, so that high-pressure hydrogen gas and toxic CO are not used and reaction conditions are mild; the sodium borohydride and the ammonium sulfate are cheap and easy to obtain and the economic applicability is high; a reagent is stable in the air and is convenient to operate; the organic solvent with the low boiling point is used and is easy to remove and a product is convenient to separate. A method for preparing formamide, provided by the invention, takes greenhouse gas, i.e., carbon dioxide, as a carbon source and has the advantages of relatively low cost, simplicity in operation and mild reaction conditions; the yield of the prepared formamide product is good and a green synthesis method is provided for N-acylation reaction.
Owner:DALIAN UNIV OF TECH
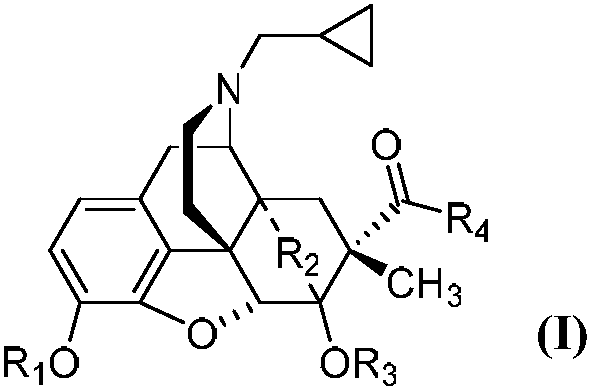
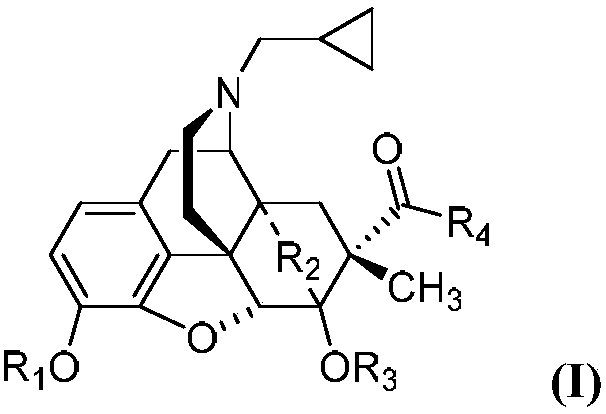
7Beta-methyl-tetralone derivative and preparation method and application thereof
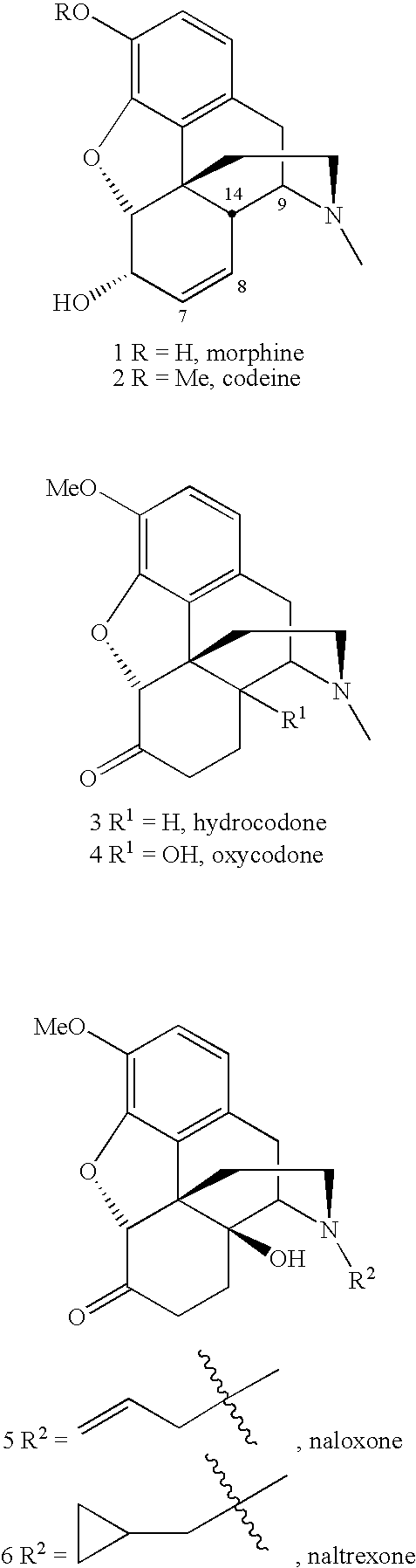
The invention belongs to the field of chemical pharmacy and relates to a 7beta-methyl-tetralone derivative shown in a general formula (I) and a preparation method thereof. According to the compound shown in the general formula (I), with thebaine as a raw material, through related conversion methods such as N-demethylation, N-acylation, a Diels-Alder reaction and reduction, oxidation and Grignard reactions, the compound is synthesized. The compound shown in the general formula (I) belongs to an opioid receptor ligand, a radioactive receptor ligand combination experiment is adopted, the affinityand selectivity of the ligand to three subtypes of an opioid receptor are measured, and a [35S]GTPgammaS combination experiment is adopted for measuring the excitement inhibitory activity of the ligand; it is proved through a result that the compound has the functions of alleviating pain, resisting depression, withdrawing opioid addiction, relieving itching and the like and can be applied to preparation of an opioid receptor treatment medicine and applied to clinical analgesia or depression resistance or opioid addiction withdrawal treatment or itching relieving treatment.
