Method for preparing beta-amino acid
An amino acid and amino acid acylase technology, applied in the field of preparing beta-amino acids, can solve problems such as undeveloped, and achieve the effects of stable method, high optical purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Preferred embodiments of the present invention will be described in detail below in conjunction with the accompanying drawings.
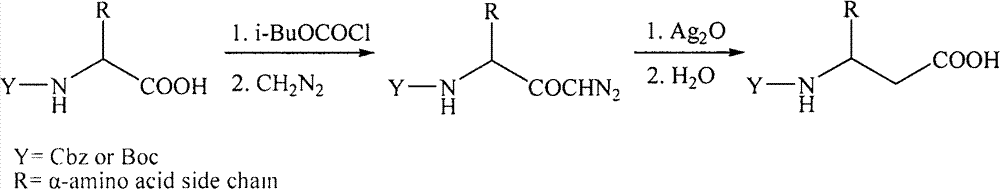
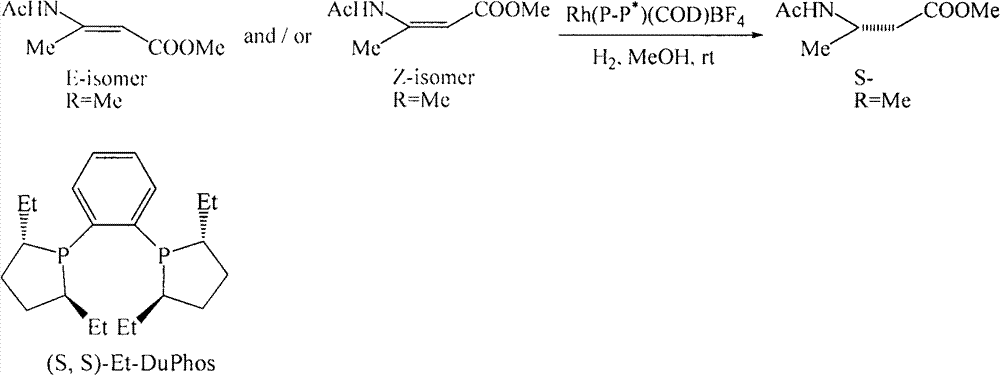
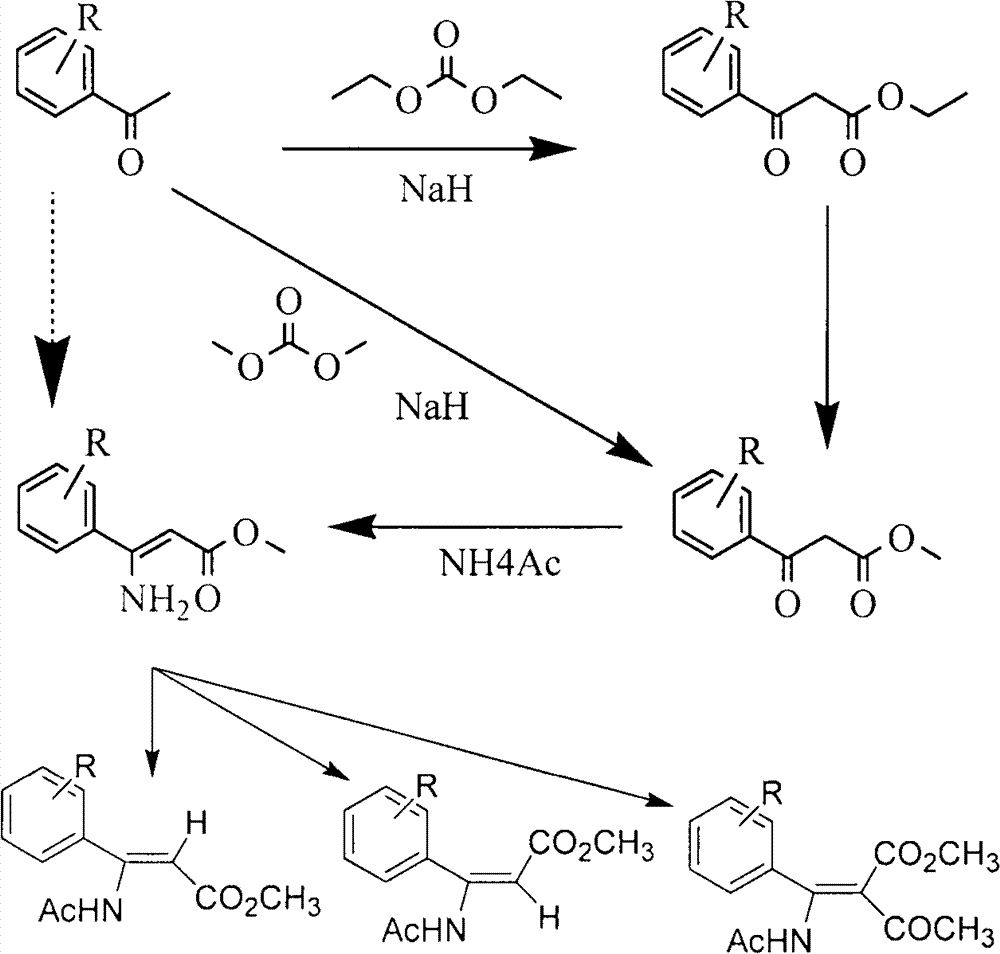
[0028] After N-acylation of amino acid, through enzymatic hydrolysis, the acyl group of one configuration of N-acylated β-amino acid is hydrolyzed to obtain the corresponding β-amino acid, while its corresponding configuration of N-acylated β-amino acid is not hydrolyzed , so as to separate by extraction according to the difference in the lipid-water solubility of the above substances; and then select another enzyme to hydrolyze the N-acylated β-amino acid in the corresponding configuration to obtain the corresponding amino acid, thereby obtaining a single configuration β-amino acid.
[0029] Specifically, β-amino acid acylase (β-Amino acylase) specifically hydrolyzes the amide group of (R) configuration N-acyl β-amino acids to generate free R-β-amino acids, while (S) configuration The amide group of the N-acyl β-amino acid is not hydrolyzed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com