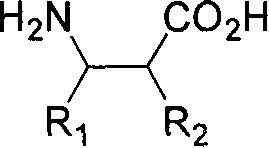
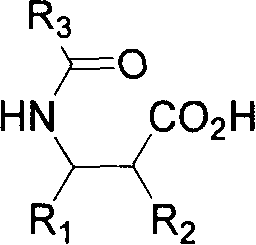
Resolution method of beta-amino acid
An amino acid, amino acid acylase technology, applied in the field of organic synthesis, can solve problems such as inability to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment one (the resolution of comparative example 1 3-phenyl-3-amino-propionic acid ethyl ester)
[0064] a, Synthesis of the substrate: 10g of 3-phenyl-3-amino-propionic acid (purchased) was put into a 100mL reaction bottle, 50mL of ethanol was added, 5g of thionyl chloride was added dropwise at 0°C, and the reaction was carried out at room temperature for about 2-8 hours. complete. The solvent was evaporated to dryness to obtain 13 g of hydrochloride of ethyl 3-phenyl-3-amino-propionate, with a yield of 95%;
[0065] b, split reaction and detection:
[0066] 10mmol (mmol) racemic compound 3-phenyl-3-amino-propionic acid ethyl ester hydrochloride was dissolved in 50mL water, and the pH was adjusted to 7.5-8.5. 10 mg Amano lipase PS was added at a reaction temperature of 30° C., and after a reaction time of 3 hours, part of the reaction liquid was taken out for processing. Add 50 mL of ethyl acetate to extract (R) ethyl 3-phenyl-3-amino-propionate, evaporate to d...
Embodiment 2
[0067] Embodiment two (the resolution of comparative example 2 3-phenyl-3-phenylacetamido-propionic acid)
[0068] a. Synthesis of racemic substrate: 10g of 3-phenyl-3-amino-propionic acid (purchased) was put into a 100mL reaction bottle, 50mL of water was added, 12.5g of potassium carbonate was added, and 14.5g of phenylacetyl chloride was added dropwise at -20°C , low temperature (below 0°C) for 2 hours. The pH was adjusted to 1, and 16 g of white solid was precipitated, with a yield of 93%.
[0069] b. Split reaction and detection:
[0070] 10 mmol of the racemate compound 3-phenyl-3-phenylacetamido-propionic acid was dissolved in 50 mL of NaOH solution (1 mol / L), and the pH was adjusted to 7.5-8.5. 10 mg of penicillin G acylase was added at a reaction temperature of 30° C., and after a reaction time of 30 minutes, part of the reaction solution was taken to separate the product. Use 1M HCl to adjust the pH to 1, extract unreacted S-3-phenyl-3-phenylacetamido-propionic ac...
Embodiment 3
[0073] Embodiment three splits 3-phenyl-3-acetamido-propionic acid with two kinds of enzymes (small test)
[0074] a. Synthesis of racemate substrate: 3-phenyl-3-amino-propionic acid can be purchased conveniently from the market. Dissolve 10 mmol of the racemic compound 3-phenyl-3-amino-propionic acid in 10 mL of NaOH solution (2 mol / L). 12 mmoles of acetic anhydride was added dropwise at 0°C, and the reaction was complete after 5 minutes. Adjust the pH to 1, extract the product with MTBE (methyl tert-butyl ether), and evaporate to dryness on a rotary evaporator to obtain 3-phenyl-3-acetamido-propionic acid.
[0075] b. β-Amino acylase resolution reaction and detection
[0076] 10 mmol of the racemic compound 3-phenyl-3-acetylamino-propionic acid was dissolved in 50 ml of NaOH solution, and the pH was adjusted to 7.0-8.0. Add 10 mg of β-Amino acylase at a reaction temperature of 40°C, and after 30 minutes of reaction time, adjust the pH to 1 with 1M HCl, and extract unreact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com