Owner:FUDAN UNIV
Green and environment-friendly method for preparing alkaline water-soluble chitosan derivative
The invention discloses a green and environment-friendly method for preparing an alkaline water-soluble chitosan derivative, and belongs to the technical field of macromolecular synthesis. The method comprises the steps that 1, a chitosan grafted polymer is prepared, wherein chitosan macromolecules are mixed with vinyl monomers with hydrophilic groups, degassing is conducted, an initiator is added, degassing is conducted continuously, the reaction is stopped, sedimentation and filtration are conducted, washing separation and purification are conducted, drying and grinding are conducted, and the chitosan grafted polymer powder is obtained; 2, N-acylation chitosan grafted polymer is obtained, wherein the chitosan grafted polymer powder is completely dissolved in water and added to an acetic acid solution, 1,2-propylene glycol is added, standing and degassing are conducted at room temperature for one day, an N-acylation solution is added to a reaction mixture, and the pH is adjusted to 7-13, evaporative crystallization is conducted, and the alkaline water-soluble chitosan derivative is obtained. The green and environment-friendly method for preparing the alkaline water-soluble chitosan derivative is friendly to environment, the chitosan derivative which is mild in reaction condition, large in molecular weight and good in water solubility and basically accords with industrial application, water solubility can be achieved within the pH range of 7-11, and the solubility reaches up to 10 mg / mL.
Owner:SOUTHEAST UNIV
Separation and recovery technology for yolk and egg white of crushed duck eggs
InactiveCN109287840AScientific separation and recyclingReasonable separation and recoveryProtein composition from eggsFiberYolk
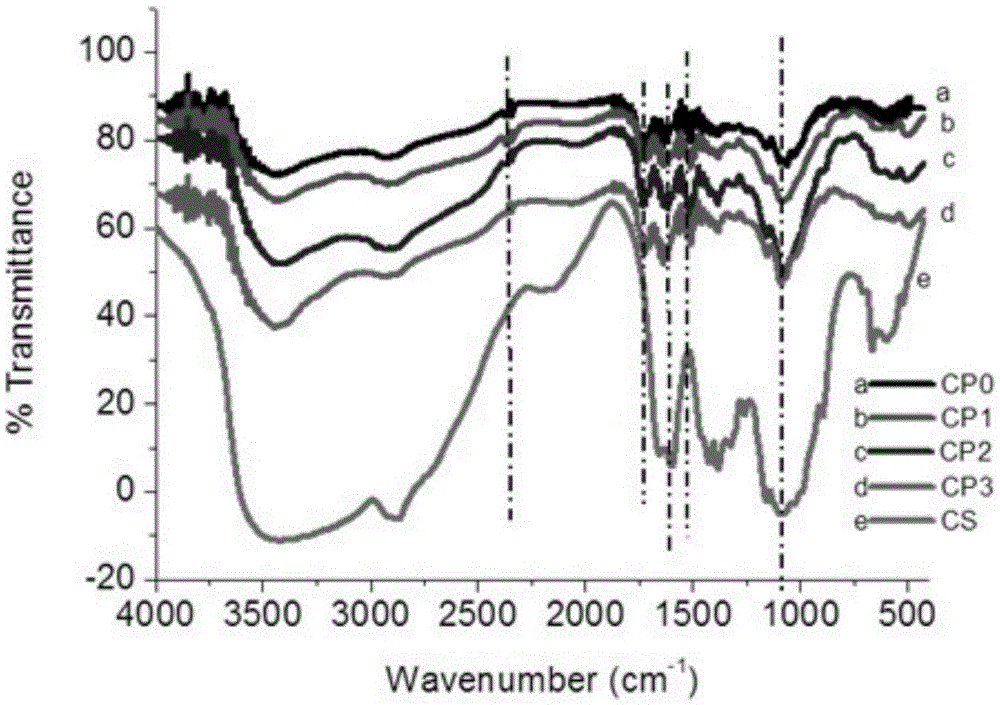
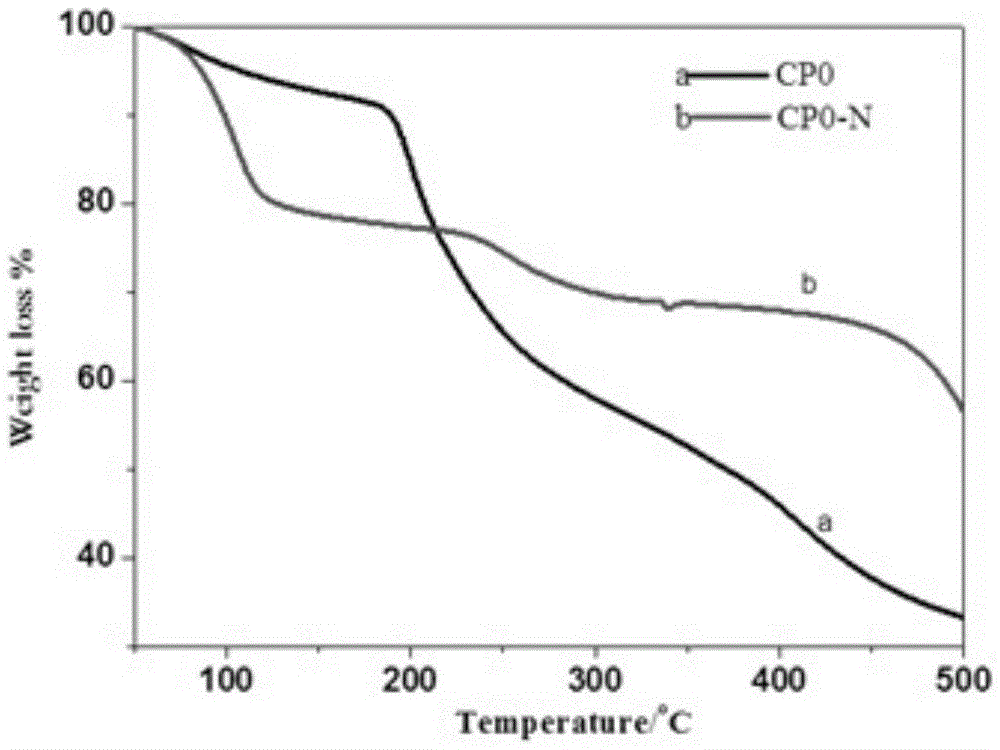
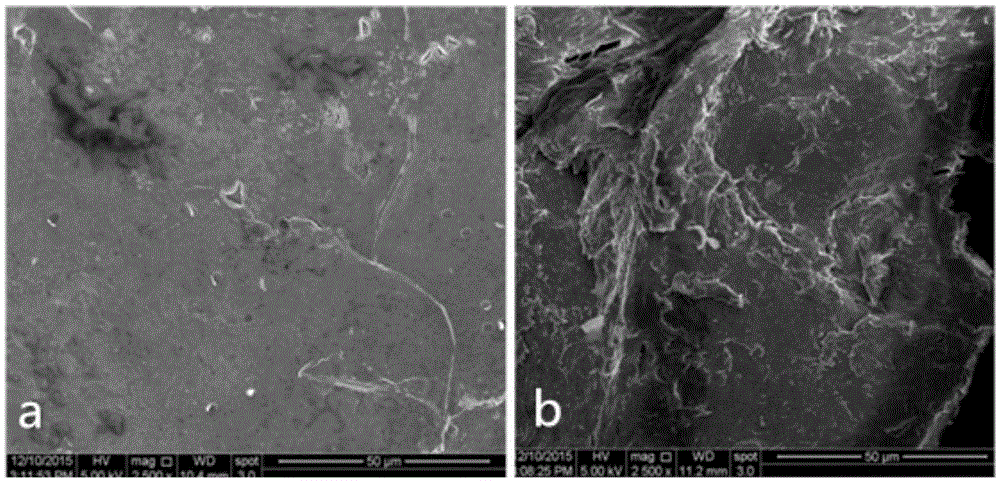
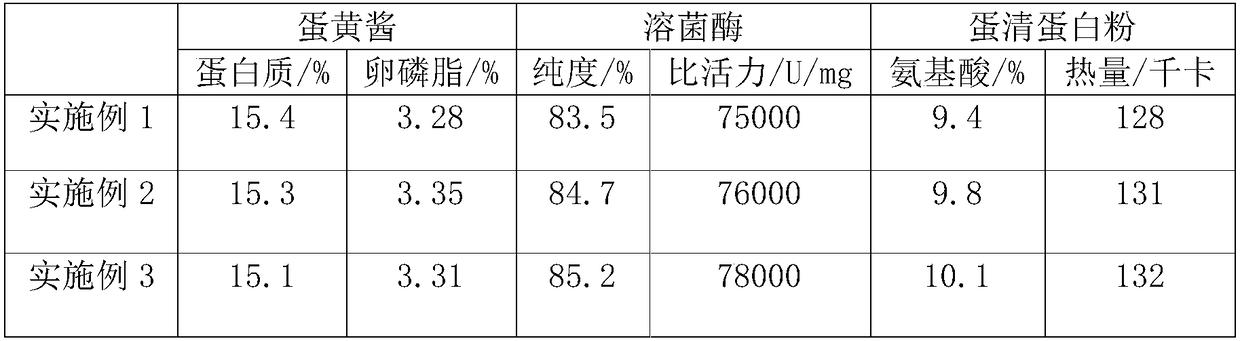
The invention discloses a separation and recovery technology for yolk and egg white of crushed duck eggs. The separation and recovery technology comprises the following steps of S1, separating egg white from yolk under a high voltage pulse assisted electric field; S2, adding dihydromyricetin to the yolk, performing thermocoagulation, grinding and emulsifying, and then performing uniform mixing andhomogenization with lemon juice and white sugar so as to obtain mayonnaise; S3, diluting the egg white, adjusting PH with edible acid to 4-6.5, and performing precipitation, centrifuging and filtration to obtain supernatant; S4, performing hollow fiber ultrafiltration treatment on the supernatant, performing infiltration with 2-6wt% N-acylation chitosan gel, performing affinity chromatography atthe flow velocity of 1.5-2ml / cm<2>, performing eluting, performing concentration, and performing drying so as to obtain purified lysozyme agents; and S5, adding pineapple protease and mint juice to egg white flocculate, performing enzymolysis at 55 DEG C for 2h, and performing vacuum freeze drying so as to obtain egg white and protein powder. According to the separation and recovery technology disclosed by the invention, the yolk and the egg white of the crushed duck eggs are efficiently recycled, economic losses caused by egg liquid deterioration can be avoided, and a new way is opened up forprocessing and utilizing of crushed eggs in poultry egg industry.
Owner:安徽靳氏食品有限公司
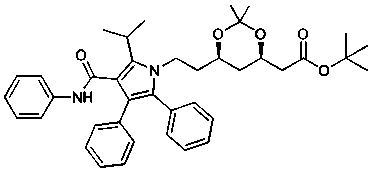
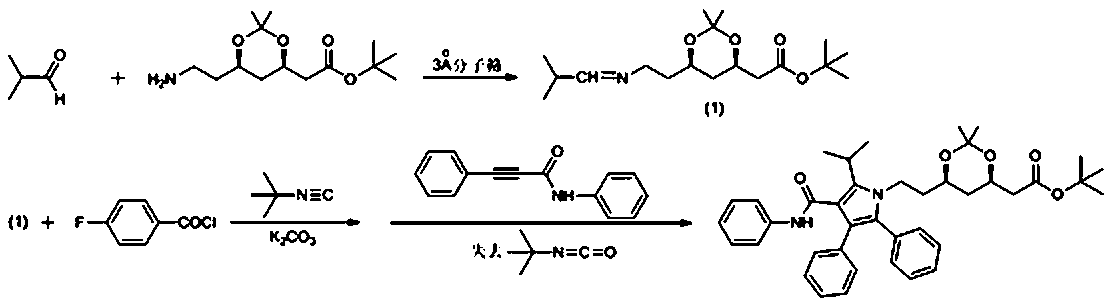
Method for preparing atorvastatin calcium intermediate
The invention discloses a method for preparing an atorvastatin calcium intermediate, and belongs to the technical field of synthesis of medicinal intermediates. The problems that an existing synthesismethod of the atorvastatin calcium intermediate is long in synthesis line and complicated in operation and raw materials are expensive can be solved. By adoption of a 'one-pot' method, firstly, isobutyraldehyde and ATS-9 are condensed to generate imine, and the imine and fluorobenzoyl chloride generate N-acylation reaction and then react with tertiary butyl isonitrile; and finally, the product and 3-phenyl propylene aniline generate 1,3-dipolar cycloaddition-removal reaction to generate a target (4R-cis)-6-[2-[2-(4-fluorinated phenyl)-5-(1-isopropyl)-3-phenyl-4-[( anilino) carboxyl]-1H-pyrrole-1-radical]-ethyl]-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate. The yield of the final product obtained by the synthesis method disclosed by the invention is as high as 72 to 75 percent.
Owner:SHANXI DATONG UNIV
Method of analyzing c-terminal amino acid sequence of peptide
InactiveUS7651859B2Inhibition is effectiveHigh possibility that undesired side reactions are advanced therebyPeptide/protein ingredientsMicrobiological testing/measurementChemical treatmentArginine
Owner:NEC CORP
Method for analyzing c-terminal amino acid sequence of peptide using mass spectrometry
InactiveUS20110183428A1Inhibition is effectiveHigh possibility that undesired side reactions are advanced therebyPeptide/protein ingredientsMicrobiological testing/measurementChemical treatmentArginine
The present invention provides a method for analyzing the C-terminal amino acid sequence of a peptide by using a reaction for successively releasing the C-terminal amino acids of the peptide, which method can suppress, when successively releasing the C-terminal amino acids of a peptide of long amino acid length, such a undesirable side reaction as cleavage of peptide bond in the intermediate position of the peptide and can carry out the chemical treatment thereof under widely applicable conditions; In the method, a dry sample of a peptide with long amino acid length is beforehand subjected to an N-acylation treatment; by using a reaction reagent where an alkanoic acid anhydride is combined with a small amount of a perfluoroalkanoic acid, successive release of C-terminal amino acids is conducted under mild conditions; a hydrolysis treatment is applied; then, selective fragmentization at site of arginine residue is performed by digestion by trypsin; thereafter, decreases in molecular weight are measured for the C-terminal side fragments derived from a series of reaction products with use of a MALDI-TOF-MS apparatus; thereby, the C-terminal amino acid sequence of the peptide sample is identified.
Owner:NEC CORP
Temperature control-type biology-based facial mask
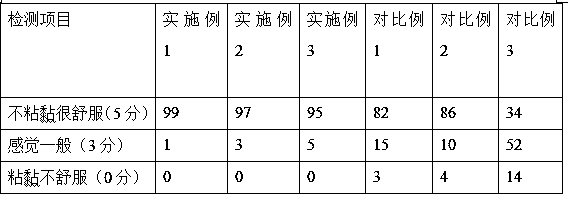
InactiveCN108635284AEasy to captureReduce churnCosmetic preparationsToilet preparationsCross-linkN acylation
The invention discloses a temperature control-type biology-based facial mask and belongs to the field of skin nursing products. Pretreatment is conducted on chitosan to activate functional groups of chitosan,nano TiO2 is adopted as a phase-change accelerant,lauric acid serves as a grafting monomer,and a copolymer is prepared through an N-acylation reaction of chitosan; the moistening degree and nutrient preservation capability of a system can be well improved,chemical bonding with degradative gelatin can be achieved,a three-dimensional cross-linked network with high molecular weight can be formed on the face during use,loss of nutrient components is reduced,and the nutrient components can be better caught by means of a bridging function in a nutrient solution and stably and comfortably acton the face. Added birch juice is abundant in different kinds of essential fructose,amino acid,vitamins,biotin and mineral substances for the human body,has the efficacy of resisting fatigue and aging and achieves a great moisture preservation effect,and the efficacy of tightening the skin and improving the state of skin around pores is achieved; moreover,users cannot have a sticky feeling afterusing the facial mask,and the facial mask is high in comfort degree. The problems that the sticky feeling is strong after existing frequently-used facial masks are used and the comfort degree is low are solved.
Owner:FOSHAN SENANG BIO TECH CO LTD
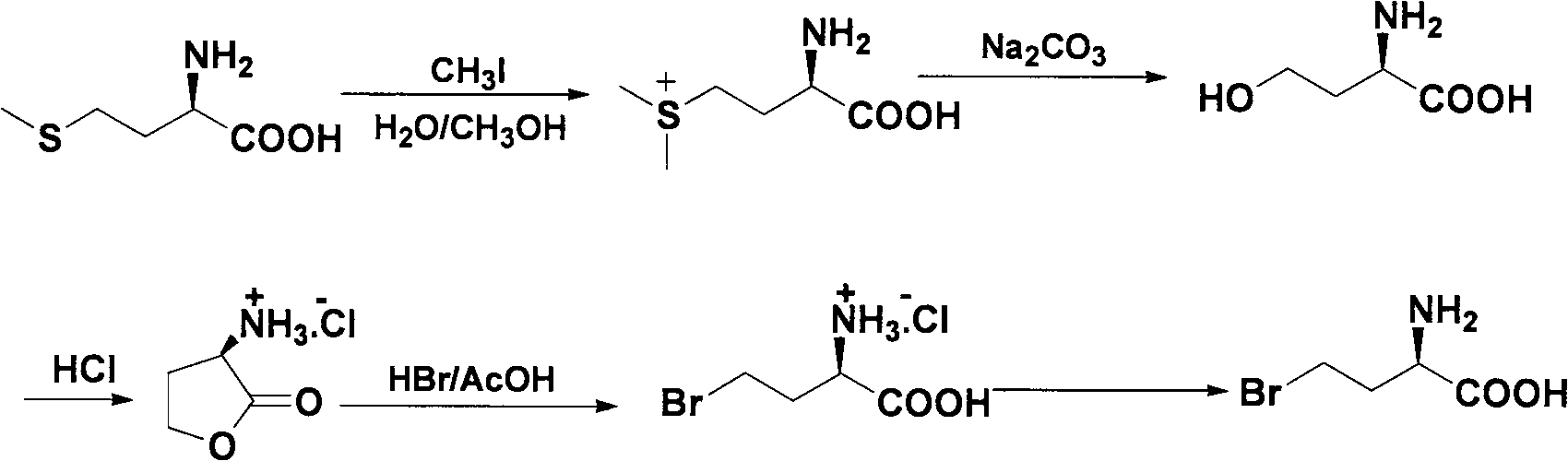
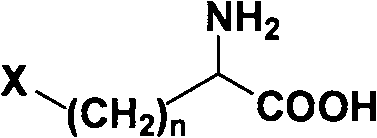
Method for splitting halogenated alpha-amino acid
The invention relates to a method for splitting halogenated alpha-amino acid. According to the invention, after N-acylation of halogenated alpha-amino acid, an acyl group of N-acylated alpha-amino acid with one structure is highly selectively hydrolyzed through selection of a proper enzyme, while a counterpart of the N-acylated alpha-amino acid with the structure does not undergo hydrolysis of acyl group; then separation is carried out based on difference between halogenated alpha-amino acid and N-acyl halogenated alpha-amino acid to obtain optically pure halogenated alpha-amino acid; and optically pure N-acyl halogenated alpha-amino acid undergoes chemical hydrolysis so as to obtain corresponding optically pure halogenated alpha-amino acid. The method provided by the invention has the advantages of stability, environment-friendliness, safety, mild production conditions, capacity of obtaining a product with optical purity as high as 99.9% and low production cost. The method provides a novel approach for industrial production of halogenated alpha-amino acid with high optical purity.
Owner:ZHANGJIAGANG JIUMU TECH +1
Resolution method of beta-amino acid
The beta-amino acid resolving process includes the first N acylation of amino acid; hydrolyzing the acyl radical of the N acylated beta-amino acid in some one configuration in high selectivity with properly selected enzyme while not affecting its antimer and extracting according to the difference of different matters in water solubility and fat solubility to obtain one kind of corresponding optically pure beta-amino acid; and hydrolyzing the acyl radical of the N acylated beta-amino acid in one other configuration with one other kind of selected enzyme to obtain one other kind of optically pure beta-amino acid similarly. The said process is stable, environment friendly, safe, low in cost and with mild production conditions, and the product reach optical purity of 99.9 wt%. The present invention provides one new way for producing optically pure beta-amino acid.
Owner:ASTATECH CHENGDU BIOPHARM CORP
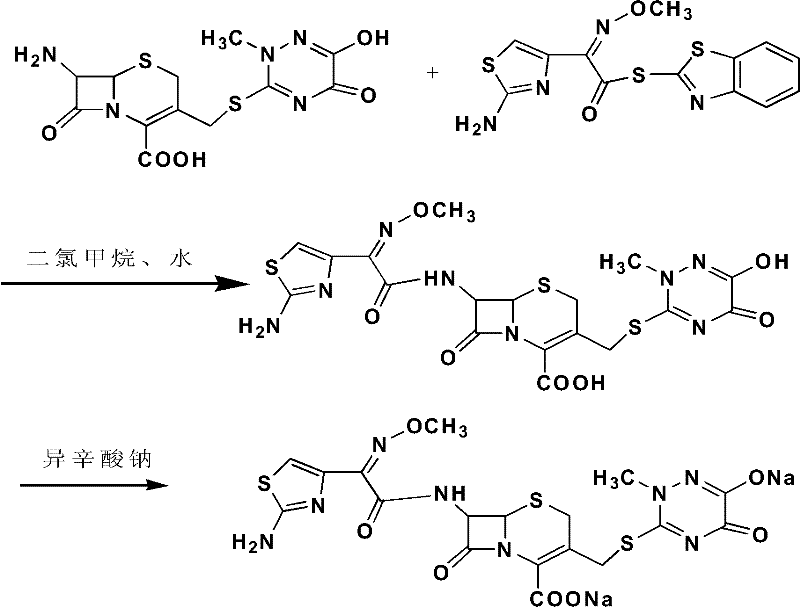
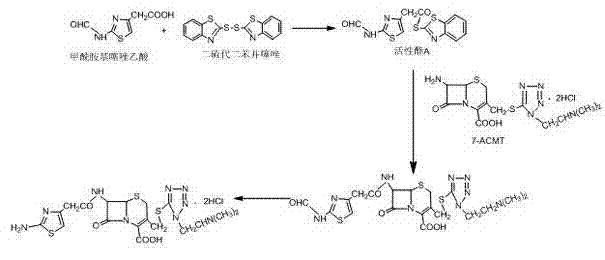
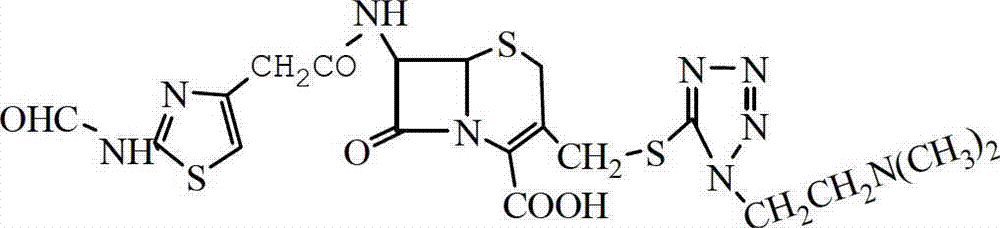
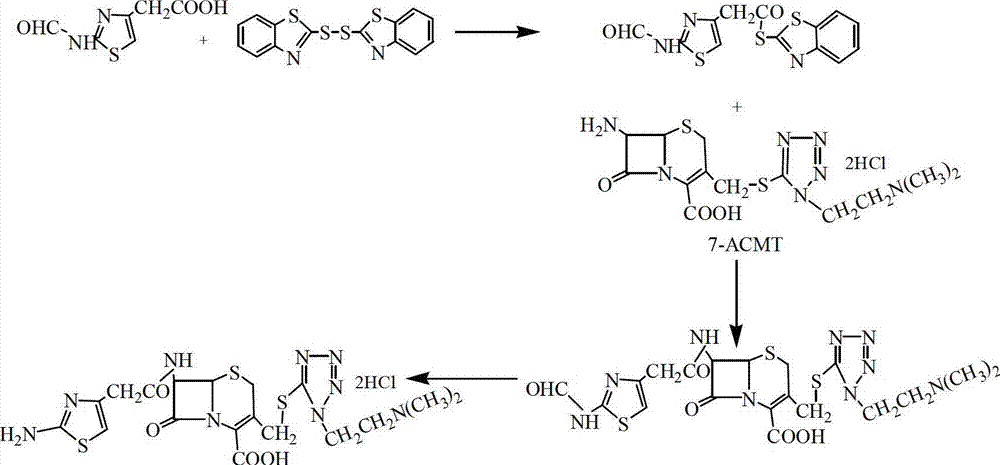
Preparation method of cefotiam hydrochloride
The invention belongs to the technical field of antibiotic drug synthesis, and particularly relates to a preparation method of cefotiam hydrochloride. The preparation method comprises the following steps of: adding formamido thiazole acetic acid to an organic solvent, adding base and dithio-bisbenzothiazole, stirring, then adding a catalyst, and carrying out condensation reaction, thus obtaining an active ester A, wherein the catalyst is triethyl phosphate; dissolving 7-ACMT in an alkali liquor of the organic solvent, adding the active ester A for carrying out N acylation reaction, adding a water solution after reaction is ended, getting a water layer, adding hydrochloric acid for hydrolysis, and dropwise adding a hydrophilic solvent to separate out the cefotiam hydrochloride. The preparation method has the advantages that reaction conditions are mild, strict anhydrous and anaerobic conditions are not needed, the cost is low, the synthesis process is simple, and the purity and the yield are high.
Owner:YIYUAN XINQUAN CHEM
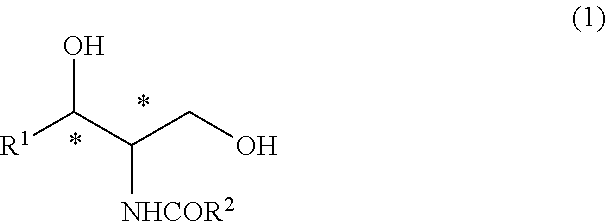
Method for producing high-purity ceramide
ActiveUS9079826B2High yieldHigh purityOrganic compound preparationOrganic chemistry methodsHydrocarbon solventsAlcohol
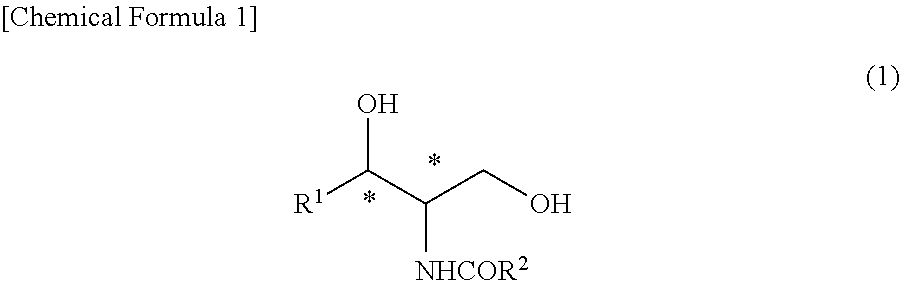
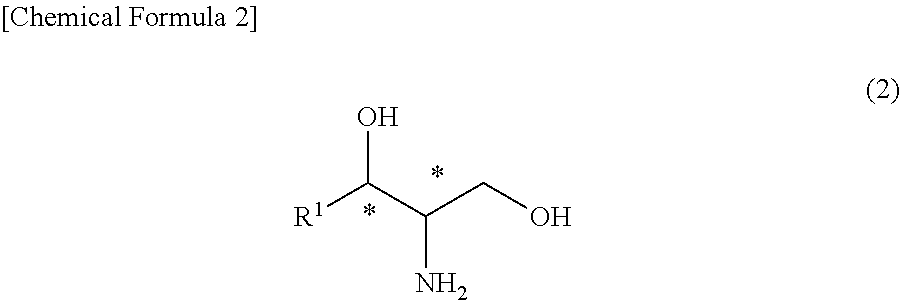
Provided is a method for producing an optically active ceramide by an N-acylation (amidation) reaction of an optically active aminodiol, wherein a crude ceramide produced therein is purified by an industrially advantageous process. Namely, provided is a method for producing a high-purity ceramide that has high diastereo purity with high yield. A high-purity ceramide is produced by: a step wherein a ceramide represented by general formula (1) is produced by reacting an aminodiol with an alkyl ester having 1-5 carbon atoms of an aliphatic carboxylic acid having 12-24 carbon atoms, said aliphatic carboxylic acid optionally having a hydroxyl group, in a hydrocarbon solvent having 5-10 carbon atoms; and a step wherein an alcohol having 1-3 carbon atoms is added into the reaction mixture obtained in the preceding step, thereby causing crystals to precipitate.(In the formula, R1 represents an alkyl group which has 13-17 carbon atoms and optionally has a carbon-carbon unsaturated bond; R2 represents an alkyl group which has 11-23 carbon atoms and optionally has a hydroxyl group; and * represents an optically active state.).
Owner:TAKASAGO INTERNATIONAL CORPORATION
N-acylation of amines
ActiveUS20130012728A1Organic compound preparationCarboxylic acid amides preparationN acylationCarboxylic acid
Provided herein are processes for the preparation of N-acylated amines. In particular, the processes comprise contacting an amine with an acid comprising a carboxylic acid group to form the N-acylated amine.
Owner:NOVUS INTERNATIONAL INC
Method for preparing beta-amino acid
The invention discloses a method for preparing beta-amino acid. The method comprises the following steps of: carrying out N acylation on amino acids, making acyl groups of N acylated beta-amino acids of one configuration to be hydrolyzed by enzymolysis to obtain corresponding beta-amino acids and not making N acylated beta-amino acids of a corresponding configuration to be hydrolyzed, separating the above amino acids according to their differences in fat water solubility properties by extraction, choosing another enzyme and hydrolyzing N acylated beta-amino acids of a corresponding configuration to obtain corresponding beta-amino acids, so as to obtain the beta-amino acid of a single configuration. The method provided by the invention is stable, environmentally friendly and safe, requires low cost, and has high optical purity. The invention also provides a new approach to producing optically-pure beta-amino acid.
Owner:SUNCHEM SHANGHAI CO LTD
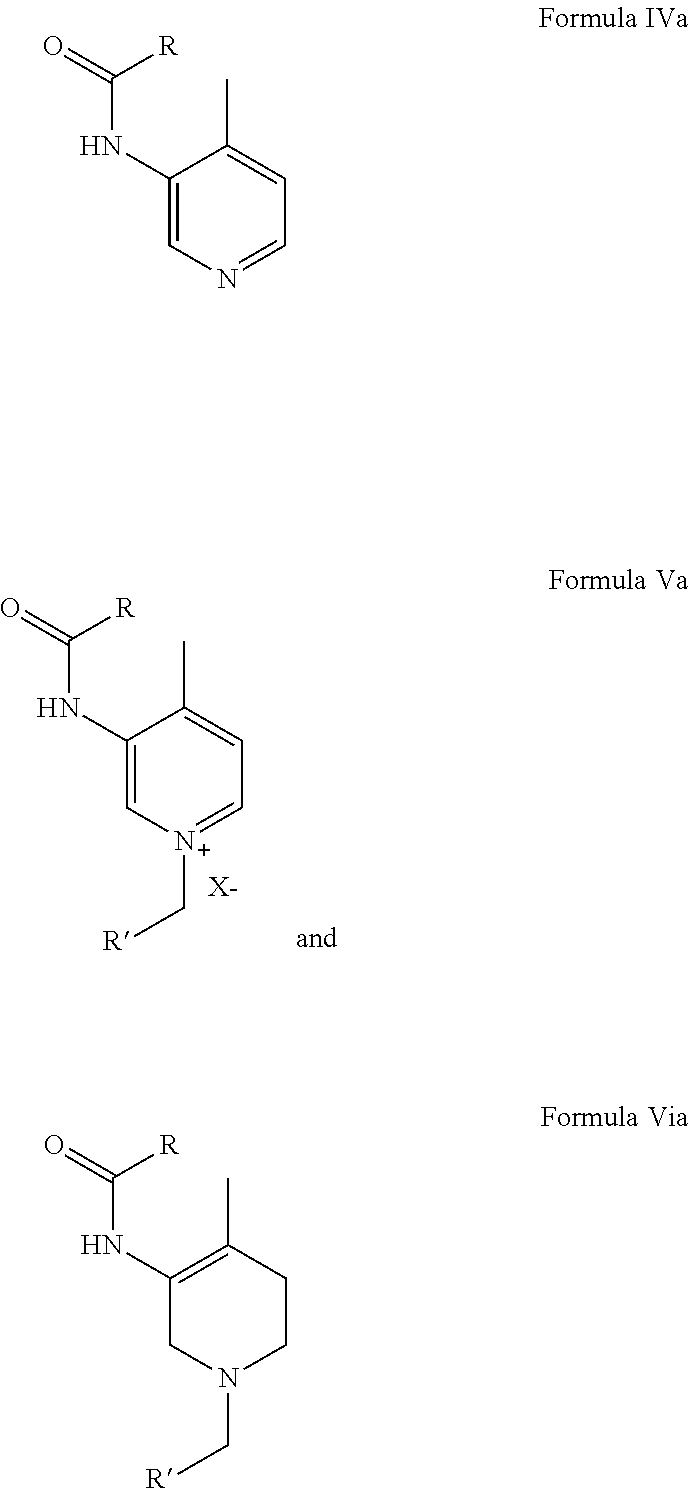
Process for the preparation of (3r,4r)-(1-benzyl-4-methylpiperidin-3-yl)-methylamine
The present disclosure is related to an improved and efficient process for preparation of (3R,4R)-(1-benzyl-4-methylpiperidin-3-yl)-methylamine which comprises:(a) N-acylation of 3-Amino-4-methyl pyridine; (b) Quarternization of 3-Acetylamino-4-methyl pyridine using benzyl halide; (c) Partial reduction of quarternized 3-Acetylamino-4-methyl pyridine by Sodium borohydride in Methanol or water; (d) Hydrolysis of partially reduced product to 1-Benzyl-4-methylpiperidin-3-one in presence of acid; (e) Reductive amination of 1-Benzyl-4-methylpiperidin-3-one using Methanolic methylamine in presence of Titanium(IV) isopropoxide in Methanol; (f) Resolution of 1-Benzyl-4-methylpiperidin-3-yl)-methylamine using Ditoluoyl (L) tartaric acid to get (3R,4R)-(1-benzyl-4-methylpiperidin-3-yl)-methylamine. The disclosure is also related to novel intermediates:wherein R, R′ and X are as described in the specification.
Owner:UNICHEM LAB LTD
Preparation method of infrared camouflage textile
ActiveCN108330677AEasy to operateGood combination fastnessVegetal fibresAluminum doped zinc oxideN acylation
The invention provides a preparation method of an infrared camouflage textile. The method comprises the following steps: firstly, introducing amino into the surface of aluminum-doped zinc oxide by utilizing low-temperature plasma; then enabling amino on the surface of the aluminum-doped zinc oxide and carboxyl on octacarboxylic metal phthalocyanine to be subjected to N-acylation reaction by utilizing reaction activity of carbodiimide, so as to prepare a phthalocyanine compound / aluminum-doped zinc oxide composite material; then treating the phthalocyanine compound / aluminum-doped zinc oxide composite material on a textile through a coating finishing manner. The textile has a relatively good camouflage effect on near-infrared and mid and far-infrared rays and has washing resistance.
Owner:CHANGZHOU UNIV
Resolution method of beta-amino acid
The beta-amino acid resolving process includes the first N acylation of amino acid; hydrolyzing the acyl radical of the N acylated beta-amino acid in some one configuration in high selectivity with properly selected enzyme while not affecting its antimer and extracting according to the difference of different matters in water solubility and fat solubility to obtain one kind of corresponding optically pure beta-amino acid; and hydrolyzing the acyl radical of the N acylated beta-amino acid in one other configuration with one other kind of selected enzyme to obtain one other kind of optically pure beta-amino acid similarly. The said process is stable, environment friendly, safe, low in cost and with mild production conditions, and the product reach optical purity of 99.9 wt%. The present invention provides one new way for producing optically pure beta-amino acid.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com